Abstract
Human cytomegalovirus (CMV) has evolved numerous strategies for evading host immune defenses, including piracy of cellular cytokines. A viral homolog of interleukin-10, designated cmvIL-10, binds to the cellular IL-10 receptor and effects potent immune suppression. The signaling pathways employed by cmvIL-10 were investigated, and the classic IL-10R/JAK1/Stat3 pathway was found to be activated in monocytes. However, inhibition of JAK1 had little effect on cmvIL-10-mediated suppression of tumor necrosis factor alpha (TNF-α) production. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway had a more significant impact on TNF-α levels but did not completely relieve the immune suppression, demonstrating that cmvIL-10 stimulates multiple signaling pathways to modulate cell function.
Human cytomegalovirus (CMV) is a widespread pathogen that persists as a lifelong latent infection. While infection is often asymptomatic in healthy individuals, life-threatening disease may occur in immunocompromised individuals, particularly transplant recipients, AIDS patients, or neonates (9, 11). The establishment of latency in immune-competent hosts is enhanced by numerous strategies for avoiding immune clearance (5, 16). Viral gene products US2, US3, US6, and US11 prevent expression of class I major histocompatibility complex (MHC) molecules on the surface of infected cells, evading cytotoxic T-lymphocyte detection (1, 2). To minimize natural killer cell-mediated lysis, an MHC I homolog is expressed (UL18), and nonclassical MHC molecules are up-regulated by gpUL40 (3, 31). In addition, HCMV encodes an interleukin-10 (IL-10) homolog, cmvIL-10, which inhibits immune cell proliferation, inflammatory cytokine synthesis, and MHC antigen expression (30).
Human IL-10 (hIL-10) binds the cellular IL-10 receptor (IL-10R), engaging tyrosine kinases JAK1 and Tyk2 (23). This leads to phosphorylation of IL-10R chains and recruitment of Stats, which become phosphorylated, form homo- or heterodimers, and translocate to the nucleus (13, 14, 20, 29). cmvIL-10 was found to strongly activate Stat3, but not Stat1, in monocytes. Stat3 phosphorylation occurred at two distinct sites, Y701 and S727. Suppression of cytokine synthesis by cmvIL-10 was only minimally affected by inhibiting JAK1 but was instead found to require phosphatidylinositol 3-kinase (PI3K) activity.
cmvIL-10 activates Stat3.
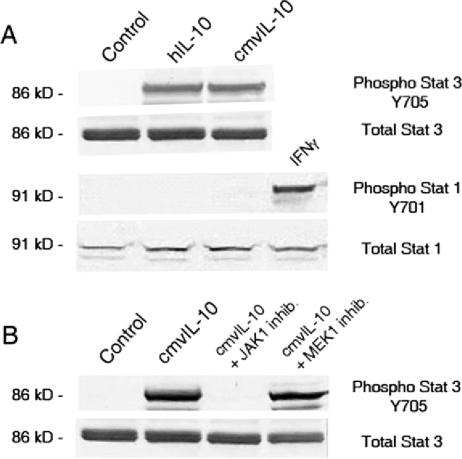
Previous studies demonstrated that cmvIL-10 engages the IL-10R (18), resulting in phosphorylation of Stat1 and Stat3 (21). However, these results were obtained using peripheral blood mononuclear cells, and hIL-10 induces differential activation of Stats in monocytes and T cells (14). To examine cmvIL-10 signaling in a pure population, monocytes were isolated from healthy human donors, treated with hIL-10 or cmvIL-10, and analyzed for the activation of Stat3. Western blotting revealed that Stat3 became phosphorylated on Y705 after treatment with either cytokine (Fig. 1A). Polyclonal antiserum directed to Stat3 showed the total amount of unmodified protein in each lysate and verified equal sample loading. Lysates were also probed for activation of Stat1, but no phosphorylation was observed in response to either hIL-10 or cmvIL-10. Polyclonal antiserum revealed unmodified Stat1 in the lysate, and treatment with gamma interferon induced phosphorylation of Stat1 at Y701. These results demonstrate that both cmvIL-10 and hIL-10 stimulate phosphorylation of Stat3 in monocytes.
FIG. 1.
cmvIL-10 activates Stat3 but not Stat1 in monocytes. A. Cell lysates from primary human CD14+ monocytes treated with 10 ng/ml recombinant hIL-10 or cmvIL-10 (R&D Systems) for 10 min were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with polyclonal antiserum (Cell Signaling) to detect phosphorylated or total unmodified Stat protein as indicated. Monocytes were isolated from buffy coats of healthy human donors by Ficoll density gradient centrifugation and magnetic separation with anti-CD14 microbeads (Miltenyi). B. Cells were pretreated with 10 μM JAK1 inhibitor {2-(1,1-dimethylethyl)-9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one, dissolved in dimethyl sulfoxide; Calbiochem} or MEK1 inhibitor (U0126; Cell Signaling) for 1 hour prior to treatment with 10 ng/ml cmvIL-10, and then lysates were immunoblotted. The total protein in each lysate was quantified (Bio-Rad protein assay kit), and 10 μg total protein was loaded in each well. Results are representative of experiments performed on cells from three different donors.
To confirm that cmvIL-10-mediated Stat3 activation requires JAK1, monocytes were pretreated with a JAK1-specific inhibitor before incubation with cmvIL-10. Stat3 was not phosphorylated in cells treated with JAK1 inhibitor (Fig. 1B), while treatment with MEK1 inhibitor or dimethyl sulfoxide had no impact on Stat3 phosphorylation. Binding of cmvIL-10 to the cellular IL-10R leads to JAK1-dependent Stat3 activation; thus, the viral cytokine employs the same intracellular signaling pathway as hIL-10.
JAK1 activity is not essential for cmvIL-10-mediated immune suppression.
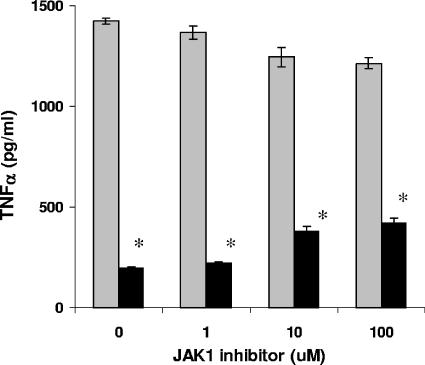
Because JAK1 activity was required for Stat3 activation, JAK1 was predicted to be necessary for suppression of inflammatory cytokines, a hallmark property of hIL-10 and cmvIL-10 (12, 30, 32). To investigate this, monocytes were incubated with JAK1 inhibitor prior to treatment with cmvIL-10 and lipopolysaccharide (LPS), and supernatants were analyzed for TNF-α levels by enzyme-linked immunosorbent assay (ELISA). LPS, a strong inflammatory stimulator, triggered high levels of TNF-α (Fig. 2). Treatment with cmvIL-10 reduced TNF-α levels by 86%. Unexpectedly, treatment with JAK1 inhibitor did not cause TNF-α levels to rise significantly. At the highest dose of inhibitor, TNF-α levels were still at 65% of the control LPS level, and only 18% inhibition of cmvIL-10 suppression was observed. While cmvIL-10 activates the JAK1/Stat3 pathway, this cascade plays only a minor role in inhibiting cytokine synthesis in monocytes.
FIG. 2.
Inhibition of TNF-α does not require JAK1 activity. Monocytes were incubated with JAK1 inhibitor for 1 h prior to treatment with 1 ng/ml LPS in the presence or absence of 10 ng/ml cmvIL-10. After 18 h, supernatants were collected and TNF-α levels were detected via sandwich ELISA (R&D Systems). Gray bars, LPS treatment only; black bars, LPS plus cmvIL-10. Error bars show standard deviations. Statistical analysis was performed using Student's t test, and differences in TNF-α production between cmv-IL-10-treated and untreated monocytes were significant regardless of inhibitor dose. *, P < 0.01. Results are representative of experiments performed on cells from five different donors in 96-well plates with 5 × 104 cells per well.
TNF-α suppression is sensitive to PI3K inhibition.
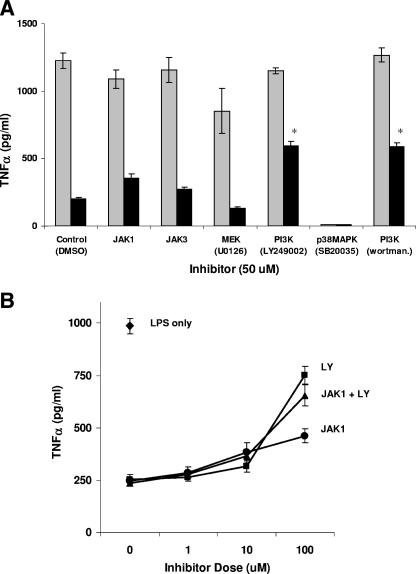
To examine other signaling pathways involved in cmvIL-10 immune suppression, various inhibitors were tested for the ability to restore TNF-α production by cmvIL-10-treated monocytes (Fig. 3A). Treatment with p38 mitogen-activated protein kinase inhibitor SB20035 resulted in undetectable levels of TNF-α in the presence or absence of cmvIL-10. Only LY249002 and wortmannin caused increases in TNF-α levels, indicating that PI3K mediates inhibition of cytokine synthesis by cmvIL-10.
FIG. 3.
PI3K plays a role in cmvIL-10-mediated cytokine suppression. (A) LPS-stimulated monocytes (1 ng/ml) were incubated with a panel of different commercially available signal transduction inhibitors [JAK3 inhibitor 4-(4′-hydroxyphenyl)amino-6,7-dimethoxyquinazoline (Calbiochem), MEK1 inhibitor U0126 and PI3K inhibitor LY249002 (Cell Signaling), or wortmannin and p38 mitogen-activated protein kinase inhibitor SB203580 (Sigma)] in the presence or absence of 10 ng/ml cmvIL-10. Trypan blue analysis confirmed cells from each treatment were viable and then supernatants were analyzed for TNF-α levels by ELISA after 18 h. Gray bars, LPS treatment only; black bars, LPS plus cmvIL-10. Statistical analysis showed that TNF-α levels increased significantly in the presence of cmvIL-10 only with treatment with PI3K inhibitors LY249002 and wortmannin. *, P < 0.01, Student's t test. Results are representative of experiments performed on cells from five different donors. (B) Monocytes were incubated in the presence of the JAK1 inhibitor (circle), the PI3K inhibitor (LY249002; square), or both inhibitors (triangle) for 1 hour prior to LPS stimulation in the presence of 10 ng/ml cmvIL-10 and then TNF-α levels were measured by ELISA. A single black diamond indicates the level of TNF-α in the presence of LPS only. Results are representative of experiments performed on cells from three different donors. Error bars represent standard deviations.
To assess additive or synergistic effects of the two pathways, monocytes were incubated with JAK1 inhibitor and LY249002 in combination (Fig. 3B). This treatment did not relieve cmvIL-10 suppression of TNF-α any more than PI3K inhibitor alone. These findings indicate that either JAK1 plays no role in inhibition of cytokine synthesis or that there may be convergence of the JAK1 and PI3K signaling pathways. If so, the most likely point of convergence would be Stat3, which can be phosphorylated at Y705 and also at a second site, S727 (10, 33).
cmvIL-10 stimulates Akt activation and Stat3 (S727) phosphorylation.
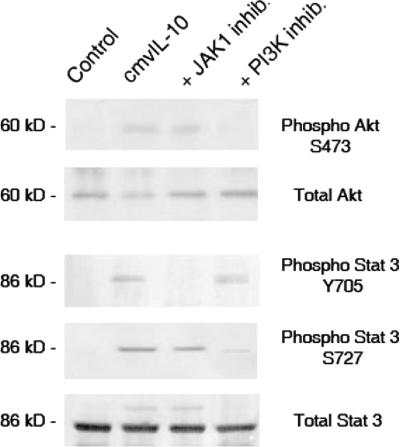
To further investigate the role of PI3K in cmvIL-10 signaling, Akt, a downstream effector of PI3K, was examined. Treatment of monocytes with cmvIL-10 led to Akt activation, as evidenced by S473 phosphorylation (Fig. 4). While JAK1 inhibitor pretreatment did not impact Akt activation, LY249002 prevented phosphorylation of Akt. Phosphorylation of Stat3 S727 occurred in cmvIL-10-treated monocytes and was dependent on PI3K, but not on JAK1 activity, indicating that Stat3 is a common target in both pathways.
FIG. 4.
cmvIL-10 induces PI3K-dependent phosphorylation of Akt and Stat3 (S7272). Human monocytes were treated with 50 μM JAK1 or PI3K inhibitor (LY249002) for 1 h prior to incubation with 10 ng/ml cmvIL-10 and then lysates were immunoblotted with polyclonal antiserum (Cell Signaling) to phosphorylated or total unmodified Stat3 or Akt protein. Results are representative of experiments performed on cells from three different donors.
Virus persistence is enhanced by viral proteins that manipulate cellular signaling pathways, particularly those that suppress immune function, like the IL-10 cascade. Immunoblotting revealed that cmvIL-10 used the same cellular signaling pathway as hIL-10 and activated Stat3, but not Stat1, via JAK1 in monocytes. While a more sensitive assay might reveal weak Stat1 activation in cmvIL-10-treated monocytes, it is more likely that Stat1 activation seen previously in peripheral blood mononuclear cells occurred in T cells (21). Interestingly, although cmvIL-10 activates Stat3 through JAK1 activity in monocytes, JAK1 activity was nonessential for inhibition of TNF-α production. This evidence suggests a role for PI3K, with the most likely scenario being that PI3K activates Akt, which activates mTOR, which targets Stat3 on S727 (24, 34). Although the importance of S727 phosphorylation for full transcriptional activity of Stat3 has previously been reported (10, 33), it is noteworthy that phosphorylation at this site alone (in the absence of Y705 phosphorylation) resulted in significant changes in cytokine gene expression.
While activation of PI3K by hIL-10 has been well documented (4, 22, 26, 28, 35), this pathway was thought to be required for proliferative but not antiinflammatory effects of the cytokine (8). Treatment with LY249002 consistently increased TNF-α relative to the cvmIL-10-suppressed levels, clearly indicating PI3K is important for this inhibition. The differing results may reflect variations in cell preparation or treatment protocol but are unlikely to represent a true signaling difference between hIL-10 and cmvIL-10. Similar results were obtained with each inhibitor using hIL-10-treated monocytes (data not shown), and no significant signaling differences between hIL-10 and cmvIL-10 have been reported to date (6, 7, 27).
Because none of the inhibitors used here restored TNF-α to control levels, it seems likely that additional signaling mechanisms still contribute to cmvIL-10-induced suppression. The inhibition of inflammatory cytokine synthesis by hIL-10 has been reported to be both Stat3 dependent (29) and independent (25), supporting the notion that cmvIL-10 may still exert immunomodulatory effects through other cellular pathways or receptors. Recent evidence has suggested that LAcmvIL-10, an alternately spliced form of cmvIL-10 expressed during latency (17), down-modulates MHC expression independent of IL-10R (15), and a whole family of IL-10-related receptors have been identified in recent years (19). Further investigation will be required to fully understand the function of cmvIL-10 in virus infection.
Acknowledgments
This work was supported by NIH grant AI063529 and USF Faculty Development Funds.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Ahn, K., A. Angulo, P. Ghazal, P. A. Peterson, Y. Yang, and K. Fruh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basta, S., and J. R. Bennink. 2003. A survival game of hide and seek: cytomegaloviruses and MHC class I antigen presentation pathways. Viral Immunol. 16:231-242. [DOI] [PubMed] [Google Scholar]
- 3.Beck, S., and B. G. Barrell. 1988. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature 331:269-272. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya, S., P. Sen, M. Wallet, B. Long, A. S. Baldwin, Jr., and R. Tisch. 2004. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IκB kinase activity. Blood 104:1100-1109. [DOI] [PubMed] [Google Scholar]
- 5.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 6.Chang, W., N. Baumgarth, J. P. Greg, C. A. Baron, and P. A. Barry. 2004. HCMV-encoded IL-10 alters dendritic cell functionality: a comprehensive comparison with cellular IL-10, abstr. 6.40. 29th Int. Herpesvirus Workshop, Reno, NV.
- 7.Chang, W. L., N. Baumgarth, D. Yu, and P. A. Barry. 2004. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J. Virol. 78:8720-8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawley, J. B., L. M. Williams, T. Mander, F. M. Brennan, and B. M. Foxwell. 1996. Interleukin-10 stimulation of phosphatidylinositol 3-kinase and p70 S6 kinase is required for the proliferative but not the antiinflammatory effects of the cytokine. J. Biol. Chem. 271:16357-16362. [DOI] [PubMed] [Google Scholar]
- 9.Damato, E. G., and C. W. Winnen. 2002. Cytomegalovirus infection: perinatal implications. J. Obstet. Gynecol. Neonatal Nurs. 31:86-92. [DOI] [PubMed] [Google Scholar]
- 10.Decker, T., and P. Kovarik. 2000. Serine phosphorylation of STATs. Oncogene 19:2628-2637. [DOI] [PubMed] [Google Scholar]
- 11.de la Hoz, R. E., G. Stephens, and C. Sherlock. 2002. Diagnosis and treatment approaches of CMV infections in adult patients. J. Clin. Virol. 25(Suppl. 2):S1-S12. [DOI] [PubMed] [Google Scholar]
- 12.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly, R. P., H. Dickensheets, and D. S. Finbloom. 1999. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19:563-573. [DOI] [PubMed] [Google Scholar]
- 14.Finbloom, D. S., and K. D. Winestock. 1995. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J. Immunol. 155:1079-1090. [PubMed] [Google Scholar]
- 15.Godwin, M., A. Cheung, C. Jenkins, J. Lewis Stern, B. Plachter, S. Pepperl-Klindworth, A. Abendroth, and B. Slobedman. 2006. Immunomodulation by a viral homolog of IL-10 expressed by human cytomegalovirus during the latent phase of infection, abstr. 2.01. 31st Int. Herpesvirus Workshop, Seattle, WA.
- 16.Hengel, H., W. Brune, and U. H. Koszinowski. 1998. Immune evasion by cytomegalovirus—survival strategies of a highly adapted opportunist. Trends Microbiol. 6:190-197. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins, C., A. Abendroth, and B. Slobedman. 2004. A novel viral transcript with homology to human interleukin-10 is expressed during latent human cytomegalovirus infection. J. Virol. 78:1440-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, B. C., N. J. Logsdon, K. Josephson, J. Cook, P. A. Barry, and M. R. Walter. 2002. Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL-10R1. Proc. Natl. Acad. Sci. USA 99:9404-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotenko, S. V. 2002. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 13:223-240. [DOI] [PubMed] [Google Scholar]
- 20.Kotenko, S. V., and S. Pestka. 2000. Jak-Stat signal transduction pathway through the eyes of cytokine class II receptor complexes. Oncogene 19:2557-2565. [DOI] [PubMed] [Google Scholar]
- 21.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirmonsef, P., C. P. Shelburne, C. Fitzhugh Yeatman II, H. J. Chong, and J. J. Ryan. 1999. Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: role of STAT6 and phosphatidylinositol 3′-kinase. J. Immunol. 163:2530-2539. [PubMed] [Google Scholar]
- 23.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 24.Morgensztern, D., and H. L. McLeod. 2005. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 16:797-803. [DOI] [PubMed] [Google Scholar]
- 25.O'Farrell, A. M., Y. Liu, K. W. Moore, and A. L. Mui. 1998. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 17:1006-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pahan, K., M. Khan, and I. Singh. 2000. Interleukin-10 and interleukin-13 inhibit proinflammatory cytokine-induced ceramide production through the activation of phosphatidylinositol 3-kinase. J. Neurochem. 75:576-582. [DOI] [PubMed] [Google Scholar]
- 27.Raftery, M. J., D. Wieland, S. Gronewald, A. A. Kraus, T. Giese, and G. Schonrich. 2004. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J. Immunol. 173:3383-3391. [DOI] [PubMed] [Google Scholar]
- 28.Ricchetti, G. A., L. M. Williams, and B. M. Foxwell. 2004. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J. Leukoc. Biol. 76:719-726. [DOI] [PubMed] [Google Scholar]
- 29.Riley, J. K., K. Takeda, S. Akira, and R. D. Schreiber. 1999. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J. Biol. Chem. 274:16513-16521. [DOI] [PubMed] [Google Scholar]
- 30.Spencer, J. V., K. M. Lockridge, P. A. Barry, G. Lin, M. Tsang, M. E. Penfold, and T. J. Schall. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomasec, P., V. M. Braud, C. Rickards, M. B. Powell, B. P. McSharry, S. Gadola, V. Cerundolo, L. K. Borysiewicz, A. J. McMichael, and G. W. Wilkinson. 2000. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science 287:1031. [DOI] [PubMed] [Google Scholar]
- 32.Vieira, P., R. de Waal-Malefyt, M. N. Dang, K. E. Johnson, R. Kastelein, D. F. Fiorentino, J. E. deVries, M. G. Roncarolo, T. R. Mosmann, and K. W. Moore. 1991. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc. Natl. Acad. Sci. USA 88:1172-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen, Z., Z. Zhong, and J. E. Darnell, Jr. 1995. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241-250. [DOI] [PubMed] [Google Scholar]
- 34.Yau, C. Y., J. J. Wheeler, K. L. Sutton, and D. W. Hedley. 2005. Inhibition of integrin-linked kinase by a selective small molecule inhibitor, QLT0254, inhibits the PI3K/PKB/mTOR, Stat3, and FKHR pathways and tumor growth and enhances gemcitabine-induced apoptosis in human orthotopic primary pancreatic cancer xenografts. Cancer Res. 65:1497-1504. [DOI] [PubMed] [Google Scholar]
- 35.Zhou, J. H., S. R. Broussard, K. Strle, G. G. Freund, R. W. Johnson, R. Dantzer, and K. W. Kelley. 2001. IL-10 inhibits apoptosis of promyeloid cells by activating insulin receptor substrate-2 and phosphatidylinositol 3′-kinase. J. Immunol. 167:4436-4442. [DOI] [PubMed] [Google Scholar]