Abstract
Disruption of cellular adhesion is an essential pathobiologic step leading to tumor dissemination. Mucin 1 (MUC1) is a mucinous glycoprotein expressed at the surfaces of epithelial cells in many tissues and their carcinomas. MUC1 plays crucial roles in tumor invasion and metastasis, especially in opposing cell adhesion. We have shown that virus infection, specifically by the human tumor virus Epstein-Barr virus (EBV) induces a spectrum of cellular invasiveness and metastasis factors. Here we show that expression of MUC1 is increased in diverse latently EBV-infected cell lines that express latent membrane protein 1 (LMP1), the main viral oncoprotein, and that the level of MUC1 was suppressed by expression of a dominant-negative mutant of LMP1. Expression of LMP1 in EBV-negative nasopharyngeal cell lines induces expression of MUC1 through activation of the MUC1 promoter via binding of STAT1 and STAT3. Finally, LMP1 reduces cell adhesion ability, which is restored by inhibition of MUC1 expression with MUC1 small interfering RNA (siRNA). In addition, LMP1 increases cell invasiveness, which is suppressed by MUC1 siRNA. Thus, LMP1 induces MUC1, a factor important in an early step of detachment and release of tumor cells, which along with induction of other invasiveness and angiogenic factors may combine to act in a complex sequential process that culminates in metastasis of EBV-infected tumor cells.
The close association of Epstein-Barr virus (EBV) with several invasive malignancies, especially nasopharyngeal carcinoma (NPC), B-cell lymphoproliferative diseases, Hodgkin's disease, and some invasive breast cancers (5, 33), has raised the question of whether a tumor virus could contribute to the invasive character of tumors. Invasion into surrounding tissue is a characteristic of malignant tumors—strikingly so in the case of NPC, which is closely linked to EBV infection. Both invasion and metastasis are programmed through sequential multistep pathobiologic processes characterized by disruption of many aspects of normal cell behavior. In papers beginning in 1998 (for a review, see reference 54), we have reported that latent membrane protein 1 (LMP1) induces the expression of a series of cellular invasion and metastasis factors, such as matrix metalloproteinase 9 (MMP9), which plays a critical role in the invasion of the basement membrane (30, 44, 53). LMP-1 also induces the MMP-1 promoter (22). Furthermore, MMP9 is involved in production of vascular endothelial factor (VEGF) (4). In addition, LMP1 induces angiogenic factors, such as VEGF through induction of cyclooxygenase 2 (COX-2) and hypoxia-inducible factor 1α (HIF-1α) (23, 31, 50). Moreover, LMP1 induces and causes release of fibroblast growth factor 2 (FGF-2) into extracellular fluid (49; S. Ceccarelli, V. Visco, N. Wakisaka, J. S. Pagano, and M. R. Torrisi, submitted for publication). LMP1 expression also promotes cell migration and invasive growth via Ets-1 expression (19, 21).
In most EBV-associated tumors, infection is predominantly latent. The EBV genes expressed in latent infection are restricted to six EBV nuclear antigens (EBNA-1, -2, -3A, -3B, -3C, and -LP), three latent membrane proteins (LMP1, -2A, and -2B), and two small nonpolyadenylated RNAs (EBER1 and -2). The patterns of expression of the genes that encode these proteins determine latency type. In type I latency, such as Burkitt's lymphoma, only EBNA1, EBERs, and sometimes LMP2A are expressed. In type II latency, such as NPC and Hodgkin's disease, EBNA1 and the three latent membrane proteins are expressed. In type III latency, typified by EBV lymphoproliferative disease, all of the EBNAs and LMPs are expressed (33). LMP1 is considered the principal EBV oncogene and can produce lymphomas in transgenic mice (25). The carboxyl-terminal portion of LMP1 in the cytoplasmic domain of the protein contains two functional signaling regions: COOH-terminal activation region 1 (CTAR1) and CTAR2. Both activate nuclear factor κB (NF-κB) (20, 44).
In addition, there is a Janus kinase 3 (JAK3) activation and binding domain positioned between CTAR1 and CTAR2 that results in activation of signal transducer and activator of transcription 1 (STAT1) and STAT3 in fibroblasts (15). STATs are latent transcription factors that become activated by phosphorylation on a single tyrosine, typically in response to extracellular ligands (9, 42). Various cytokines and growth factors can cause STAT phosphorylation through receptor or associated kinases. Once phosphorylated, STATs can form homo- or heterodimers that accumulate in the nucleus, recognize specific DNA sequences, and activate transcription of target genes (9, 42). However, the pattern of activation of STATs by LMP1 is unclear, perhaps because it is influenced by cell type (7, 55). Chen et al. reported that adherent cell lines stably transfected with LMP1 induced tyrosine-phosphorylated STAT3 and -5, but not STAT1 (7). On the other hand, in suspension cell lines, Zhang et al. showed that LMP1 can induce expression of STAT1 protein as well as serine but not tyrosine phosphorylation of the protein (55). LMP1 also induced expression of STAT2 and -3 proteins (55).
A series of reports have built a picture of how a tumor virus may have a major impact on invasion and metastasis. This process also requires an earlier step that involves antiadhesive functions, such as dissociation of cell-cell and cell-matrix adhesion, which we now address. The main regulator of the cell adhesion system is E-cadherin. Epithelial E-cadherin, a component of adherens junctions, is crucial for the organization and maintenance of differentiated epithelia (17). This protein mediates cell adhesion via a homophilic and Ca2+-dependent pathway. In cancer, the maintenance and integrity of epithelia are lost, and the resulting dissociation of cells leads ultimately to metastatic dissemination. Dissociation of cells can occur through a decrease in the local expression level of E-cadherin. It is well documented that LMP1 downregulates E-cadherin gene expression through cellular DNA methylation machinery by activation of DNA methyltransferase (48). However, the mechanism of antiadhesion is otherwise unclear except for E-cadherin itself. The E-cadherin-mediated cell adhesion system is modulated by the mucin-like glycoprotein MUC1, one of the mucin protein families. In breast cancer cells, the cytoplasmic domain of MUC1 binds β-catenin, leading to a decrease of E-cadherin-β-catenin complex and resulting in an antiadhesive effect (27).
MUC1 is a large surface glycoprotein expressed by epithelial cells that is overexpressed and aberrantly glycosylated in several carcinomas, such as breast cancer (13, 46, 47). The biological functions of MUC1 are inferred from in vitro experiments (38, 47). The long and rigid extracellular domain of MUC1 can shield adhesion molecules and diminish cellular adhesion if the glycoprotein is present at a high enough density on the cell surface. In addition, MUC1 represses T-cell proliferation, resulting in immunosuppressive effects (14). Recent papers indicate that MUC1 is regulated by cytokines that signal through NF-κB, STAT1, and STAT3 (12, 26). In addition, because MUC1 may be an optimal candidate for active immunotherapy (1), specifically as a target for dendritic cell therapy and vaccination for MUC1-positive cancers, it has increasing potential medical importance.
Here, we show the first evidence that the EBV oncoprotein LMP1 induces MUC1 expression. We demonstrate that LMP1 activates STAT1 and STAT3, which in turn results in induction of MUC1, and finally enhances dissociation of adherent cells and cell invasiveness.
MATERIALS AND METHODS
Cell culture.
KH-1 and KH-2 lines are EBV-positive type II cell lines derived from fusion of KR-4 (an EBV-positive type III lymphoblastoid cell line) and HeLa cells (human cervical carcinoma) (gifts of Maria Masucci, Karolinska Institute, Stockholm, Sweden) (8). Ad-AH cells, provided by Erik K. Flemington (Tulane University, New Orleans, LA), are an EBV-negative human nasopharyngeal cell line (45). T-47D (a human breast cancer cell line) and HeLa cells were obtained from the American Type Culture Collection. MDA-MB-231 (a human breast cancer cell line) and EBV-infected MDA-MB-231 clones (C4A3, C1D12, C2G6, and C3B4) were described previously (3, 50). The parental MDA-MB-231 cells and C4A3 clone are LMP1-negative cells. C1D12, C2G6, and C3B4 are LMP1-positive clones. LMP1 expression is strongest in the C3B4 clone and not detected in C4A3. MDA-MB-231 cells were maintained in RPMI 1640, with 10% fetal bovine serum (FBS), 4 μM l-glutamine, penicillin, and streptomycin. EBV-infected MDA-MB-231 clones were maintained in the same medium, but with 700 μg/ml G418 (Life Technologies, Grand Island, NY). The other cells were maintained in Dulbecco's modified Eagle's medium with 10% FBS and penicillin and streptomycin.
Antibodies and reagents.
Mouse LMP1 monoclonal antibody was purchased from DAKO (Glostrup, Denmark). Mouse MUC-1 monoclonal antibody, clone VU2G7 was from Chemicon International Inc. (Temecula, CA). Mouse monoclonal antibody against STAT1, STAT3, tyrosine-phosphorylated STAT1 (pY-STAT1) (phosphorylated tyrosine at position 701 [pY-701]), and pY-STAT3 (pY-705) were from Transduction Laboratories (San Diego, CA). Mouse monoclonal antibody against γ-tubulin was from Sigma (St. Louis, MO). Mouse monoclonal hemagglutinin antibody and rabbit polyclonal histone antibody (H2B) were from Santa Cruz Biotechnology (Santa Cruz, CA). Collagen type I gelatin, laminin, and fibronectin, were purchased from Sigma. Recombinant interleukin 6 (IL-6) was from R & D Systems (Minneapolis, MN). MUC1 siRNA was purchased from Dharmacon (Chicago, IL).
Plasmids and site-directed mutagenesis.
pcDNA3-based LMP1 has been described elsewhere (53). LMP1-DM, which is doubly mutated in both the CTAR1 and CTAR2 regions and acts as an LMP1 dominant-negative mutant, has been described elsewhere (56). To monitor expression of LMP1-DM, we used Western blots with LMP1 antibodies. Hemagglutinin-tagged STAT1 dominant-negative and STAT3 dominant-negative (STAT1-DN and STAT3-DN) mutants were generous gifts from Toshio Hirano (Osaka University, Osaka, Japan) (32). cDNA of the tandem repeat domain of the MUC1 gene in Bluescript vector for Northern analysis was the generous gift from Joyce Taylor-Papadimitriou (Imperial Cancer Research Fund, London, United Kingdom) (24).
Transient and stable transfection.
Cells were transfected with 1 μg of appropriate plasmid(s) with the use of Effectene transfection reagent (QIAGEN, Valencia, CA) following the manufacturer's instructions. Stable cell lines were established by cultivating Ad-AH cells in the presence of 800 μg/ml G418. For siRNA transfection, stable Ad-AH cell lines with or without pcLMP1 were transfected with the use of Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA).
Western blot analysis.
Transfected cells were cultured in appropriate medium with 10% FBS for 2 days and then cultured in appropriate medium without FBS for 24 h. Whole-cell lysates were analyzed by Western blotting as described previously (50). Nuclear extracts were prepared by NE-PER nuclear and cytoplasmic extraction reagents according to the manufacturer's protocol (Pierce, Rockford, IL).
Northern blot analysis.
RNA preparation and Northern analysis were carried out as described previously (24, 51).
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared from pcDNA3- or pcLMP1-transfected (transiently transfected) Ad-AH cells as described previously (50). The following double-stranded 32P-labeled oligonucleotide probes were synthesized: wild-type STAT-MUC1 oligonucleotide, 5′-GGCTATTCCGGGAAGTGGT-3′; mutant STAT-MUC1 oligonucleotide, 5′-GGCTACTCGAGAAGTGGT-3′ (12). For reaction, equal amounts of nuclear extract (15 μg) were incubated in DNA-binding buffer as described elsewhere (12). Samples were loaded on 5% native polyacrylamide gels in a ice-cold 0.5× Tris-borate EDTA (TBE) buffer. Gels were vacuum dried and exposed for autoradiography. For competition assays, excess amounts of wild-type or mutant cold oligonucleotides were used (×100). For supershift assays, we used antibodies against STAT1 and STAT3 as described earlier (12).
In vitro cell invasiveness assay.
Invasiveness assays were performed with the use of Biocoat Matrigel invasion chambers (Becton Dickinson Labware, Bedford, MA) as described previously (30). Biocoat cell culture inserts were used for uncoated assays. Stably pcLMP1-transfected Ad-AH cells and stably pcDNA3-transfected Ad-AH cells were transfected with either control siRNA or MUC1 siRNA and cultivated for 48 h for the assays which were done in triplicate.
Cell adhesion assay.
The cell adhesion assay was based on an established method (38). Briefly, microwells were coated with type I collagen, gelatin, laminin, or fibronectin (10 μg/cm2) overnight at 4°C and then washed once with a phosphate-buffered saline solution. Bovine serum albumin (1.5%) was added to the coated wells and incubated for 1 h at 37°C to block the remaining binding sites. Triplicate suspended aliquots of stably pcLMP1-transfected Ad-AH cells and control cells transfected with either control siRNA or MUC1 siRNA and cultivated for 48 h were added to each coated well and allowed to incubate for 1 h at 37°C. After the wells were washed three times with a phosphate-buffered saline solution, adherent cells were stained with 0.04% crystal violet solution. The crystal violet was solubilized by 50% methanol and 0.1% Triton X-100 (Sigma). Finally, absorbance was measured at 590 nm. Results were expressed as a percentage of the initial number of cells.
Statistical analysis.
Significant differences were determined by the paired t test. A P value of <0.05 was considered statistically significant.
RESULTS
The level of MUC1 protein is increased in latently infected type II adherent cells.
We first determined endogenous levels of MUC1 in EBV-infected cell lines. Type II latently infected cells express EBNA1 and latent membrane proteins (33). KH-1 and KH-2 are type II adherent cell lines derived by fusion of an EBV-infected lymphoblastoid suspension cell line, KR-4, and adherent HeLa cells (8). The expression level of MUC1 protein is clearly greater in KH-1 and KH-2 cells, which express EBV latency proteins, including LMP1, than in HeLa cells (Fig. 1A). EBV-negative T47-D cells treated with IL-6 were used as a positive control for expression of MUC1 (12). MUC1 has two allelic mature forms with an apparent molecular mass of more than 400 kDa (12, 26).
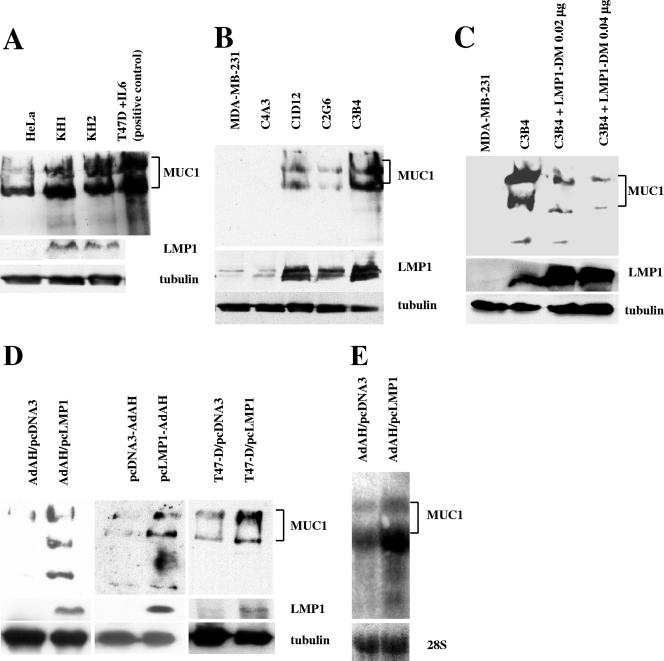
FIG. 1.
Levels of MUC1 protein are increased in LMP1-expressing type II latently EBV-infected cells and are induced by LMP1. (A) KH-1 and KH-2 are type II cell lines derived by fusion of KR-4 and adherent HeLa cells (8); HeLa cells are used as a negative control. Subconfluent cells were lysed for Western blots. Half of the protein from T47-D cells treated with IL-6 was used as a positive control (12). (B) MDA-MB-231 is an EBV-negative human breast carcinoma cell line. C1D12, C2G6, and C3B4 are its EBV-infected derivatives that express LMP1. (C) Suppression of LMP1 in type II latently infected cells resulted in suppression of expression of MUC1. An LMP1 dominant-negative mutant (LMP1-DM) was transfected into C3B4 cells; MDA-MB-231 is used as a negative control. Expression of LMP1-DM was confirmed by Western blotting with LMP1 antibody. (D) LMP1 induces expression of MUC1 protein. Lysates from Ad-AH or T47-D cells transiently transfected with either pcDNA3 or pcLMP1 expression plasmids were used, as well as Ad-AH cells stably transfected with either pcDNA3 (pcDNA3-AdAH) or pcLMP1 (pcLMP1-AdAH). MUC1 has an apparent molecular mass of more than 400 kDa. Brackets indicate the two major mature allelic forms of MUC1. (E) Induction of MUC1 by LMP1 is at the transcriptional level. RNA from Ad-AH cells transfected with either pcDNA3 or pcLMP1 expression plasmid was used. RNA was analyzed by Northern blotting for MUC1 tandem repeat; 28S rRNA was used as a loading control.
MDA-MB-231 is an EBV-negative breast cancer cell line, and C4A3, C1D12, C2G6, and C3B4 are EBV-infected clones derived from it. As shown in Fig. 1B, the MUC1 level is increased in the LMP1-positive clones, C1D12, C2G6, and C3B4, that express LMP1, but not in LMP1-negative C4A3 cells or in the parental line, MDA-MB-231. Beads migrating with LMP1 in the first two lanes of Fig. 1B are nonspecific.
We then examined whether the increase in the level of MUC1 in type II latently cells is due to LMP1. LMP1-DM expression plasmid was transfected into the C3B4 cell line, which expresses both LMP1 and MUC1 at high levels. LMP1-DM suppressed expression of MUC1 as shown in Fig. 1C. The level of suppression correlated with the amount of LMP1 dominant-negative mutant used.
Thus, the level of MUC1 protein corresponds to the level of LMP1 in the two quite different sets of cell lines. These results prompted investigation of the role of LMP1 in the induction of MUC1 protein.
LMP1 induces expression of MUC1 protein and mRNA.
Ad-AH is an EBV-negative nasopharyngeal epithelial cell line. Stable or transient transfectants of Ad-AH cells expressing LMP1 and controls were lysed for analysis by Western blotting. The expression level of MUC1 was significantly higher in LMP1-expressing cells whether stably or transiently transfected than in those cells not expressing the viral protein (Fig. 1D). We also detected a higher level of MUC1 in the LMP1-transfected T47-D breast carcinoma cells than in pcDNA3-transfected T47-D cells (Fig. 1D).
Next, we investigated whether LMP1 induces transcription of MUC1. As shown in Fig. 1E, LMP1-transfected Ad-AH cells had higher levels of MUC1 RNA than the control cells did. Whether induction of MUC1 by LMP1 is direct or indirect remains to be ascertained.
LMP1-induced activated STAT1 and STAT3 are required for induction of MUC1.
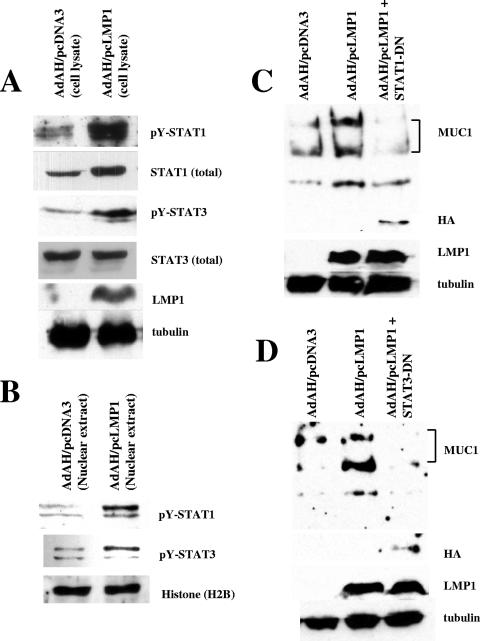
Whether LMP1 can induce tyrosine-phosphorylated STAT1 is uncertain (7, 15, 55). The effects of activated STAT1 and -3 are critical for MUC1 transcription (12). First, we examined whether LMP1 can induce activated STAT1 and -3, i.e., their tyrosine-phosphorylated forms. Using recently developed monoclonal antibodies, we detected activated STAT1 and -3 in whole-cell lysates and nuclear extracts of Ad-AH cells expressing LMP1 (Fig. 2A and B). In addition to these results, LMP1 expression increases the level of STAT1 itself. Next we examined the effects of STAT1-DN and STAT3-DN mutants on the levels of MUC1 induced by LMP1. Cotransfection of STAT1-DN mutants with LMP1 repressed induction of MUC1 by LMP1 (Fig. 2C). Similarly, STAT3-DN suppressed MUC1 expression by LMP1 (Fig. 2D). Thus, STAT1 and -3 signaling appears to affect expression of MUC1 induction by LMP1.
FIG. 2.
Activated STAT1 and STAT3 are required for the induction of MUC1. (A) LMP1 induces tyrosine-phosphorylated STAT1 and STAT3 (pY-STAT1 and pY-STAT3) in whole-cell lysates of Ad-AH cells. (B) LMP1 induces tyrosine-phosphorylated STAT1 and STAT3 (pY-STAT1 and pY-STAT3) in nuclear extracts of Ad-AH cells. Histone H2B was used as a loading control. (C) Ad-AH cells were transfected with pcDNA3 or pcLMP1, or pcLMP1 plus STAT1-dominant-negative (DN) mutant. Cell lysates were used for Western blotting. HA, hemagglutinin. (D) Ad-AH cells were transfected with pcDNA3 or pcLMP1 with or without STAT3-DN. Cell lysates were used for Western blotting.
Gamma interferon together with tumor necrosis factor alpha stimulates expression of MUC1 (26). However, with LMP1 as the inducing factor, we did not detect a consistent effect on MUC1 by cotransfection of the IκB superrepressor srIκBα (S32A S36A) with the viral protein (30, 35; data not shown).
LMP1 induces binding activities of STATs for the MUC1 promoter.
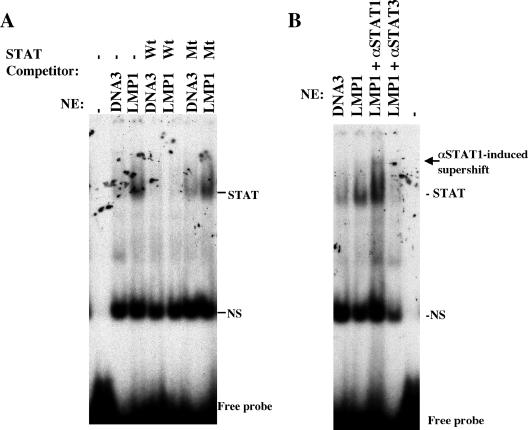
We confirmed whether the LMP1-inducible factors STAT1 and STAT3 can bind to a target sequence in the MUC1 promoter by an EMSA. When oligonucleotide probes containing STAT-binding sites (Fig. 3A) from the MUC1 promoter were incubated with nuclear extracts from Ad-AH cells transiently transfected with or without LMP1, binding of complexes containing STAT (Fig. 3A) was detected clearly in the LMP1-expressing cells. An excess amount of unlabeled oligonucleotides with wild-type sequence competed with the probe for binding, whereas oligonucleotides with mutated sequence did not.
FIG. 3.
LMP1 induces binding of nuclear factor to STAT1 and STAT3 sequences in the MUC1 promoter. (A) Induction of nuclear factor binding to the STAT sequence. Nuclear extracts (NE) from Ad-AH cells were mixed with 32P-labeled STAT probe and analyzed by EMSAs. Nonlabeled STAT (wild type [Wt], ×100) or mutated (Mt) probe were used as competitors. (B) Identification of STAT complexes. Nuclear extracts (NE) were incubated with antibodies specific for STAT1 (αSTAT1) or STAT3, and complexes were resolved by EMSAs. The arrow indicates a band supershifted by STAT1 antibody. Binding of STAT3 is attenuated by a STAT3 antibody. NS, nonspecific binding.
Next since a previous report showed that both STAT1 and STAT3 are required for complex formation on the STAT DNA-binding site, we tested for changes in migration or detection of STAT1- and STAT3-containing binding complexes that could be produced by STAT antibodies. STAT1 antibody produced a supershifted band, whereas STAT3 antibody disrupted the protein-DNA binding complex (Fig. 3B). These results suggest that both STAT1 and -3 can bind in a complex to the same STAT-specific sequences in the MUC1 promoter.
LMP1 enhances matrix-cell dissociation.
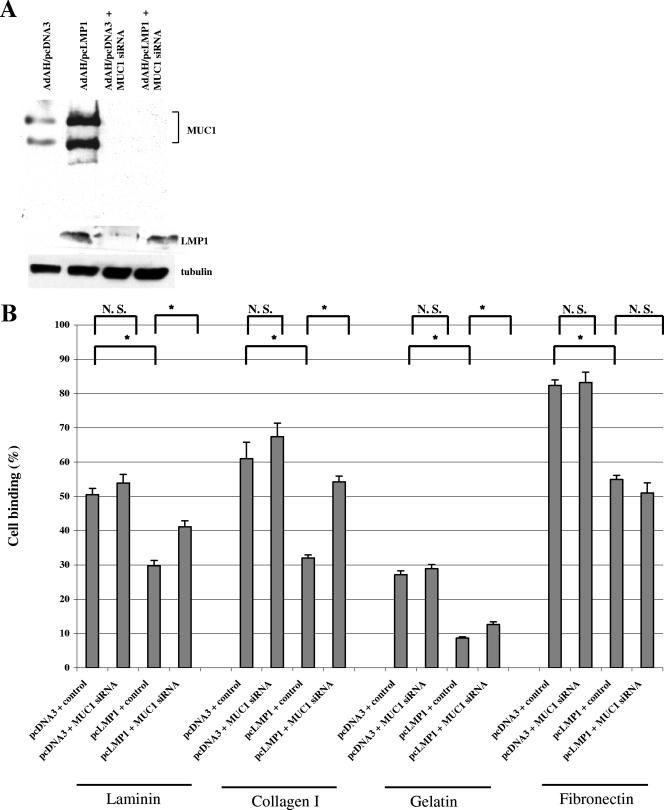
To examine whether adhesion of cells is affected in cells that express LMP1, we used a cell-matrix adhesion assay. First, to repress expression of MUC1, we transfected MUC1 siRNA into stably pcLMP1 or pcDNA3-transfected Ad-AH cells. Figure 4A shows that MUC1 siRNA completely abolished expression of both endogenous and LMP1-induced MUC1 protein. The same samples were used to appraise the effects of LMP1 and MUC1 siRNA on cell adhesion in the matrix adhesion assays. As shown in Fig. 4B, LMP1-expressing Ad-AH cells lost their ability to bind to main components of matrix (collagen type I, gelatin, laminin, and fibronectin) to a significant extent even though the binding rates are different for each matrix (Fig. 4B, P < 0.05). However, by use of MUC1 siRNA to inhibit MUC1 expression, the antiadhesive effect was reversed, and cell adhesion was restored except with fibronectin (P < 0.05). Transfection of MUC1 siRNA into pcDNA3-transfected cells did not affect binding significantly (see the leftmost two bars for each matrix component in Fig. 4B). Thus, expression of MUC1 induced by LMP1 appears to be required for the antiadhesive function of LMP1-expressing cells.
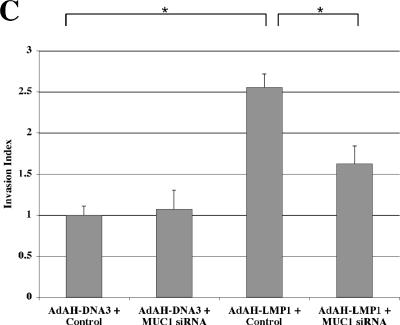
FIG. 4.
LMP1-induced MUC1 reduces cell adhesion to extracellular matrix and enhances cell invasiveness in vitro. (A) Stably pcDNA3- or pcLMP1-transfected Ad-AH cells with or without MUC1 siRNA (100 nM) were analyzed for MUC-1 by Western blotting. (B) Cell binding to matrix and inhibition by MUC1 siRNA indicated by the percentage of Ad-AH cells initially added. Data were calculated as the means of three replicate samples plus standard deviations (error bars). Values that were significantly different from each other (P < 0.05) are indicated ( ). N.S., not significantly different. (C) Increase in invasiveness of Ad-AH cells produced by LMP1 in Matrigel assays is inhibited by MUC1 siRNA. The invasion index is calculated as described previously (29). Values are shown as averages plus standard deviations (error bars) from triplicate experiments. Ad-AH cells were stably transfected with pcDNA3 or pcLMP1. Values that were significantly different from each other (P < 0.05) are indicated (
). N.S., not significantly different. (C) Increase in invasiveness of Ad-AH cells produced by LMP1 in Matrigel assays is inhibited by MUC1 siRNA. The invasion index is calculated as described previously (29). Values are shown as averages plus standard deviations (error bars) from triplicate experiments. Ad-AH cells were stably transfected with pcDNA3 or pcLMP1. Values that were significantly different from each other (P < 0.05) are indicated ( ).
).
LMP1 induces cell invasiveness via MUC1.
We have shown that LMP1-induced MMP9 enhances invasiveness of LMP1-expressing adherent cells in vitro (30). There are also reports of cell invasiveness related to expression of MUC1 in in vitro invasion assays (38). A Matrigel invasion chamber system is commonly used to assay tumor cell invasiveness in vitro (30, 38). LMP1-expressing Ad-AH cells were more than twice as invasive in this assay compared with pcDNA3-transfected Ad-AH cells (Fig. 4C). The results were statistically significant (P < 0.05), suggesting that LMP1 is a possible invasiveness-inducing factor in these cells. However, since several reports have shown that MUC1 itself also contributes to cell invasiveness, we examined the contribution of MUC1 induced by LMP1 to this property. With the use of MUC1 siRNA, we showed that LMP1-induced invasiveness could be clearly inhibited (compare the two rightmost bars in Fig. 4C). The results suggest that LMP1 also contributes to cell invasiveness through induction of MUC1.
DISCUSSION
LMP1 expression is not only essential for B-cell immortalization by EBV, but it is the only EBV protein that transforms nonlymphoid cells, such as rodent fibroblasts (20), human epithelial cells (10), and human keratinocytes (11). Consequently, the primary oncogenic properties of LMP1 have been the focus of innumerable investigations. Here we continue our investigations of another aspect of LMP1 function, namely, its ability ultimately to affect tumor progression. NPC is a highly invasive malignancy, and LMP1 is expressed in at least 70% of NPCs and in all EBV preinvasive NPC lesions (34). We have presented varied and mounting evidence that LMP1 promotes cell invasion and metastasis as well as angiogenesis (23, 30, 31, 44, 49, 50, 53, 54). However, tumor invasion and metastasis also require counteraction of cell-adhesive functions.
The extracellular mucin-like domain of MUC1 can shield adhesion molecules and diminish cellular adhesion (13, 52). Overexpression of MUC1 in carcinoma cells (18) is expected to have an effect on cellular behavior similar to that of loss of function of the major epithelial adhesion molecule E-cadherin, which has been shown recently to promote invasion and metastasis of carcinoma cells (48). Overexpression of MUC1 in several cancers is reported to correlate with poor prognosis (16). MUC1 has been used as an antigen for detection of recurrence of breast cancer, and detection of MUC1 mRNA may be useful as a marker for metastasis of gastric cancer (2). In addition, MUC1 is widely considered one of the most promising candidates for immunization against several cancers (6). In this paper, we demonstrate that LMP1 activates STAT1 and STAT3, resulting in expression of MUC1, and that inhibition of expression of these factors inhibits expression of MUC1 by LMP1 at the protein and transcriptional levels.
The first report concerning induction of STATs by LMP1 showed that the viral protein increases DNA-binding activities for STAT1 and STAT3 in fibroblasts detected by an EMSA (15). Chen et al. showed that LMP1 induces activated STAT3 and STAT5 in adherent cell lines, but not STAT1 (7). A subsequent report by Zhang et al. revealed that LMP1 induced increases in the total amount of STAT1, but not the tyrosine-phosphorylated form, in suspension cell lines (56). However, in our system, LMP1 induces activated STAT1 and -3, as well as increases in the total amount of STAT1 in Ad-AH cell lines consistent with the first report in this field (15). Moreover, the EMSA results showing that LMP1 can induce both activated STAT1 and -3 support results shown by Western blotting. We conclude that although whether LMP1 activates STAT1 is still controversial to some extent, the differences observed may be attributed to the different cell lines used or to experimental parameters, such as specificity of antibodies, LMP1 constructs, and transfection methods.
A recent report shows that activated STAT1 and STAT3 are critical for MUC1 transcription (12). STAT1-DN and STAT3-DN inhibited LMP1-induced MUC1 at the protein level (Fig. 2C and D). We confirmed these results by showing that both STAT1 and STAT3 could bind to the MUC1 promoter construct in cells in which LMP1 has expressed (Fig. 3A). We also found that LMP1-induced STAT1 and -3 could bind in a complex to the MUC1 promoter by supershift complex analyses (Fig. 3B).
In the last part of the study, we examined whether LMP1-induced MUC1 has potential biological significance through the use of cell adhesion and in vitro invasion assays. First, we showed that LMP1-expressing cells reduce the ability of cells to adhere to the main components of matrix. MUC1 siRNA restored the ability to bind to several matrices except for fibronectin. A recent paper revealed that MUC1 does not affect binding activity to fibronectin (38). At this point, we conclude that even though LMP1 reduces cell-binding activity to fibronectin, this property may be affected by unknown LMP1-inducible factors, not by MUC1. That LMP1 reduces cell adhesion functions and that this reduction may be caused by MUC1 provide the first such evidence in this field.
Since MUC1 expression may induce cell invasiveness by interacting with E-cadherin or β-catenin (43, 47), with the use of MUC1 siRNA, we showed that LMP1-induced cell invasiveness could be repressed by reducing expression of MUC1. The results suggest that at least MUC1 induced by LMP1 is required for this phenomenon. Thus, LMP1-induced MUC1 affects cell invasiveness and dissociation of adherent cells. LMP1 can also induce cell invasiveness via induction of MMP9 (30).
Recently, MUC1 has gained attention in another sphere, as a number of proto-oncogenes that interact with MUC1, such as epidermal growth factor receptor (EGFR), c-Src, and β-catenin, have been identified (27, 28, 43). In vitro studies show that defined sequence in the cytoplasmic domain of MUC1 interacts with β-catenin and can compete with E-cadherin at adherens junctions for binding of β-catenin when expressed at high levels. On the other hand, c-Src and EGFR phosphorylate MUC1, and these events promote interactions between β-catenin and MUC1 (28, 41). Interestingly, LMP1 induces EGFR in adherent cell lines (29), which may promote formation of MUC1-β-catenin complexes. Also, in other work we have shown that type III latently infected lymphocytes express high levels of β-catenin (39, 40), which may also contribute to formation of these complexes.
This study is the first to show that LMP1 induces MUC1 expression by STAT1 and STAT3 signaling. Furthermore, we detected activated STAT1 at the protein level, and these interactions including STAT1 and STAT3 may enhance MUC1 expression. Finally, we found that MUC1 induction by LMP1 affects cellular invasive and antiadhesive functions. Thus, LMP1 induces key factors required in the multistep process of invasion and metastasis: destruction of basement membrane, angiogenesis, cell motility (19, 21, 23, 30, 31, 44, 49, 50, 53, 54), and now a newly identified factor, cell adhesion capacity. EBV is the first tumor virus shown to induce this array of factors crucial in late stages of oncogenesis, adding significantly to its oncogenic properties (54).
Acknowledgments
This work was supported by grant P01CA19014 from the NCI.
We thank Toshio Hirano and Joyce Taylor-Papadimitirou for providing STAT1 and STAT3 dominant-negative plasmids, and Northern probe for MUC1, respectively. We are grateful to Erik K. Flemington and Maria Masucci for providing cell lines.
Footnotes
Published ahead of print on 6 December 2006.
REFERENCES
- 1.Agrawal, B., S. J. Gendler, and B. M. Longnecker. 1998. The biological role of mucins in cellular interactions and immune regulation: prospects for cancer immunotherapy. Mol. Med. Today 4:397-403. [DOI] [PubMed] [Google Scholar]
- 2.Ajisaka, H., and K. Miwa. 2003. Micrometastases in sentinel nodes of gastric cancer. Br. J. Cancer 89:676-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbach, H., V. Viglasky, F. Lefeu, J. M. Guinebretiere, V. Ramirez, N. Bride, N. Boualaga, T. Bauchet, J. P. Peyrat, M. C. Mathieu, S. Mourah, M. P. Podgorniak, J. M. Seignerin, K. Takada, and I. Joab. 2006. Epstein-Barr virus (EBV) genome and expression in breast cancer tissue: effect of EBV infection of breast cancer cells on resistance to paclitaxel (Taxol). J. Virol. 80:845-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergers, G., R. Brekken, G. McMahon, T. H. Vu, T. Itoh, K. Tamaki, K. Tanzawa, P. Thorpe, S. Itohara, Z. Werb, and D. Hanahan. 2000. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet, M. J. M., E. Guinebretiere, V. Kremmer, E. Grunewald, G. Benhamou, G. Contesso, and I. Joab. 1999. Detection of Epstein-Barr virus in invasive breast cancers. J. Natl. Cancer Inst. 91:1376-1381. [DOI] [PubMed] [Google Scholar]
- 6.Chen, D., J. Xia, Y. Tanaka, H. Chen, S. Koido, O. Wernet, P. Mukherjee, S. J. Gendler, D. Kufe, and J. Gong. 2003. Immunotherapy of spontaneous mammary carcinoma with fusions of dendric cells and mucin 1-positive carcinoma cells. Immunology 109:300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, H., L. Hutt-Fletcher, L. Cao, and S. D. Hayward. 2003. A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. J. Virol. 77:4139-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras-Brodin, B. A., M. Anvret, S. Imreh, E. Altiok, G. Klein, and M.G. Masucci. 1991. B cell phenotype-dependent expression of the Epstein-Barr virus nuclear antigens EBNA-2 to EBNA-6: studies with somatic cell hybrids. J. Gen. Virol. 72:3025-3033. [DOI] [PubMed] [Google Scholar]
- 9.Darnell, J. E. J., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415-1421. [DOI] [PubMed] [Google Scholar]
- 10.Dawson, C. W., A. B. Rickinson, and L. S. Young. 1990. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature (London) 344:777-780. [DOI] [PubMed] [Google Scholar]
- 11.Fahraeus, R., L. Rymo, J. S. Rhim, and G. Klein. 1990. Morphological transformation of human keratinocytes expressing the LMP gene of Epstein-Barr virus. Nature (London) 345:447-449. [DOI] [PubMed] [Google Scholar]
- 12.Gaemers, I. C., H. L. Vos, H. H. Volders, S. W. van der Valk, and J. A. Hilkens. 2001. STAT-responsive element in the promoter of the episialin/MUC1 gene is involved in its overexpression in carcinoma cells. J. Biol. Chem. 276:6191-6199. [DOI] [PubMed] [Google Scholar]
- 13.Gendler, S. J., T. Duhig, D. Lamport, R. White, M. Parker, and J. Taylor-Papadimitriou. 1987. Cloning of partial cDNA encoding differentiation antigen and tumor associated mucin glycoproteins expressed by human epithelia. Proc. Natl. Acad. Sci. USA 84:6060-6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimmi, C. D., B. W. Morrison, B. A. Mainprice, J. G. Gribben, V. A. Boussiotis, G. J. Freeman, S. Y. L. Park, M. Watanabe, J. Gong, D. F. Hayes, D. W. Kufe, and L. M. Nadler. 1996. Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human T cells. Nat. Med. 2:1367-1370. [DOI] [PubMed] [Google Scholar]
- 15.Gires, O., F. Kohlhuber, E. Kilger, M. Bauman, A. Kieser, C. Kaiser, R. Zeider, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guddo, F., A. Giatromanolaki, M. I. Koukourakis, C. Reina, A. M. Vignola, G. Chloverakis, J. Hilkens, K. C. Gatter, A. L. Harris, and G. Bonsignnore. 1998. MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlated with poor survival in node positive patients. J. Clin. Pathol. 51:667-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumbiner, B., B. Stevenson, and A. Grimaldi. 1988. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 107:1575-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilkens, J., F. Buijs, J. Hilgers, P. Hageman, J. Calafat, A. Sonnenberg, and M. van der Valk. 1984. Monoclonal antibodies against human milk-fat globule membranes detecting differentiation antigens of the mammary gland and its tumors. Int. J. Cancer 34:197-206. [DOI] [PubMed] [Google Scholar]
- 19.Horikawa, T., T. S. Sheen, H. Takeshita, H. Sato, M. Furukawa, and T. Yoshizaki. 2001. Induction of c-Met proto-oncogene by Epstein-Barr virus latent membrane protein 1 and the correlation with cervical lymph node metastasis of nasopharyngeal carcinoma. Am. J. Pathol. 159:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, K. R., T. Yoshizaki, H. Miyamori, K. Hasegawa, T. Horikawa, M. Furukawa, S. Harada, M. Seiki, and H. Sato. 2000. Transformation of Madine-Darby canine kidney (MDCK) epithelial cells by Epstein-Barr virus latent membrane protein 1 (LMP1) induces expression of Ets 1 and invasiveness. Oncogene 19:1764-1771. [DOI] [PubMed] [Google Scholar]
- 22.Kondo, S., N. Wakisaka, M. J. Schell, T. Horikawa, T. S. Sheen, H. Sato, M. Furukawa, J. S. Pagano, and T. Yoshizaki. 2005. Epstein-Barr virus latent membrane protein 1 induces the matrix metalloproteinase-1 promoter via an Ets binding site formed by a single nucleotide polymorphism: enhanced susceptibility to nasopharyngeal carcinoma. Int. J. Cancer 115:368-376. [DOI] [PubMed] [Google Scholar]
- 23.Kondo, S., S. Y. Seo, T. Yoshizaki, N. Wakisaka, M. Furukawa, I. Joab, K. L. Jang, and J. S. Pagano. 2006. EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1α through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res. 66:9870-9877. [DOI] [PubMed] [Google Scholar]
- 24.Kovarik, A., N. Peat, D. Wilson, S. J. Gendler, and J. Taylor-Papadimitriou. 1993. Analysis of the tissue-specific promoter of the MUC1 gene. J. Biol. Chem. 268:9917-9926. [PubMed] [Google Scholar]
- 25.Kulwichit, W., R. H. Edwards, E. M. Davenport, J. F. Baskar, V. Godfrey, and N. Raab-Traub. 1998. Expression of Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc. Natl. Acad. Sci. USA 95:11963-11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagow, E. L., and D. D. Carson. 2002. Synergistic stimulation of MUC1 expression in normal breast epithelia and breast cancer cells by interferon-γ and tumor necrosis factor-α. J. Cell Biol. 86:759-772. [DOI] [PubMed] [Google Scholar]
- 27.Li, Y., A. Bharti, D. Chen, J. Gong, and D. Kufe. 1998. Interaction of glycogen synthase kinase 3 beta with DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol. Biol. Cell 18:7216-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, Y., H. Kuwahara, J. Ren, G. Wen, and D. Kufe. 2001. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3β. J. Biol. Chem. 276:6061-6064. [DOI] [PubMed] [Google Scholar]
- 29.Miller, W. E., J. L. Cheshire, and N. Raab-Traub. 1998. Interaction of tumor necrosis factor receptor-associated factor signaling proteins with the latent membrane protein 1 PXQXT motif is essential for induction of epidermal growth factor receptor expression. Mol. Cell. Biol. 18:2835-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murono, S., T. Yoshizaki, H. Sato, H. Takeshita, M. Furukawa, and J. S. Pagano. 2000. Aspirin inhibits tumor cell invasiveness induced by Epstein-Barr virus latent membrane protein 1 through suppression of metalloproteinase-9 expression. Cancer Res. 60:2555-2561. [PubMed] [Google Scholar]
- 31.Murono, S., H. Inoue, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajima, K., Y. Yamanaka, K. Nakae, H. Kojima, M. Ichida, N. Kiuchi, T. Kitaoka, T. Fukada, M. Hibi, and T. Hirano. 1996. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 15:3651-3658. [PMC free article] [PubMed] [Google Scholar]
- 33.Pagano, J. S., M. Blaser, M. A. Buendia, B. Damania, K. Khalili, N. Raab-Traub, and B. Roizman. 2004. Infectious agents and cancer: criteria for a causal relation. Semin. Cancer Biol. 14:453-471. [DOI] [PubMed] [Google Scholar]
- 34.Pathmanathan, R., U. Prasad, R. Sadler, K. Flynn, and N. Raab-Traub. 1995. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N. Engl. J. Med. 333:693-698. [DOI] [PubMed] [Google Scholar]
- 35.Reuther, J. Y., G. W. Reuther, D. Cortez, A. M. Pendergast, and A. S. Baldwin. 1998. A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 12:968-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reference deleted.
- 37.Reference deleted.
- 38.Satoh, S., Y. Hinoda, T. Hayashi, M. D. Burdick, K. Imai, and M. A. Hollingsworth. 2000. Enhancement of metastatic properties of pancreatic cancer cells by MUC1 gene encoding an anti-adhesion molecule. Int. J. Cancer 88:507-518. [DOI] [PubMed] [Google Scholar]
- 39.Shackelford, J., C. Maier, and J. S. Pagano. 2003. Epstein-Barr virus activates β-catenin in type III latently infected lymphocyte lines: association with deubiquitinating enzymes. Proc. Natl. Acad. Sci. USA 100:15572-15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shackelford, J., and J. S. Pagano. 2005. Targeting of host-cell ubiquitin pathways by viruses. Essays Biochem. 41:139-156. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder, J. A., M. C. Thompson, M. M. Gardner, and S. J. Gendler. 2001. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J. Biol. Chem. 276:13057-13064. [DOI] [PubMed] [Google Scholar]
- 42.Stark, G. R. 1997. Genetic analysis of interferon and other mammalian signaling pathways. Harvey Lect. 93:1-16. [PubMed] [Google Scholar]
- 43.Steelant, W. F. A., J. L. Goeman, J. Philippe, L. C. J. M. Oomen, J. Hilkens, M.-A. Krzewinski-Recchi, G. Huet, J. van der Eycken, P. Delannoy, E. A. Bruyneel, and M. M. Mareel. 2001. Alkyl-lysophospholipid-1-O-octadecyl-2-O-methyl-glycerophophocholine induces invasion through episialin-mediated neutralization of E-cadherin in human mammary MCF-7 cells in vitro. Int. J. Cancer 92:527-536. [DOI] [PubMed] [Google Scholar]
- 44.Takeshita, H., T. Yoshizaki, W. E. Miller, H. Sato, M. Furukawa, J. S. Pagano, and N. Raab-Traub. 1999. Matrix metalloproteinase 9 expression is induced by Epstein-Barr virus latent membrane protein 1 C-terminal activation regions 1 and 2. J. Virol. 73:5548-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takimoto, T., H. Sato, H. Ogura, and R. Glaser. 1986. Rescue of a biologically active Epstein-Barr virus from nonproducer cells. Cancer Res. 46:2085-2087. [PubMed] [Google Scholar]
- 46.Taylor-Papadimitriou, J., J. Burchell, D. W. Miles, and M. Dalziel. 1999. MUC1 and cancer. Biochem. Biophys. Acta 1455:301-313. [DOI] [PubMed] [Google Scholar]
- 47.Truant, S., E. Bruyneel, V. Gouyer, O. De Wever, F.-R. Pruvot, M. Mareel, and G. Huet. 2003. Requirement of both mucins and proteoglycans in cell-cell dissociation and invasiveness of colon carcinoma HT-29 cells. Int. J. Cancer 104:683-694. [DOI] [PubMed] [Google Scholar]
- 48.Tsai, C. N., C. L. Tsai, K. P. Tse, H. Y. Chang, and Y. S. Chang. 2002. The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the down-regulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc. Natl. Acad. Sci. USA 99:10084-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakisaka, N., S. Murono, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2002. Epstein-Barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer Res. 62:6337-6344. [PubMed] [Google Scholar]
- 50.Wakisaka, N., S. Kondo, T. Yoshizaki, S. Murono, M. Furukawa, and J. S. Pagano. 2004. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1α. Mol. Cell. Biol. 24:5223-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, D., D. Liebowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831-840. [DOI] [PubMed] [Google Scholar]
- 52.Wesseling, J., S. W. van der Valk, H. L. Vos, A. Sonnenberg, and J. Hilkens. 1995. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol. 129:255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizaki, T., H. Sato, M. Furukawa, and J. S. Pagano. 1998. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc. Natl. Acad. Sci. USA 95:3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshizaki, T., N. Wakisaka, and J. S. Pagano. 2005. Epstein-Barr virus, invasion and metastasis, p. 171-196. In E. S. Robertson (ed.), Epstein Barr virus. Caiser Academic Press, Norwich, United Kingdom.
- 55.Zhang, L., K. Hong, J. Zhang, and J. S. Pagano. 2004. Multiple signal transducers and activators of transcription are induced by EBV-LMP1. Virology 323:141-152. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, L., L. Wu, K. Hong, and J. S. Pagano. 2001. Intracellular signaling molecules activated by Epstein-Barr virus for induction of interferon regulatory factor 7. J. Virol. 75:12393-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]