Abstract
Avian influenza virus (AIV) A/turkey/Oregon/71-SEPRL (TK/OR/71-SEPRL) (H7N3) encodes a full-length NS1 protein and is a weak inducer of interferon (IFN). A variant, TK/OR/71-delNS1 (H7N3), produces a truncated NS1 protein and is a strong inducer of IFN. These otherwise genetically related variants differ 20-fold in their capacities to induce IFN in primary chicken embryo cells but are similar in their sensitivities to the action of IFN. Furthermore, the weak IFN-inducing strain actively suppresses IFN induction in cells that are otherwise programmed to produce it. These phenotypic differences are attributed to the enhanced IFN-inducing capacity that characterizes type A influenza virus strains that produce defective NS1 protein. The pathogenesis of these two variants was evaluated in 1-day-old and 4-week-old chickens. The cell tropisms of both viruses were similar. However, the lesions in chickens produced by the weak IFN inducer were more severe and differed somewhat in character from those observed for the strong IFN inducer. Differences in lesions included the nature of inflammation, the rate of resolution of the infection, and the extent of viral replication and/or virus dissemination. The amelioration of pathogenesis is attributed to the higher levels of IFN produced by the variant encoding the truncated NS1 protein and the antiviral state subsequently induced by that IFN. The high titer of virus observed in kidney tissue (≈109 50% embryo lethal doses/g) from 1-day-old chickens infected intravenously by the weak IFN-inducing strain is attributed to the capacity of chicken kidney cells to activate the hemagglutinin fusion peptide along with their unresponsiveness to inducers of IFN as measured in vitro. Thus, the IFN-inducing capacity of AIV appears to be a significant factor in regulating the pathogenesis, virulence, and viral transmission of AIV in chickens. This suggests that the IFN-inducing and IFN induction suppression phenotypes of AIV should be considered when characterizing strains of influenza virus.
Type A influenza viruses (avian influenza viruses [AIVs]) pose a threat to both avian and animal populations because of their capacity to initiate epornitics, to mutate from low to high pathogenicity in poultry, and to be transmitted as strains with the potential to initiate pandemics (13, 16, 19, 24, 33, 53, 58, 59). The challenge is to understand how this orthomyxovirus, with its eight gene segments (22), each independently transcribed (1), is genetically constituted to generate AIVs that express extremes for both virulence and host specificity (14, 17, 19, 39, 48, 52) and what means can be used to reduce or prevent the acquisition of a high-pathogenicity (HP) phenotype from a low-pathogenicity (LP) progenitor with its propensity to jump the species barrier to a new host. It is clear that sufficient free circulation of H5 and H7 low-pathogenic avian influenza virus (LP-AIV) often leads to mutational changes that result in the appearance of highly pathogenic avian influenza virus (HPAI) (23). Although the probability of generating HP strains from LP strains of AIV during a single replication cycle is intrinsically low, the probability of occurrence increases when the number of cycles is repeated as the virus spreads through the high density of chickens that characterize commercial poultry populations. In this context, there are some 9 billion chickens produced annually in the United States alone (www.ers.usda.gov/News/broilercoverage.htm), with worldwide poultry production estimated at over 30 billion birds. Poultry can serve as important intermediate hosts to generate HPAI with the potential to initiate pandemics.
Clearly, mutations or insertions at the cleavage site in the hemagglutinin (HA) gene increase the range of cellular proteases that make a virus infectious and thus enlarge the range of susceptible tissues in the host; however, the cleavage site is not the only factor that affects the virulence of AIV (22). It is apparent that other AIV genes contribute to the severity of the HP phenotype, including those that encode the polymerase (12, 14, 17, 27, 57) and receptor specificity (33). Another gene, the nonstructural (NS) gene, and one of its products, NS1, enhances AIV pathogenicity by preventing the induction and/or production of interferon (IFN), an early step in the innate defense of the host against the virus (7, 9, 12, 27, 58, 60). By blocking IFN induction and/or production, NS1 effectively prevents the activation of the IFN action pathway and the development of an antiviral state to which AIV is intrinsically sensitive (41, 43, 45). Alterations in NS1, including single amino acid changes, truncations, or deletions, often produce populations of AIV that replicate poorly in cells that are competent for producing IFN (7, 8, 60), grow to low titers in animals, express marked reductions in pathogenesis, and are attenuated (7, 9, 49). These altered phenotypes in mammals correlate with the enhanced IFN-inducing capacity of the virus and its presumed activation of an IFN-mediated antiviral state (7, 9, 25, 49). Alterations of the NS1 protein, a D92E change, also confer resistance to the antiviral effects of IFN-α and other cytokines in a pig lung cell line and exacerbate pathogenesis in pigs (48). However, this same virus is sensitive to IFN action in primary chicken embryonic cells (47a). This report compares for the first time the pathogenesis in chickens that results from infection with two genetically related avian influenza viruses that differ 20-fold in their IFN-inducing capacity. The original A/turkey/Oregon/71 (TK/OR/71) (H7N3) is a field isolate of an AIV that caused mild disease signs in turkeys and about 0.5% mortality (4). When chickens were experimentally inoculated with this virus, no disease signs were observed (3, 4). The initial genetic characterization of TK/OR/71 was carried out on a stock that had undergone multiple egg passages. Analysis of the NS segment revealed a 10-nucleotide deletion in the coding region of NS1. This virus is referred to herein as TK/OR/71-delNS1 (37). This deletion results in a frameshift mutation that produces a truncated NS1 protein of 124 amino acids (Fig. 1); the NS2 protein is unaffected (38). Subsequent sequence analysis of the NS1 gene from a previous low-passage stock of TK/OR/71 revealed an isolate that encoded a full-length NS1 gene. This virus is referred to herein as TK/OR/71-SEPRL (51). This study compares the characteristics of these two genetically related variants in chickens and in cell culture.
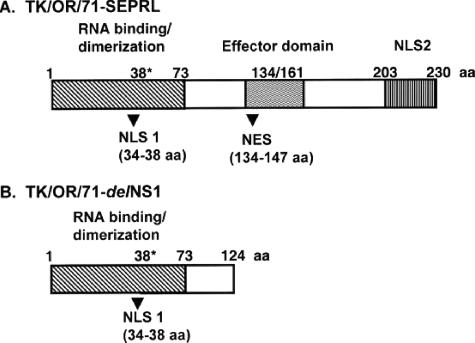
FIG. 1.
Schematic of NS1 proteins encoded by TK/OR/71-SEPRL and TK/OR/71-delNS1. (A) The full-length TK/OR/71-SEPRL NS1 protein consists of 230 amino acids. (B) The truncated NS1 protein from TK/OR/71-delNS1 consists of 124 amino acids. NLS, nuclear localization signal; NES, nuclear export signal; aa, amino acids; 38* represents the only amino acid required for RNA binding but not dimerization (42).
The study of AIV pathogenesis by these two variants that differ in their IFN-inducing capacities but not in their sensitivities to IFN action, as is demonstrated here, gains added importance from previous reports that showed an amelioration of clinical signs in chickens that were administered recombinant chicken alpha interferon (rChIFN-α) continuously in drinking water and challenged with avian viruses causing Newcastle disease (31, 35), infectious bronchitis (40), and infectious bursal disease (35).
(This work was presented in part at the 25th Annual Meeting of the American Society for Virology in Madison, WI, 15 to 19 July 2006 [47a].)
MATERIALS AND METHODS
Viruses and cells.
TK/OR/71-SEPRL (H7N3) encodes a full-length NS1 gene (51), whereas TK/OR/71-delNS1 (H7N3) (kindly provided by Peter Palese, Mount Sinai School of Medicine, NY) encodes a truncated NS1 protein (37). Viruses were propagated by passage in specific-pathogen-free (SPF) 10-day-old embryonating chicken eggs. These virus stocks were aliquoted and stored at −70°C. Primary chicken embryo cells (CEC) were prepared from 9- to 10-day-old embryos, and chicken embryo kidney (CEK) cells were prepared from 18-day-old embryos. Both were obtained as cell suspensions from SPF eggs (Charles River SPAFAS, Inc., North Franklin, CT).
Molecular cloning, PCR amplification, and sequencing of viral isolates.
The complete coding sequences of all eight gene segments were determined for both TK/OR/71-SEPRL and TK/OR/71-delNS1 using methods described previously (5). Briefly, reverse transcriptase PCRs (RT-PCRs) were performed on RNA isolated from TK/OR/71-SEPRL or TK/OR/71-delNS1 stocks. The matrix (M), nucleoprotein (NP), hemagglutinin, and neuraminidase (NA) genes were cloned and sequenced. The NS gene segment was cloned and sequenced previously (51). The three polymerase genes were each amplified in three overlapping pieces, and the PCR products were sequenced directly. All plasmids and PCR products were sequenced using a PRISM Ready Reaction Dye Deoxy Terminator cycle sequencing kit (Perkin-Elmer) and run on an ABI 3700 automated sequencer (Perkin-Elmer).
Sequence analysis.
DNASTAR (Madison, WI) software was used to create sequence contigs, multiple sequence alignments, and percent sequence identities of the gene segments from TK/OR/71-SEPRL and TK/OR/71-delNS1.
Animal experiments.
The standard Office International des Epizooties World Organization for Animal Health pathotyping test was employed by using 4-week-old SPF Plymouth White Rock chickens (39). Briefly, eight birds were intravenously inoculated with a 10-fold dilution of either TK/OR/71-SEPRL or TK/OR/71-delNS1. Birds were monitored 10 days for death. If 75% or more of the birds died, the virus was considered to be highly pathogenic. If less than 75% of the birds died, the virus was considered to be low pathogenic.
Additionally, 4-week-old SPF Plymouth White Rock chickens were inoculated with 107 50% embryo lethal doses (ELD50) of either TK/OR/71-SEPRL or TK/OR/71-delNS1 via the intrachoanal cleft route. Eight birds per virus were sacrificed at both 3 and 6 days postinfection (dpi), and tissues were taken for evaluation by histopathology and immunohistochemistry. Tissues included brain, bursa, thyroid, thymus, lung, kidney with adrenal gland, gonad, heart, liver, spleen, pancreas with duodenum/jejunum, cecal tonsil, upper and lower trachea, nasal cavity, and sinus. Oral and cloacal swabs were obtained from the birds at 3 and 6 dpi and stored at −70°C in sterile brain heart infusion broth containing 100 U/ml penicillin G, 2 μg/ml amphotericin B, and 20 μg/ml gentamicin. SPF 10-day-old embryonated chicken eggs were inoculated with swab fluid to screen for the presence of virus.
TK/OR/71-SEPRL and TK/OR/71-delNS1 were evaluated using 4-week-old SPF Plymouth White Rock chickens for their ability to transmit virus to uninoculated cage mates. Four birds were each inoculated with 107 ELD50 of one of the two viruses by the intrachoanal cleft route. At 1 dpi, four uninoculated birds were placed into cages with the respective virus-inoculated birds. Blood was drawn from the wing vein of all birds at 7, 14, 21, and 28 dpi, and seroconversion was monitored by hemagglutinin inhibition (HI) assay. Oral and cloacal swabs were obtained from all birds beginning at 3 dpi and continuing every 3 days until the end of the experiment. Virus was detected as described above. Two experimentally inoculated birds per cage were euthanized at 14 dpi after blood was drawn to prevent overcrowding. The remaining chickens were euthanized at 28 dpi after blood was drawn.
One-day-old SPF Plymouth White Rock chicks were inoculated intravenously or by intrachoanal cleft with 107 ELD50 of either TK/OR/71-SEPRL or TK/OR/71-delNS1. Chicks were monitored daily for clinical signs of disease and death. Moribund chicks were euthanized with sodium pentobarbital. To prevent bias, three chicks per group were tagged prior to inoculation and euthanized at 3 dpi. Tissues from tagged chicks that died prior to 3 dpi were taken on the day of death for evaluation by histopathology and immunohistochemistry and included nasal cavity, trachea, lung, kidney, and brain. One kidney and one lung were obtained aseptically for virus isolation. The mean death time was calculated by adding the day of death postinoculation for each chick divided by the total number of chicks that died.
Lungs and kidneys from the 1-day-old chicks were transferred to tubes containing brain heart infusion broth with 100 U/ml penicillin G, 2 μg/ml amphotericin B, and 20 μg/ml gentamicin. The weight of the organ was determined, and the organs were ground mechanically using an aseptic technique. Ten-day-old SPF embryonated chicken eggs were inoculated with the organ solutions and monitored for death. The ELD50 for each positive sample was then determined with 10-day-old-embryonated eggs, and the ELD50/gram of tissue was calculated.
All animals were housed in Horsfal-Bauer stainless steel isolation units ventilated under negative pressure with HEPA-filtered air in biosafety level 3 agriculture facilities. Animal care was provided as required by the Institutional Animal Care and Use Committee based on the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (1a) Food and water were provided ad libitum.
Histopathology and immunohistochemistry.
Tissues were fixed in 10% neutral buffered formalin solution, sectioned, and stained with hematoxylin and eosin. Duplicate sections were stained immunohistochemically to determine influenza viral antigen distribution in individual tissues. A monoclonal antibody against influenza virus A nucleoprotein (P13C11) (5), developed in our laboratory, was used as the primary antibody in a streptavidin-biotin-alkaline phosphatase complex immunohistochemical method as previously described (52).
IFN action: virus plaque reduction assays.
The sensitivities of the low- and high-IFN-inducing strains of TK/OR/71 to interferon action were determined by both a yield reduction assay on primary CEC that required trypsin to propagate the virus and a plaque reduction (50% plaque reduction) assay on primary CEK cells prepared from 18-day-old embryos (Charles River SPAFAS, Storrs, CT) (47). In the latter case, confluent cell monolayers were established and treated overnight with rChIFN-α in terms of effective vesicular stomatitis virus (VSV) 50% plaque reduction units/ml. CEK cells did not require trypsin in the agarose overlay during plaque formation, nor did they produce significant amounts of IFN upon infection with a strong inducer. This meant that the survival curves of AIV plaque-forming particles (PFP) were generated as a function of IFN dose under conditions where only the exogenously added rChIFN-α contributed to the antiviral state developed during the assay. This is particularly important when the sensitivities of two closely related viruses that differ significantly in their IFN-inducing capacities are compared. VSV Indiana is sensitive to rChIFN-α and was used as a standard to determine the effective dose of IFN (45).
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this paper were deposited in the GenBank database under accession numbers DQ870885 to DQ870898. The NS1 and matrix sequences for TK/OR/71-SEPRL were previously reported to GenBank under accession numbers U96740 and M16623.
RESULTS
Sequence analysis of TK/OR/71-SEPRL and TK/OR/71-delNS1.
Table 1 provides a complete sequence analysis of the genomes of both AIV variants used in this study. The data were generated by PCR amplifying, cloning, and sequencing the HA, NA, NP, M, and NS genes and by directly sequencing the PCR products produced by amplifying the polymerase genes (PA, PB1, and PB2). These data show that in addition to the differences already noted in the NS1 genes of these two variants of TK/OR/71, there are also differences in the nucleotide sequences that result in amino acid changes in the HA, NP, and M genes. There are two amino acid changes each predicted in the HA and M1 proteins and one amino acid change in the NP protein (Table 1). These five changes are point mutations and are likely a reflection of the quasispecies nature of AIV and other RNA viruses. Since the two variants are equally sensitive to the action of IFN (see Fig. 4), these amino acids changes do not affect the IFN action pathways. However, the variant with the large deletion in the NS1 gene induces 20 times more IFN than the variant with the intact NS1 gene, in keeping with the enhancement of the IFN-inducing capacity that has been shown to accompany mutations or deletions in the NS1 gene (7, 27, 60). Thus, the phenotypic differences observed between these viruses in the experiments described herein are most likely related to genotypic differences in the NS1 gene.
TABLE 1.
Percent nucleotide and amino acid identities for TK/OR/71-SEPRL and TK/OR/71-delNS1
| Gene | % Nucleotide identity (no. of nucleotides that are different) | Nucleotide(s)a | % Amino acid identity (no. of amino acids that are different) | Amino acid(s)a |
|---|---|---|---|---|
| PA | 100 (0) | 100 (0) | ||
| PB1 | 100 (0) | 100 (0) | ||
| PB2 | 99.9 (1) | 1350 | 100 (0) | |
| HA | 99.8 (3) | 45, 638, 677 | 99.6 (2) | 213, 226 |
| NA | 99.9 (1) | 372 | 100 (0) | |
| NP | 99.8 (3) | 27, 573, 1518 | 99.8 (1) | 495 |
| M | 99.7 (3) | 505, 537, 587 | 99.2 (2) (M1) | 175, 192 |
| 100 (0) (M2) | ||||
| NS | 99.4 (10) | 365-374b | 94.4 (—)c (NS1) | 118-230 |
| 100 (0) (NS2) |
Nucleotide numbers or amino acid residues that are different.
Corresponds with nucleotides 377 to 386 reported previously by Norton et al. (37).
The NS1 proteins of TK/OR/71-SEPRL and TK/OR/71-delNS1 are identical until the nucleotide deletion in the latter variant introduced a frameshift mutation. The NS1 protein is truncated after 124 amino acids; thus, the number of differences was not determined.
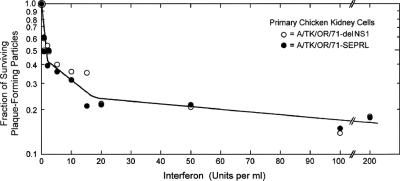
FIG. 4.
Comparison of the survival of TK/OR/71-SEPRL and TK/OR/71-delNS1 infectious virus measured by plaque formation on primary CEK cell monolayers as a function of ChIFN-α dose. Confluent monolayers of CEK cells prepared from 18-day-old embryos were treated for 24 h with various doses of rChIFN-α, infected with virus, incubated at 37.5°C for 3 days, and stained with neutral red; the number of plaques were determined relative to untreated cells; and the fraction of surviving PFP was determined. The triphasic slopes of the curves generated are considered experimentally indistinguishable and have been observed and analyzed previously with other AIV strains (45).
Figure 1 represents a schematic drawing of the NS1 protein produced by TK/OR/71-SEPRL (A) and TK/OR/71-delNS1 (B), its truncated counterpart. Important binding and functional sites and nuclear transport signals that have been mapped (42) are shown on the diagram. The NS1 protein of TK/OR/71-delNS1 consists of amino acids 1 to 124. The full-length NS1 encoded by TK/OR/71-SEPRL contains signals for nuclear localization and nuclear export in an effector domain at the C terminus (42).
Reduction in virus shedding from TK/OR/71-delNS1-infected chickens.
Both TK/OR/71-SEPRL and TK/OR/71-delNS1 were determined to be LP-AIVs using the standard Office International des Epizooties pathotyping test (39). When 4-week-old chickens were inoculated intravenously with 107 ELD50 of TK/OR/71-SEPRL, one out of eight birds died by 10 days postinfection, and none of the TK/OR/71-delNS1-infected birds died (0/8 birds). The pathogenesis of TK/OR/71-SEPRL and TK/OR/71-delNS1 was then evaluated using 4-week-old chickens to determine if the enhanced IFN-inducing capacity of TK/OR/71-delNS1 as measured in vitro affected the pathogenesis of the virus as determined in vivo. When chickens were inoculated in the intrachoanal cleft with 107 ELD50 of either the low- or high-IFN-inducing strains, no birds from either group exhibited clinical signs of disease. However, the number of birds shedding virus in oral and cloacal samples 3 and 6 dpi was reduced for birds inoculated with TK/OR/71-delNS1 compared to those inoculated with TK/OR/71-SEPRL (Table 2).
TABLE 2.
Frequency of virus detection from 4-week-old chickens infected with TK/OR/71-SEPRL or TK/OR/71-delNS1 via the intrachoanal cleft
| Virus | No. of positive samples/no. of bird sampled at:
|
|||
|---|---|---|---|---|
| 3 dpi
|
6 dpi
|
|||
| Oral | Cloacal | Oral | Cloacal | |
| TK/OR/71-SEPRL | 12/32 | 6/32 | 0/16 | 4/16 |
| TK/OR/71-delNS1 | 2/32 | 0/32 | 0/16 | 0/16 |
Comparison of lesions and influenza virus antigen produced in 4-week-old chickens infected with TK/OR/71-SEPRL or TK/OR/712-delNS1.
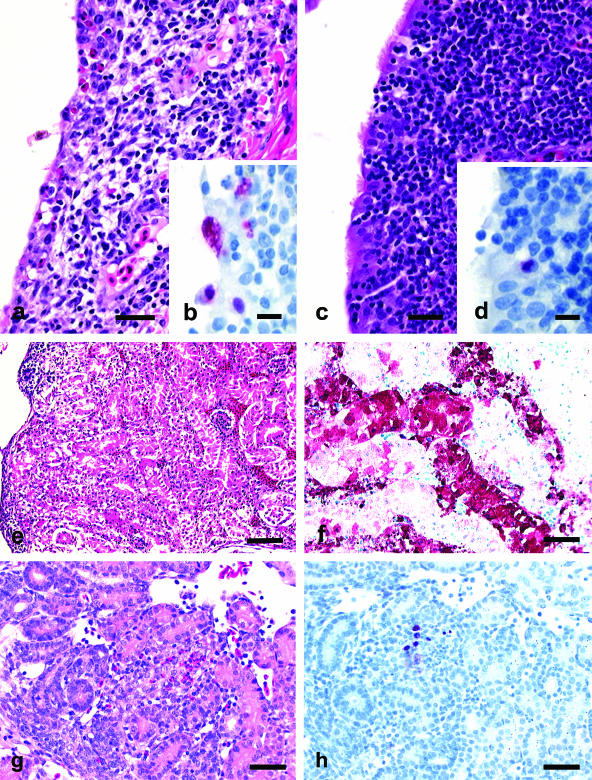
Figure 2a to d shows that TK/OR/71-SEPRL produced more severe lesions and more avian influenza virus nucleoprotein in tissues of 4-week-old chickens than TK/OR/71-delNS1. At 3 dpi in 4-week-old chickens inoculated in the intrachoanal cleft, both the TK/OR/71-SEPRL and TK/OR/71-delNS1 strains produced moderate lymphocytic rhinitis and sinusitis and hyperplasia of bronchus-associated lymphoid tissue, but the nasal epithelium of TK/OR/71-SEPRL-infected chickens had more severe necrosis and more consistent demonstration of AIV nucleoprotein. On 6 dpi, the TK/OR/71-SEPRL group had moderate lymphocytic rhinitis and sinusitis and mild tracheitis, while chickens given TK/OR/71-delNS1 had only minimal lesions within the upper respiratory tract.
FIG. 2.
Experimental studies in 1-day-old and 4-week-old chickens inoculated with TK/OR/71-SEPRL or TK/OR/71-delNS1. (a, c, e, and g) Photomicrographs of hematoxylin- and eosin-stained tissue sections. (b, d, f, and h) Photomicrographs of tissue sections stained immunohistochemically to demonstrate avian influenza virus nucleoprotein. (a) Lymphocytic to heterophilic sinusitis with necrosis of respiratory epithelium in a 4-week-old chicken challenged with TK/OR/71-SEPRL via the intrachoanal cleft at 3 dpi. Bar, 25 μm. (b) Avian influenza nucleoprotein in respiratory epithelium of the infraorbital sinus in a 4-week-old chicken challenged with TK/OR/71-SEPRL via the intrachoanal cleft at 3 dpi. Bar, 10 μm. (c) Lymphocytic sinusitis with intact ciliated respiratory epithelium from a 4-week-old chicken challenged with TK/OR/71-delNS1 by intrachoanal cleft at 3 dpi. Bar, 25 μm. (d) Rare avian influenza nucleoprotein in respiratory epithelium of the infraorbital sinus from chicken in c. Bar, 10 μm. (e) Severe necrosis in kidney tubules from a 1-day-old chick intravenously challenged with TK/OR/71-SEPRL at 3 dpi. Bar, 50 μm. (f) Extensive staining of avian influenza nucleoprotein in necrotic kidney tubules from the chick in e. Bar, 25 μm. (g) Mild focal heterophilic interstitial nephritis with associated tubule necrosis in kidney from a 1-day-old chick intravenously challenged with TK/OR/71-delNS1 at 3 dpi. Bar, 50 μm. (h) Infrequent staining for avian influenza nucleoprotein in necrotic tubule epithelium and macrophages from the chick described in g. Bar, 25 μm.
TK/OR/71-SEPRL is virulent in 1-day-old chicks.
Since 1-day-old chicks display clinical signs when infected with avian influenza viruses more often than adult birds, they were inoculated by intrachoanal cleft or intravenously with TK/OR/71-SEPRL or TK/OR/71-del to compare clinical disease signs. This would determine whether the regulation of the IFN-inducing capacity of the variants by the NS1 protein was reflected by differences in virulence in vivo. Table 3 shows the avirulence of TK/OR/71-SEPRL or TK/OR/71-delNS1 inoculated into chicks by the intrachoanal cleft route: one of eight and zero of eight birds died, respectively. However, there was a marked difference in virulence when the birds were inoculated intravenously with either of the two variants: seven of eight birds inoculated intravenously with TK/OR/71-SEPRL died, while only one of seven birds inoculated with TK/OR/71-delNS1 died. The mean death time for birds intravenously inoculated with TK/OR/71-SEPRL was 3.4 days.
TABLE 3.
Mortality of 1-day-old chicks inoculated intravenously or intrachoanally with TK/OR/71-SEPRL or TK/OR/71-delNS1
| Route of inoculation | No. of birds that died/total no. of birds (no. of birds that were euthanized when moribund) | Time (days) |
|---|---|---|
| TK/OR/71-SEPRL | ||
| Intrachoanal | 1/8 (0) | 3a |
| Intravenous | 7/8 (1) | 3.43 |
| TK/OR/71-delNS1 | ||
| Intrachoanal | 0/8 (0) | 0 |
| Intravenous | 1/7 (1) | 8a |
Only one bird died at the day postinfection noted.
A lung and a kidney were removed aseptically from the chicks that were evaluated in the pathology experiment described above at 3 dpi for virus isolation. Table 4 shows that all lungs and kidneys tested from birds inoculated with TK/OR/71-delNS1 were negative for virus regardless of the route of infection. These organs were negative for virus when chicks inoculated with TK/OR/71-SEPRL via the intrachoanal route were tested. In contrast, all lungs and kidneys from chicks inoculated intravenously with TK/OR/71-SEPRL were positive for virus and contained high titers of virus. The lungs averaged 2.6 × 106 ELD50/g of tissue, whereas the titer in kidneys was about 500-fold higher (1.3 × 109 ELD50/g).
TABLE 4.
Virus detection and titers from lung and kidneys of 1-day-old chicks inoculated with TK/OR/71-SEPRL or TK/OR/71-delNS1
| Virus | No. of positive samples/no. of samples tested at 3 dpi (avg tissue titer [ELD50/g of tissue])
|
|||
|---|---|---|---|---|
| Intrachoanal route
|
Intravenous route
|
|||
| Lung | Kidney | Lung | Kidney | |
| TK/OR/71-SEPRL | 0/3 | 0/3 | 3/3 (2.55 × 106) | 3/3 (1.33 × 109) |
| TK/OR/71-delNS1 | 0/3 | 0/3 | 0/3 | 0/3 |
Evidence of virus in the kidney indicates a systemic infection of 4-day-old chicks by TK/OR/71-SEPRL, the weak inducer of IFN, but not by TK/OR/71-delNS1, the strong inducer of IFN. In this context, monolayers of CEK cells prepared from 18-day-old embryos produced low levels of IFN when infected with TK/OR/71-delNS1 (Fig. 3, bottom curve), which otherwise functions as a good inducer of IFN on chicken whole embryonic cells aged in vitro to allow the development of the IFN system (Fig. 3, top curve) (44). CEK cells support plaque formation of these two AIV variants in the absence of trypsin in the overlay, indicating that CEK cells contain a protease capable of activating the fusion peptide of AIV that is necessary for its cell-to-cell spread during plaque formation (18, 22). Monolayers prepared from 10-day-old CEC require trypsin in the overlay to sustain plaque formation by these two variants and thus presumably do not express this protease. Stocks of both variants of TK/OR/71 were egg derived and hence contained activated fusion peptides that allowed the inoculum virus entry into the cell (18, 22, 27). These conditions show that both variants replicate well in kidney cells where IFN induction is compromised and where protease presumably can activate the fusion peptide. However, the capacity of TK/OR/71-SEPRL to suppress IFN induction as measured in vitro (27) provides an additional replication advantage by further down-regulating IFN production. This situation is thought to account for the high titers of TK/OR/71-SEPRL observed in the kidney tissue of birds (Table 4).
FIG. 3.
Comparison of the IFN induction dose (multiplicity)-response (IFN yield) curves for TK/OR/71-delNS1 in monolayers of developmentally aged primary CEC and primary CEK cells from 18-day-old embryos. CEC were made from 10-day-old embryos and incubated in vitro for 9 days (total developmental age, 19 days) before inducing IFN. CEK cells were made from the kidneys of 18-day-old embryos and incubated in vitro for 2 days (total developmental age, 20 days) before inducing IFN. At the time of induction, there were 107 cells per monolayer. The infected/induced cells were incubated at 40.5°C for 24 h, and the medium was harvested, processed, and assayed for IFN as described previously (47).
Severe lesions and abundant avian influenza virus antigen in TK/OR/71-SEPRL-infected 1-day-old chicks.
In 1-day-old chicks infected by the intrachoanal cleft route, TK/OR/71-SEPRL and TK/OR/71-delNS1 produced inflammatory lesions in the upper respiratory tract similar to those described above for the 4-week-old challenged birds. The necrosis of the respiratory epithelium was more frequent and more severe in chicks inoculated with TK/OR/71-SEPRL. In contrast, 1-day-old chicks inoculated intravenously with TK/OR/71-SEPRL produced moderate to severe multifocal necrosis in kidney tubules (Fig. 2e), mild to severe heterophilic interstitial pneumonia and edema, and severe necrosis of nasal gland epithelium (data not shown). Figure 2f shows abundant levels of nucleoprotein in necrotic cells of tissues from TK/OR/71-SEPRL-infected chicks. In contrast, chicks inoculated with TK/OR/71-delNS1 developed only minimal nephritis with the sporadic appearance of avian influenza virus nucleoprotein.
TK/OR/71-delNS1 does not transmit efficiently from infected chickens to uninoculated cage mates.
Both TK/OR/71-SEPRL and TK/OR/71-delNS1 were compared for their ability to spread naturally from experimentally infected chickens to contact control birds. Hemagglutination inhibition assays were used to measure the levels of serum antibody titers for all birds in the study and showed that all birds inoculated with either TK/OR/71-SEPRL or TK/OR/71-delNS1 seroconverted (HI titer of ≥8) (Table 5). Birds that were infected with TK/OR/71-SEPRL had higher HI titers than those infected with TK/OR/71-del. Although this difference was not statistically significantly (P = 0.06), it may be biologically relevant. When sera from uninoculated cage mates were tested, all birds housed with the TK/OR/71-SEPRL-infected chickens seroconverted to high titers. In marked contrast, none of the uninoculated birds housed with the TK/OR/71-delNS1-infected chickens seroconverted (Table 5). This difference was highly statistically significant (P = 0.0004).
TABLE 5.
Transmissibility of TK/OR/71-SEPRL and TK/OR/71-delNS1 in 4-week-old white Plymouth Rock chickens
| Virus | Intrachoanally infected birds
|
Uninoculated cage mates
|
||||||
|---|---|---|---|---|---|---|---|---|
| Seroconversiona
|
No. of positive swabs/total no. of swabse
|
Seroconversiona
|
No. of positive swabs/total no. of swabse
|
|||||
| No. positive/total no. | HI titer (geo mean)b,c | Tracheal | Cloacal | No. positive/total no. | HI titer (geo mean)b,d | Tracheal | Cloacal | |
| TK/OR/71-SEPRL | 4/4 | 256 | 3/4 | 2/4 | 4/4 | 861 | 4/4 | 4/4 |
| TK/OR/71-delNS1 | 4/4 | 38 | 1/4 | 0/4 | 0/4 | 0 | 1/4 | 0/4 |
Samples were considered positive if the HI titer was ≥8.
Geometric mean (geo mean) was determined as previously described (4a). A t test was performed using two-sample equal variance with two-tailed distribution comparing HI titers from TK/OR/71-SEPRL- and TK/OR/71-delNS1-infected birds or comparing the HI titers from the uninocultated cage mates housed with either TK/OR/71-SEPRL- or TK/OR/71-delNS1-infected birds.
P = 0.06 and is not significant at the level of P = 0.05.
P = 0.0004 and is significant at the level of P = 0.05
Birds were swabbed every 3 days from 3 to 28 dpi. Data shown were obtained through 15 dpi, when the last positive swab was recorded.
Sensitivity of TK/OR/71-SEPRL and TK/OR/71-delNS1 to the action of IFN.
One possible explanation for the reduced pathogenesis observed in chickens infected with TK/OR/71-delNS1 relative to that expressed by TK/OR/71-SEPRL is that the former virus is more sensitive to the action of IFN than is the latter virus. To test this possibility, the survival of infectious virus, measured as PFP, for each virus was determined in monolayers of primary CEK cells as a function of the IFN dose. Figure 4 shows the survival curves generated under these conditions: they are considered experimentally indistinguishable. The initial exponential loss of PFP activity reveals that about 60% of the population of both viruses is equally and highly sensitive to the action of IFN at low doses (≤2 IFN U/ml). The slope of the survival curve is comparable to that observed for VSV, which was used as a standard because of its high sensitivity to IFN action (46). The slope of the PFP survival curve at higher IFN doses (≈2 to 20 U/ml) shows that about 15% of the virus in both populations is ≈30 times more resistant to the action of the ChIFN-α than the majority population. At doses of >20 U/ml, about 25% of the AIV populations was over 100 times more resistant to IFN action. The nature of this “resistant” fraction was not determined, but in comparable studies with an HPAI (H5N2) strain, the resistance was transient in nature and was shown to regain sensitivity in the next round of gene packaging (45). Since these experiments were carried out in cells that did not respond well to inducers of IFN (Fig. 3), the contribution of endogenously induced (autocrine) IFN was nil, and the observed survival curves represent a true measure of the IFN dose. Similar results were obtained using PFP yield reduction assays on trypsin-treated CEC (data not shown).
DISCUSSION
Mutations, truncations, or deletions of the NS gene in type A influenza viruses that lead to the synthesis of aberrant NS1 protein, or its absence, have been shown to enhance the IFN-inducing capacity of the virus both in vitro (7, 11, 27, 58) and in vivo (9). Viruses altered in this manner replicate poorly and display attenuated pathogenesis in mice (7, 9) and swine (49). Data presented here provide evidence that the NS1 protein and its regulation of the IFN-inducing capacity of avian influenza virus (10, 21, 27) play a comparable role in chickens. Both TK/OR/71-SEPRL and TK/OR/71-delNS1 are genetically related LP type A avian influenza viruses that caused no clinical signs of disease in 4-week-old birds. However, TK/OR/71-SEPRL, a suppressor of IFN induction and itself a weak inducer of IFN (≈400 U/107 cells), was shed and transmitted more efficiently from infected birds and produced more severe pathological lesions in both 4-week-old chickens and 1-day-old chicks than did TK/OR/71-delNS1, a strong inducer of IFN (≈9,000 U/107 cells) in developmentally aged chicken cells (27).
Chicken kidney embryo cells were relatively nonresponsive to the induction of IFN compared to aged CEC in vitro (Fig. 3) and supported plaque formation from both variants of TK/OR/71 in the absence of the trypsin required to produce plaques in CEC (Fig. 4). From these attributes, we infer that the CEK cells express a protease that cleaves the hemagglutinin protein of AIV and produces virions that can sustain plaque formation in these permissive host cells. In this context, quantification of infectious AIV in kidneys from 1-day-old chicks 3 days after intravenous inoculation may serve as a rapid means of assessing the pathogenesis of AIV.
Survival curves of AIV PFP on CEK cells generated as a function of the IFN dose demonstrate that both TK/OR/71-SEPRL and TK/OR/71-delNS1 are equally sensitive to the action of IFN (Fig. 4). AIV yield reduction assays also showed that the two variants were similarly sensitive to IFN action but did not produce a triphasic curve (data not shown). Thus, the variant that induces the most IFN results in the amelioration of pathogenesis. The IFN induced by that virus, TK/OR/71-delNS1, would result in the development of a latent antiviral state occurring with the highest probability in adjacent host cells. That latent antiviral state would become activated upon the exposure of cells to newly synthesized AIV double-stranded RNA (dsRNA) (26) or virus containing or producing dsRNA upon introduction into the cell (27). The effectiveness of free dsRNA is likely to be low because of the coinduction and secretion along with IFN of a double-stranded RNase (dsRNase) from chicken cells (34). Internalized particles of AIV that activate the latent antiviral state need not be infectious, and the 20-fold excess of noninfectious IFN-inducing particles (IFP) in TK/OR/71-delNS1 populations (27) means that there is a large number of virus particles released from infected cells that can both induce an antiviral state and activate it in cells surrounding the original focus of infection. These IFP may play a significant role in the extent of pathogenesis expressed in chickens infected by AIV. In the case of a strong IFN inducer like TK/OR/71-delNS1, virus replication would be compromised, and virus shedding would be reduced or eliminated, resulting in an ameliorated disease state. In contrast, the large excess of IFN induction-suppressing particles (ISP) present in populations of TK/OR/71-SEPRL (27) would suppress IFN production in surrounding cells, favor replication of the virus, and enhance pathogenesis, as was observed.
The histopathological and immunohistochemical data support this view and show that although the main sites of replication for TK/OR/71-SEPRL and TK/OR/71-delNS 1 were similar, the histopathologies and abundances of antigen for the two viruses differed markedly (Fig. 2): TK/OR/71-SEPRL, which displays an ISP phenotype (27), caused moderate to severe tissue damage and produced abundant antigen at the sites of infection (Fig. 2), while TK/OR/71-delNS1, which displays an IFP phenotype (27), had little or no effect on tissues and rarely produced detectable levels of antigen (Fig. 2). Since TK/OR/71-SEPRL (ISP) and TK/OR/71-delNS1 (IFP) grew to equivalent titers in 10-day-old embryonated eggs and in trypsin-treated young primary CECs and Vero and MDCK cells (data not shown), it appears that in cells compromised for IFN-producing capacity, TK/OR/71-delNS1 is as infectious as TK/OR/71-SEPRL, as was reported previously for other strains of type A influenza virus (8, 11). Furthermore, the titer of TK/OR/71-delNS1 is >10-fold less when grown in 14-day-old embryonated eggs (A. N. Cauthen and D. L. Suarez, unpublished observation), consistent with the enhanced expression of the IFN system as the chicken embryo develops (44) and the enhanced replication of AIV compromised in its expression of the NS1 gene in younger embryonated eggs (55, 60).
Since stocks of TK/OR/71 are produced in eggs, the fusogenic peptide is activated and hence is not rate limiting in the infectious process (22). Consequently, the reduced ability of TK/OR/71-delNS1 to initially infect cells is not likely to be the cause of the reduced pathogenesis of the virus in chickens. It seems more likely that the large excess of IFN-inducing particles that make up the TK/OR/71-delNS1 population induces high levels of IFN, which in turn induces an antiviral state in cells surrounding the initial site of infection, thereby compromising the yield of virus and further transmission of this IFN-sensitive virus. Another mechanism may also contribute to reduced yields of virus. Type I interferons were previously shown to be essential to initiate apoptotic death in virus-infected mammalian cells (56). In this context, the combined sequential addition of IFN to cells followed by exposure to dsRNA hours later exacerbates cell killing (50) and reduces virus replication in chicken cells (30) by activating apoptosis (31a), a demonstrated mode of cell killing by AIV (15, 54). This condition is likely to prevail during infection of chickens with a virus capable of inducing high levels of IFN and containing large numbers of IFP. Consider the following: IFN is detectable within a few hours after infection of chicken cells with TK/OR/71-delNS1, and by 10 h postinfection, the cells surrounding the originally infected cell are bathed in high concentrations of IFN (27). The latent antiviral state induced under these conditions, and the sensitization of cells by IFN to apoptosis mediated in part by dsRNA, would be activated upon exposure to AIV dsRNA released during infection (26) or in the form of the large excess of IFP produced by TK/OR/71-delNS1 (27). These IFP and any AIV dsRNA released by the disintegrating infected cell (26) should be a rich source of dsRNA, an otherwise rate-limiting reactant to which the chicken cell is exquisitely responsive (27, 28, 32). In contrast, TK/OR/71-SEPRL induces low amounts of IFN during infection, and its 20-fold excess of noninfectious ISP/PFP would be expected to prevent IFN induction in any cell that is otherwise competent to produce IFN (27).
For these reasons, and extrapolating from data acquired in vitro, differences observed between TK/OR/71-SEPRL and TK/OR/71-delNS1 in the animal experiments are best accounted for by an IFN-mediated reduction in virus replication rather than a diminished dissemination of the virus in the host per se. Studies planned for the direct measurement of IFN levels in serum would clarify this point. Although either mechanism would result in reduced virus shedding and inefficient viral transmission to a new host, it seems unlikely that the NS1 protein would affect the physical release of the virus from the infected cell or cell tropism since those functions have been attributed to the neuraminidase and hemagglutinin proteins, respectively (22).
The C-terminal domain of NS1 from TK/OR/71-SEPRL, like other AIVs, binds CPSF30, a cellular factor required for the 3′-end processing of cellular pre-mRNAs (10, 21, 36, 58). Thus, any IFN mRNA induced/transcribed during infection would not be translated, thereby effectively blocking the production of IFN and its activation of any IFN action pathway. In contrast, the C terminus of TK/OR/71-delNS1 is missing, and CPSF30 is free to properly process cellular pre-mRNAs, like that encoding IFN, setting IFN-mediated antiviral pathways and the observed amelioration of pathogenesis in chickens infected with this variant into motion. Interestingly, the dsRNA binding region of the TK/OR/71-delNS1 variant remains intact and hence might be expected to prevent IFN induction by sequestering viral dsRNA. However, the threshold for inducing IFN in chicken cells is exquisitely low, one molecule per cell (28), an amount of dsRNA that might easily escape sequestration. The induction, production, and action of IFN allowed by the TK/OR/71-delNS1 variant are thought to be responsible for the observed decreased levels of progeny virus and NP antigen and the subsequent minimal damage of tissue.
Since both variants of TK/OR/71 AIV are equally sensitive to the action of IFN, the C-terminal portion of the NS1 protein does not contribute any more, or less, protection against the antiviral effects of IFN-α. This points again to the enhanced induction of IFN by TK/OR/71-delNS1 and its action in the host as the primary underlying difference between the pathologies of these two genetically closely related viruses. This view is in accord with the observations of studies carried out with mice in which the IFN-inducing capacity of the attenuated AIV strains expressing aberrant NS1 was elevated and viral pathogenesis was attenuated (7, 9). Studies of swine gave comparable results (49).
Although the specific mechanism of the antiviral action of ChIFN-α was not addressed, evidence for two reactants in IFN action pathways that were demonstrable in mammalian cells has been documented in chicken cells: the 2′,5′-oligoadenylate synthetase (2) and the double-stranded RNA-dependent protein kinase (PKR) pathway (29, 32). The Mx system, which was shown to be important in mammalian cells, may be operative in chickens but only infrequently, being restricted to those few birds that have a unique amino acid change in the Mx protein (20). Two other reactants that avian cells produce following virus infection may also contribute to the reduction of virus shedding in AIV-infected chickens: ChIFN-γ (6, 61), with its capacity to act synergistically with ChIFN-α (46), and a secreted dsRNase unique to avian species and coinduced with IFN (34). In the case of ChIFN-γ, the synergistic action of the two types of ChIFN can enhance the antiviral state up to 10-fold over the action of either IFN acting alone, even at levels of IFN in the 1-U/ml range (46). In addition, the combined action of the two IFNs accelerates the rate at which nitric oxide is produced from chicken macrophages and the maximal levels reached (46). The dsRNA-induced and secreted dsRNase that is unique to avian species have been observed in the sera of chickens (I. C. Tomazos, L. Van Der Heide, and P. I. Marcus, unpublished observations). It is not known what role, if any, the endoribonuclease plays as a defense mechanism against avian viruses like AIV. However, we note that viral dsRNA in medium bathing AIV-infected cells has been reported (26) and that the degradation of viral dsRNA with serum dsRNase may represent a cellular mechanism to regulate the induction of IFN, a potent biological response modifier.
Other avian influenza viruses that encode full-length NS1 genes [A/chicken/Pennsylvania/13690/93 (H5N2) and A/chicken/Alabama/75 (H4N8)] were shown to be sensitive to ChIFN-α action in primary CEC, as was WSN, a human-derived nonavian laboratory strain (data not shown), along with TK/ONT/7732/66 (H5N9) in plaque reduction assays (45). Thus, TK/OR/71-SEPRL and TK/OR/71-delNS1 do not appear to be unique in their sensitivity to ChIFN-α.
These data indicate that the regulation of the IFN-inducing capacity, i.e., the expression of the IFP/ISP (ifp/isp) phenotypes by the NS1 protein, contributes significantly to the pathogenesis and natural chicken-to-chicken transmission of virus in otherwise LP avian influenza viruses. Since ChIFN-α functions as an immunostimulant/adjuvant in chickens when delivered perorally to the oromucosal region (27a), it may be appropriate to determine the ifp/isp phenotypes of viruses used in live attenuated or inactivated whole virus vaccines. The ifp/isp phenotype may aid in predicting the efficacy of the virus as a vaccine. As others have noted previously, the NS gene may be a good target for manipulation to develop live vaccines (9, 55) or antivirals (58) for avian influenza virus, with its primary advantage of the activation of the IFN system through the induction of IFN as was shown in mammals and now in chickens.
Acknowledgments
We thank Joan Beck, Liz Turpin, Suzanne DeBlois, John Latimer, and Joyce Bennett for technical assistance and Roger Brock for animal care. We also thank Peter Palese for providing TK/OR/71-delNS1, Kathy Spindler for useful comments on the manuscript, Daniel Promislow for assistance with statistical analysis, and the South Atlantic Area DNA Sequencing Facility for assistance with sequencing the TK/OR/71-SEPRL and TK/OR/71-delNS1 genes. The study benefited from use of the Animal Cell Culture Facility of the Biotechnology/Bioservices Center at the University of Connecticut.
This work was supported by USDA/ARS CRIS project number 6612-32000-022-98 and USDA grant 58-1940-0-007 through the Center of Excellence for Vaccine Research at the University of Connecticut.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Abraham, G. 1979. The effect of ultraviolet radiation on the primary transcription of influenza virus messenger RNAs. Virology 97:177-182. [DOI] [PubMed] [Google Scholar]
- 1a.Anonymous. 1999. Guide for the care and use of agricultural animals in agricultural research and teaching. Federation of Animal Science Societies, Savoy, IL.
- 2.Ball, L. A. 1979. Induction of 2′5′-oligoadenylate synthetase activity and a new protein by chick interferon. Virology 94:282-296. [DOI] [PubMed] [Google Scholar]
- 3.Beard, C. W., and B. C. Easterday. 1973. A turkey/Oregon/71, an avirulent influenza isolate with hemagglutinin of fowl plague virus. Avian Dis. 17:173-181. [PubMed] [Google Scholar]
- 4.Beard, C. W., and D. H. Helfer. 1972. Isolation of two turkey influenza viruses in Oregon. Avian Dis. 16:1133-1136. [PubMed] [Google Scholar]
- 4a.Brugh, M. 1977. A simple method for recording and analyzing serological data. Avian Dis. 22:362-365. [PubMed] [Google Scholar]
- 5.Cauthen, A. N., D. E. Swayne, S. Schultz-Cherry, M. L. Perdue, and D. L. Suarez. 2000. Comparisons of highly virulent H5N1 influenza viruses isolated from humans and chickens from Hong Kong. J. Virol. 74:6592-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Digby, M. R., and J. W. Lowenthal. 1995. Cloning and expression of the chicken interferon-gamma gene. J. Interferon Cytokine Res. 15:939-945. [DOI] [PubMed] [Google Scholar]
- 7.Donelan, N. R., C. F. Basler, and A. García-Sastre. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77:1357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egorov, A., S. Brandt, S. Sereinig, J. Romanova, B. Ferko, D. Katinger, A. Grassauer, G. Alexandrova, H. Katinger, and T. Muster. 1998. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J. Virol. 72:6437-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferko, B., J. Stasakova, J. Romanova, C. Kittel, S. Sereing, H. Katinger, and A. Egorov. 2004. Immunogenicity and protection efficacy of replication-deficient influenza A viruses with altered NS1 genes. J. Virol. 78:13037-13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortez, P., A. Beloso, and J. Ortin. 1994. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks RNA nucleocytoplasmic transport. EMBO J. 13:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 12.Goto, H., and Y. Kawaoka. 1998. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc. Natl. Acad. Sci. USA 95:10224-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan, Y., L. L. Poon, C. Y. Cheung, T. M. Ellis, W. Lim, A. S. Lipatov, K. H. Chan, K. M. Strurm-Ramirez, C. L. Cheung, Y. H. Leung, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. USA 101:8156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 15.Hinshaw, V. S., C. W. Olsen, N. Dybdahl-Sissoko, and D. Evans. 1994. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J. Virol. 68:3667-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horimoto, T., and Y. Kawaoka. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 3:591-600. [DOI] [PubMed] [Google Scholar]
- 17.Katz, J. M., L. Xiuhua, T. M. Tumpey, C. B. Smith. M. W. Shaw, and K. Subbarao. 2000. Molecular correlates of influenza A H5N1 virus pathogenesis in mice. J. Virol. 74:10807-10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klenk, H.-D., and W. Garten. 1994. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 2:39-43. [DOI] [PubMed] [Google Scholar]
- 19.Knobler, S. L., A. Mack, A. Mahmoud, and S. M. Lemon (ed.). 2005. The threat of pandemic influenza. Are we ready? National Academies Press, Washington, DC. [PubMed]
- 20.Ko, J. H., A. Takada, T. Mitsuhashi, T. Agui, and T. Watanabe. 2004. Native antiviral specificity of chicken Mx protein depends on amino acid variation at position 631. Anim. Genet. 35:119-122. [DOI] [PubMed] [Google Scholar]
- 21.Krug, R. M., W. Yuan, D. L. Noah, and A. G. Latham. 2003. Intracellular warfare between human influenza viruses and human cells: the role of the viral NS1 protein. Virology 309:181-189. [DOI] [PubMed] [Google Scholar]
- 22.Lamb, R. A., and R. M. Krug. 2001. Orthomyxoviridae: the viruses and their replication, p. 725-769. In D. M. Knipe and P. M. Howley (ed.), Fundamental virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 23.Lee, C.-W., D. E. Swayne, J. A. Linares, D. A. Senne, and D. L. Suarez. 2005. H5N2 avian influenza outbreak in Texas in 2004: the first highly pathogenic strain in the United states in 20 years? J. Virol. 79:11412-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipatov, A. S., E. A. Govorkova, R. J. Webby, H. Ozaki, M. Peiris, Y. Guan, L. Poon, and R. G. Webster. 2004. Influenza: emergence and control. J. Virol. 78:8951-8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majde, J. A., N. Guha-Thakurta, Z. Chen, S. Bredow, and J. M. Krueger. 1998. Spontaneous release of stable viral double-stranded RNA into the extracellular medium by influenza virus-infected MDCK endothelial cells: implications for the viral acute-phase response. Arch. Virol. 143:2371-2380. [DOI] [PubMed] [Google Scholar]
- 27.Marcus, P. I., J. M. Rojek, and M. J. Sekellick. 2005. Interferon induction and/or production and its suppression by influenza A viruses. J. Virol. 79:2880-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Marcus, P. I., T. Girshick, L. Van Der Heide, and M. J. Sekellick. 2006. Chicken IFN-alpha in drinking water functions as an adjuvant for influenza virus. Eur. Cytokine Netw. 17(Suppl.):69. [Google Scholar]
- 28.Marcus, P. I., and M. J. Sekellick. 1977. Defective-interfering particles with covalently linked [±]RNA induce interferon. Nature 266:815-819. [DOI] [PubMed] [Google Scholar]
- 29.Marcus, P. I., and M. J. Sekellick. 1988. Interferon induction by viruses. XVI. 2-Aminopurine blocks selectively and reversibly an early stage in interferon induction. J. Gen. Virol. 69:1637-1645. [DOI] [PubMed] [Google Scholar]
- 30.Marcus, P. I., and M. J. Sekellick. 2001. Combined sequential treatment with interferon and dsRNA abrogates virus resistance to interferon action. J. Interferon Cytokine Res. 21:423-429. [DOI] [PubMed] [Google Scholar]
- 31.Marcus, P. I., L. van der Heide, and M. J. Sekellick. 1999. Chicken interferon action on avian viruses. I. Oral administration of chicken interferon-α ameliorates Newcastle disease. J. Interferon Cytokine Res. 19:881-885. [DOI] [PubMed] [Google Scholar]
- 31a.Marcus, P. I., and M. J. Sekellick. 2002. Apoptosis in interferon-treated cells exposed to dsRNA. J. Interferon Cytokine Res. 22(Suppl. 1.):S157. [Google Scholar]
- 32.Martínez-Costas, J., C. González-López, V. N. Vakharia, and J. Benavente. 2000. Possible involvement of the double-stranded RNA-binding core protein σA in the resistance of avian reovirus to interferon. J. Virol. 74:1124-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matrosovich, M. N., S. Krauss, and R. G. Webster. 2001. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281:156-162. [DOI] [PubMed] [Google Scholar]
- 34.Meegan, J. M., and P. I. Marcus. 1989. Double-stranded ribonuclease coinduced with interferon. Science 244:1089-1091. [DOI] [PubMed] [Google Scholar]
- 35.Mo, C. W., Y. C. Cao, and B. L. Lim. 2001. The in vivo and in vitro effects of chicken interferon alpha on infectious bursal disease virus and Newcastle disease virus infection. Avian Dis. 45:389-399. [PubMed] [Google Scholar]
- 36.Noah, D. L., K. Y. Twu, and R. M. Krug. 2003. Cellular antiviral responses against influenza A virus are countered at the post-transcriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAs. Virology 307:386-395. [DOI] [PubMed] [Google Scholar]
- 37.Norton, G. P., T. Tanaka, K. Tobita, S. Nakada, D. A. Buonagurio, D. A. Greenspan, M. Krystal, and P. Palese. 1987. Infectious influenza virus A and B virus variants with long carboxyl terminal deletions in the NS1 polypeptides. Virology 156:204-213. [DOI] [PubMed] [Google Scholar]
- 38.Obenauer, J. C., J. Denson, P. K. Mehta, X. Su, S. Mukatira, D. B. Finkelstein, X. Xu, J. Wang, J. Ma, Y. Fan, K. M. Rakestraw, R. G. Webster, E. Hoffman, S. Krauss, J. Zheng, Z. Zhang, and C. W. Naeve. 2006. Large-scale sequence analysis of avian influenza isolates. Science 311:1576-1580. [DOI] [PubMed] [Google Scholar]
- 39.Office International des Epizooties. 1996. Highly pathogenic avian influenza (fowl plague), p. 155-160. In Manual of standards for diagnostic tests and vaccines, 3rd ed. Office International des Epizooties, Paris, France.
- 40.Pei, J., M. J. Sekellick, P. I. Marcus, I.-S. Choi, and E. W. Collisson. 2001. Chicken interferon type I inhibits infectious bronchitis virus (IBV) replication and associated respiratory illness. J. Interferon Cytokine Res. 21:1071-1077. [DOI] [PubMed] [Google Scholar]
- 41.Portnoy, J., and T. C. Merigan. 1971. The effect of interferon and interferon inducers on avian influenza. J. Infect. Dis. 124:545-552. [DOI] [PubMed] [Google Scholar]
- 42.Qian, X.-Y., F. Alonso-Caplen, and R. M. Krug. 1994. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J. Virol. 68:2433-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richman, D. D., B. R. Murphy, S. Baron, and C. Uhlendorf. 1976. Three strains of influenza A virus (H3N2): interferon sensitivity in vitro and interferon production in volunteers. J. Clin. Microbiol. 3:223-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekellick, M. J., W. J. Biggers, and P. I. Marcus. 1990. Development of the interferon system. I. In chicken cells development in ovo continues on time in vitro. In Vitro Cell. Dev. Biol. 26:997-1003. [DOI] [PubMed] [Google Scholar]
- 45.Sekellick, M. J., S. A. Carra, A. Bowman, D. A. Hopkins, and P. I. Marcus. 2000. Transient resistance of influenza virus to interferon action attributed to random packaging and activity of NS genes. J. Interferon Cytokine Res. 20:963-970. [DOI] [PubMed] [Google Scholar]
- 46.Sekellick, M. J., J. W. Lowenthal, T. E. O'Neil, and P. I. Marcus. 1998. Chicken interferons type I and II enhance synergistically the antiviral state and nitric oxide secretion. J. Interferon Cytokine Res. 18:407-414. [DOI] [PubMed] [Google Scholar]
- 47.Sekellick, M. J., and P. I. Marcus. 1986. Induction of high titer chicken interferon. Methods Enzymol. 119:115-125. [DOI] [PubMed] [Google Scholar]
- 47a.Sekellick, M. J., K. N. Mohni, and P. I. Marcus. 2006. Abstr. 25th Am. Soc. Virol., abstr. W2-1, p. 69.
- 48.Seo, S. H., E. Hoffmann, and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950-954. [DOI] [PubMed] [Google Scholar]
- 49.Solórzano, A., R. J. Webby, K. M. Lager, B. H. Janke, A. García-Sastre, and J. A. Richt. 2005. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J. Virol. 79:7535-7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart, W. E., II, E. DeClercq, A. Billiau, J. Desmyter, and P. DeSomer. 1972. Increased susceptibility of cells treated with interferon to the toxicity of polyribosinic-polyribocytidylic acid. Proc. Natl. Acad. Sci. USA 69:1851-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suarez, D. L., and M. L. Perdue. 1998. Multiple alignment comparison of the non-structural genes of influenza A viruses. Virus Res. 54:59-69. [DOI] [PubMed] [Google Scholar]
- 52.Swayne, D. E. 1997. Pathobiology of H5N2 Mexican avian influenza viruses for chickens. Vet. Pathol. 34:557-567. [DOI] [PubMed] [Google Scholar]
- 53.Swayne, D. E., and D. L. Suarez. 2005. U.S. strategies for controlling avian influenza in agricultural systems, p.233-253. In S. L. Knobler, A. Mack, A. Mahmood, and S. M. Lemon (ed.), The threat of pandemic influenza: are we ready? National Academies Press, Washington, DC. [PubMed]
- 54.Takizawa, T., S. Matsukkawa, Y. Higuchi, S. Nakamura, Y. Nakanishi, and R. Fukuda. 1993. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J. Gen. Virol. 74:2347-2355. [DOI] [PubMed] [Google Scholar]
- 55.Talon, J., M. Salvatore, R. E. O'Neill, Y. Nakaaya, H. Zheng, T. Muster, A. García-Sastre, and P. Palese. 2000. Influenza A and B viruses expressing altered NS1 proteins: a vaccine approach. Proc. Natl. Acad. Sci. USA 97:4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka, N., M. Sato, M. S. Lamphier, H. Nozawa, E. Oda, S. Noguchi, R. D. Schriber, Y. Tsujimoto, and T. Taniguchi. 1998. Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells 3:29-37. [DOI] [PubMed] [Google Scholar]
- 57.Taubenberger, J. K., A. H. Reid, R. M. Lourens, R. Wang, J. Guozhong, and T. G. Fanning. 2005. Characterization of the 1918 influenza virus polymerase genes. Nature 437:889-893. [DOI] [PubMed] [Google Scholar]
- 58.Twu, K. Y., D. L. Noah, P. Rao, R.-L. Kuo, and R. M. Krug. 2006. The CPSF30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J. Virol. 80:3957-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webby, R. J., and R. G. Webster. 2003. Are we ready for pandemic influenza? Science 302:1519-1522. [DOI] [PubMed] [Google Scholar]
- 60.Yewdell, J., and A. García-Sastre. 2002. Influenza still surprises. Curr. Opin. Microbiol. 5:414-418. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida, I., and P. I. Marcus. 1990. Interferon induction by viruses. XX. Acid-labile interferon accounts for the antiviral effect induced by poly(rI):poly(rC) in primary chicken embryo cells. J. Interferon. Res. 10:461-468. [DOI] [PubMed] [Google Scholar]