Abstract
Adaptive radiation is facilitated by a rugged adaptive landscape, where fitness peaks correspond to trait values that enhance the use of distinct resources. Different species are thought to occupy the different peaks, with hybrids falling into low-fitness valleys between them. We hypothesize that human activities can smooth adaptive landscapes, increase hybrid fitness and hamper evolutionary diversification. We investigated this possibility by analysing beak size data for 1755 Geospiza fortis measured between 1964 and 2005 on the island of Santa Cruz, Galápagos. Some populations of this species can display a resource-based bimodality in beak size, which mirrors the greater beak size differences among species. We first show that an historically bimodal population at one site, Academy Bay, has lost this property in concert with a marked increase in local human population density. We next show that a nearby site with lower human impacts, El Garrapatero, currently manifests strong bimodality. This comparison suggests that bimodality can persist when human densities are low (Academy Bay in the past, El Garrapatero in the present), but not when they are high (Academy Bay in the present). Human activities may negatively impact diversification in ‘young’ adaptive radiations, perhaps by altering adaptive landscapes.
Keywords: adaptive radiation, adaptive divergence, speciation, reproductive isolation, rapid evolution, contemporary evolution
1. Introduction
Human activities are known to influence the evolution of natural populations (Hendry & Kinnison 1999; Hendry et al. 2000; Bradshaw & Holzapfel 2001; Koskinen et al. 2002; Coltman et al. 2003; Levinton et al. 2003; Stockwell et al. 2003; Olsen et al. 2004), but it remains uncertain as to how such activities might impact evolutionary diversification itself. One obvious impact is that humans can cause some species to go extinct. A less obvious possibility is that humans might cause species early in the process of divergence to instead fuse back together (Seehausen et al. 1997; Streelman et al. 2004; Gow et al. 2006). This particular impact should be most prevalent for ‘young’ adaptive radiations where interbreeding is still possible. One such radiation is represented by Darwin's finches of the Galápagos Islands, Ecuador. Here, a single ancestral species has evolved over a few million years into 14 recognized species that have beak sizes and shapes specialized for using different food resources (Lack 1947; Bowman 1961; Abbott et al. 1977; Schluter & Grant 1984; Grant 1986; Schluter 2000). Of particular relevance to our study, the small ground finch (Geospiza fuliginosa) has a small beak and preferentially consumes small/soft seeds, the large ground finch (Geospiza magnirostris) has a large beak and preferentially consumes large/hard seeds and the medium ground finch (Geospiza fortis) has an intermediate-sized beak and preferentially consumes intermediate-sized seeds. All of the ground finch species lack intrinsic genetic incompatibilities (Grant 1986; Grant & Grant 1996; Grant et al. 2004, 2005), and so may be susceptible to human impacts that promote species fusion.
The earliest stages of adaptive radiation in Darwin's finches are accessible for research because the same traits (beak size and shape) that vary among species can also vary dramatically within them (Lack 1947; Bowman 1961; Grant et al. 1976; Abbott et al. 1977; Grant 1986; Grant & Grant 1989). This intra-specific variation has a strong additive genetic basis (Keller et al. 2001) and important fitness consequences. In G. fortis populations, for example, individuals with larger beaks can bite harder (Herrel et al. 2005) and are known to eat harder seeds in the wild (Grant 1986; Price 1987). As would be expected for a heritable trait with fitness consequences, G. fortis beak size evolves owing to natural selection imposed by changes in food resources. In particular, average beak size increases (decreases) when small/soft seeds become less (more) abundant (Grant & Grant 1995, 2002). Given that beak size is the primary axis of adaptive radiation in ground finches (Lack 1947; Bowman 1961; Abbott et al. 1977; Schluter & Grant 1984; Grant 1986; Schluter 2000), human activities that influence selection on beak size might impact diversification. An excellent place to test for such impacts is Academy Bay on Santa Cruz Island, where the G. fortis population is highly variable in beak size and comes in contact with a rapidly growing human population.
The unusually high beak size variability in Academy Bay G. fortis (figure 1) has been recognized since at least the 1940s (Lack 1947; Bowman 1961; Grant et al. 1976; Grant & Grant 1989). This variation was historically manifest as two different modes (peaks) in the frequency distribution of beak size, with both modes falling between the larger-beaked G. magnirostris and the smaller-beaked G. fuliginosa. This within-site, within-species bimodality was persistently evident in samples from the 1940s (Lack 1947), the 1950s (Bowman 1961), and the 1960s (Snow 1966; Ford et al. 1973). Although some G. fortis with intermediate beak sizes were always present, the two modes remained distinguishable, suggesting an early step en route to speciation. Ford et al. (1973) suggested that the modes had formed owing to disruptive selection in sympatry, with another possibility being divergence in allopatry followed by secondary contact. The bimodality could also have been influenced by interbreeding between G. fortis residents and immigrants (from the same or different islands) and by hybridization with other ground finch species. In any case, the bimodality in Academy Bay G. fortis was a remarkable intra-specific mirror of the greater beak size differences among species. As such, these two scales of diversity were probably influenced by the same evolutionary processes: i.e. disruptive and directional selection owing to the abundance of seeds differing in size and hardness.
Figure 1.
Beak size variation in Academy Bay G. fortis. Both birds are mature males caught at the same time in the same mist net. Photograph by Andrew P. Hendry.
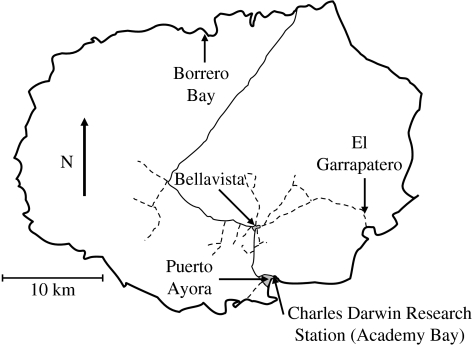
If humans influence evolutionary diversification in Darwin's finches, such impacts are most likely at Academy Bay—because it is here that human population density has increased most dramatically. Census data show that the population of Puerto Ayora in Academy Bay was 900 in 1974, 2404 in 1982, 4294 in 1990 and 9582 in 2001 (www.inec.gov.ec). Census data specific to Puerto Ayora are not available prior to 1974, but the human population of the Galápagos as a whole jumped from 2391 in 1962 to 4037 in 1974 (www.inec.gov.ec). Increases in the numbers of tourists have been even greater; from 4500 in 1970 (organized tours began in 1967) to 41 000 in 1991 (www.darwinfoundation.org). We tested whether these increases were coincident with changes in the distinctiveness of beak size modes in Academy Bay G. fortis. Our analyses were based on beak size measurements from 1964 to 2004 at the Charles Darwin Research Station, located immediately adjacent to the town of Puerto Ayora (figure 2).
Figure 2.
A map of Santa Cruz Island, Galápagos, showing paved roads (solid lines), gravel roads and major trails (broken lines), major towns (Puerto Ayora and Bellavista) and sites where G. fortis were sampled (Academy Bay, El Garrapatero and Borrero Bay).
Academy Bay is the only place where a bimodal Darwin's finch population is known to have come into contact with an increasing human population. Our inferences regarding human impacts are therefore restricted to this unreplicated circumstance, followed by speculation as to possible impacts on other aspects of the Darwin's finch radiation. As a spatial control for human impacts, we analysed bimodality in G. fortis beak size at two sites on the same island but still remote from human settlements (figure 2): Borrero Bay (1973–2004) and El Garrapatero (2003–2005). Direct human impacts are negligible at these two sites, but potential indirect impacts are certainly present, such as grazing by introduced goats and donkeys.
2. Material and methods
Mist nets and baited traps were used to capture the birds. With few exceptions (see below), each bird was measured for beak length (anterior edge of nostril to tip of upper mandible), beak depth (at the nares) and beak width (base of lower mandible). Most birds were then banded, which minimized remeasurement of the same individuals within years. After measurement, all birds were released at the site of capture. Within each time- and site-specific ‘sample’, G. fortis could be reliably distinguished from related species based on morphological discontinuities and concordance with previous work (Ford et al. 1973; Abbott et al. 1977; Grant 1986). At Academy Bay in 2004, for example, discriminant functions based on beak dimensions upheld our original species designations for 40 out of 41 G. fuliginosa, 169 out of 173 G. fortis and 11 out of 11 G. magnirostris.
Data for Academy Bay were collected by David Snow (February 1963–May 1964, N=110; beak length measured differently), Hugh Ford and colleagues (August–September 1968, N=327; beak width not measured), Ian Abbott (February–March 1973, N=35; beak depth not measured), Peter Grant (November–December 1973, N=58), Peter Boag (January–February 1988, N=53) and Jeffrey Podos, Andrew Hendry and colleagues (January–March 1999–2002, N=119; February–March 2003, N=125; January–March 2004, N=173). Data for Borrero Bay were collected by Ian Abbott and Peter Grant (April–May 1973, N=122; culmen depth rather than beak depth), Peter Grant (December 1973, N=72; July 1975, N=84) and Andrew Hendry and colleagues (March 2004, N=128). Data for El Garrapatero were collected by Jeffrey Podos, Andrew Hendry and colleagues (February–March 2003, N=55; January–March 2004, N=114; January–April 2005, N=180).
Different investigators occasionally measured beak dimensions in different ways, as noted above. We therefore avoided direct beak size comparisons among samples collected by different investigators. Our main inferences were instead based on the degree of bimodality within each sample. For each beak size dimension in each sample, we first calculated coefficients of variation, which should be robust to differences in mean values. We then used principal components analysis to combine all beak dimensions into a single composite measure of ‘beak size’ (PC1) for each bird within a given sample. Because beak width was not measured in 1968, a critical sample for our inferences, we also recalculated PC1 for each bird after excluding beak width measurements from each sample.
We used three complementary methods to infer bimodality. First, we examined frequency histograms of PC1 for samples of more than 100 birds. Second, we plotted observed cumulative proportions for PC1 against cumulative proportions expected under normality. These plots take the form of a straight line for a single normal distribution but have a characteristically curved shape for a bimodal distribution (see §3). Third, we tested statistically whether each sample was better represented by a single normal distribution or by a mixture of two normal distributions. To do this, we fitted a two-component normal mixture model, having data , with for where and are two normal density functions having means and and a common variance , and p represents the probability that observation lies in component 1 (described by ). Mixtures were fitted to the data via discretization of parameters and an efficient summation method (Brewer 2003). This approach enabled direct calculation of Bayesian estimates of the means, variance and proportions (p) for each normal distribution, which amounts to a form of numerical integration of the posterior densities (Brewer 2003).
For each sample, we next compared the fit of a single normal distribution to that of a mixture of two normal distributions based on Akaike's Information Criterion corrected for sample size (AICc; Burnham & Anderson 2002). For each sample, we calculated ΔAIC as AICc for the single normal distribution minus AICc for the fitted mixture of two normal distributions. Our interpretation of these ΔAIC values was similar to established guidelines (Burnham & Anderson 2002). Specifically, we interpreted ΔAIC<−8 as strong support for a single normal distribution, −8≤ΔAIC<−5 as moderate support for a single normal distribution, −5≤ΔAIC≤5 as roughly equivalent support for a single normal distribution or a mixture of two normal distributions, 5<ΔAIC≤8 as moderate support for a mixture of two normal distributions, and ΔAIC>8 as strong support for a mixture of two normal distributions. We also calculated AICw (Burnham & Anderson 2002) for each sample, here representing the likelihood that a mixture of two normal distributions fits the data better than a single normal distribution.
Inferring bimodality in finite samples is a notoriously difficult statistical endeavour (Brewer 2003). One problem is that overlap between the tails of two distributions can fill the gap between them, making the two modes difficult to distinguish. Another problem is that a distribution with fewer individuals can be obscured by the tail of a distribution with more individuals. Both of these properties characterized our finch data to the extent that frequency histograms were limited in their ability to discriminate between unimodal and bimodal distributions. Fortunately, the normal probability plots and Bayesian mixture models proved very effective at doing so (simulation results not shown). We therefore use frequency histograms as visual representations when sample sizes are large, but specifically base our primary inferences on the probability plots and mixture models.
3. Results
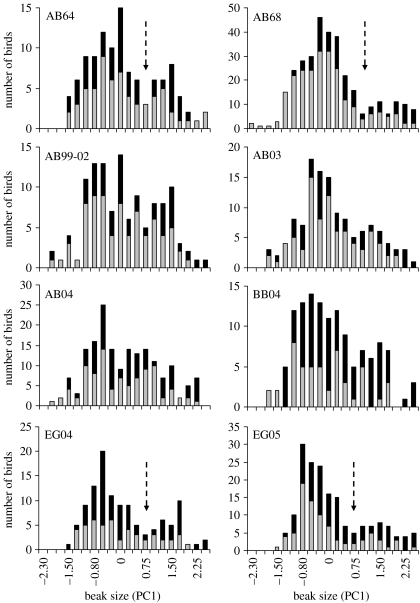
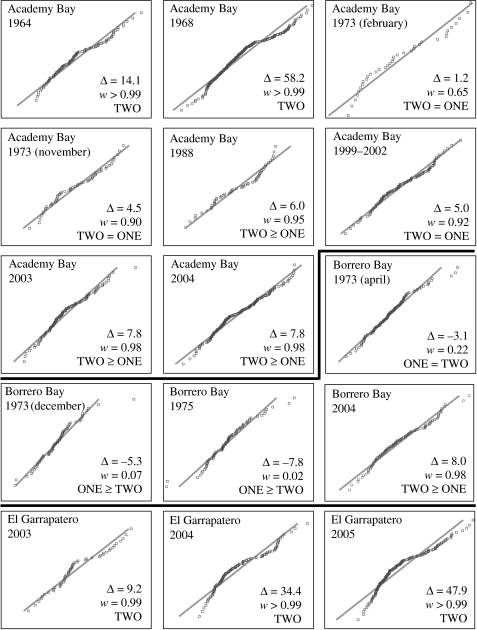
Beak sizes in Academy Bay G. fortis were strongly bimodal in the 1960s, a result that was not dependent on sex or age (figure 3; see also Ford et al. 1973). Strong bimodality in these samples was evident as obvious discontinuities in frequency histograms (figure 3), strong deviations from normality in probability plots (figure 4) and substantially better fits for mixtures of two normal distributions than for single normal distributions (ΔAIC=14.1−58.2). After the 1960s, Academy Bay G. fortis have not evinced strong bimodality, a result evident in weak or absent discontinuities in frequency histograms (figure 3), weak to moderate deviations from normality in probability plots (figure 4), and roughly equivalent fits for mixtures of two normal distributions and single normal distributions (ΔAIC=1.2−7.8). The loss of strong bimodality was not accompanied by changes in coefficients of variation for beak dimensions (electronic supplementary material A), suggesting an increase in the relative abundance of intermediate-sized birds rather than a loss of the large- or small-beaked birds (see also figure 5). Interestingly, the loss of strong bimodality was abrupt, occurring between 1968 and 1973. Only minor changes took place later, with one being a slight tendency toward bimodality in the 2003 and 2004 samples. This apparent change may be illusory because the slight bimodality in these samples falls far short of the strong bimodality at Academy Bay in the 1960s or El Garrapatero in the present. All of these interpretations continue to hold, and the 2003 and 2004 samples are no longer bimodal, in analyses that excluded beak width (electronic supplementary material B).
Figure 3.
Beak size variation in samples of more than 100 G. fortis. Shown are frequency histograms of PC1 (larger values correspond to larger beaks), with the black portions of bars representing mature males and the grey portions representing all other birds. Panels are labelled according to sampling site (Academy Bay, AB; El Garrapatero, EG; Borrero Bay, BB) and year. The 1973 Borrero Bay histogram is not shown here because of limited space, but is provided in electronic supplementary material D. Arrows show discontinuities between the small beak and large beak modes in the samples statistically confirmed to have strong bimodality (see figure 4). x-axes are not comparable across panels because PC1 was calculated within each sample.
Figure 4.
Normal probability plots showing observed cumulative proportions for PC1 (points) versus expected cumulative proportions under a single normal distribution (line). Also shown are the results of Bayesian mixture models: Δ represents AICc for a single normal distribution minus AICc for the fitted mixture of two normal distributions (i.e. ΔAIC), and w represents the likelihood that a mixture of two normal distributions fits the data better than a single normal distribution (i.e. AICw). Also shown are our conclusions regarding bimodality: ONE represents strong support for a single normal distribution, TWO represents strong support for a mixture of two normal distributions, ONE≥TWO represents moderately more support for a single normal distribution, TWO≥ONE represents moderately more support for a mixture of two normal distributions, and ONE=TWO represents roughly equivalent support for a single normal distribution or a mixture of two normal distributions.
Figure 5.
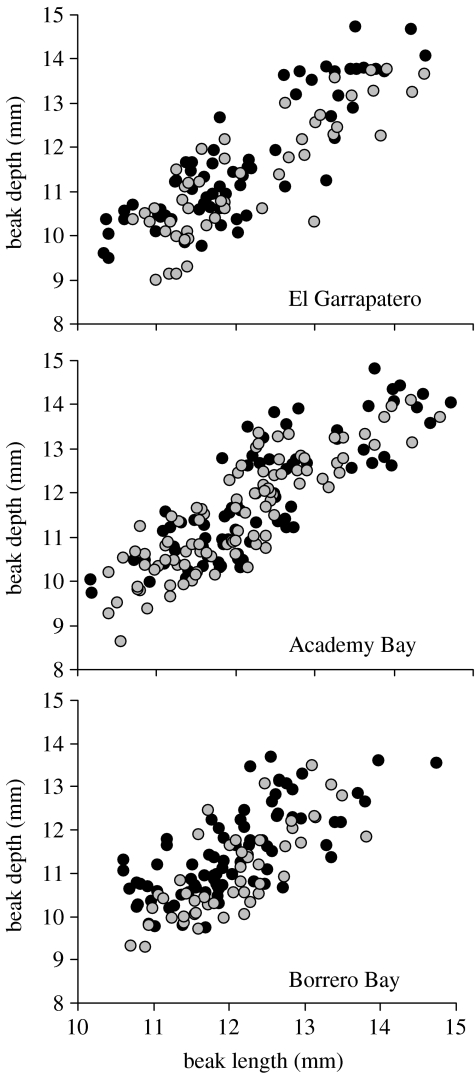
Variation in beak length and beak depth for samples of G. fortis collected by the same investigators between January and March 2004. Black points show mature males and grey points show all other birds. This figure illustrates the partial gap in beak size between modes at El Garrapatero, the filling of this gap at Academy Bay and the relative scarcity of the largest-beaked birds at Borrero Bay.
Beak sizes in El Garrapatero G. fortis were strongly bimodal in all samples (2003–2005), a result that was again independent of sex or age (figure 5). Strong bimodality in these samples was evident as obvious discontinuities in frequency histograms (figure 3), strong deviations from normality in probability plots (figure 4) and substantially better fits for mixtures of two normal distributions than for single normal distributions (ΔAIC=9.2−54.5). In all respects, the distribution of G. fortis beak sizes at this site was remarkably similar to that at Academy Bay in the 1960s (figure 3; electronic supplementary material A). All of these interpretations continue to hold, although bimodality appears weaker in the small sample from 2003, in analyses that excluded beak width (electronic supplementary material B).
Beak sizes in Borrero Bay G. fortis showed a tendency toward unimodality in the 1970s, a result evident in the lack of discontinuities in frequency histograms, weak departures from normality in probability plots (figure 4) and slightly to moderately better fits for single normal distributions than for mixtures of two normal distributions (ΔAIC=−3.1−7.8). At the same site in 2004, beak sizes tended toward bimodality (figures 3 and 4; ΔAIC=8.0). It is important to recognize, however, that this slight bimodality in the recent Borrero Bay sample is very different from the strong bimodality at Academy Bay in the 1960s and El Garrapatero in the present. The reason is that large-beaked G. fortis are rare at Borrero Bay, even in the recent samples (figure 5). This observation fits with the lower coefficients of variation for beak dimensions at Borrero Bay than at the other sites (electronic supplementary material A). All of these interpretations continue to hold, and are strengthened, in analyses that excluded beak width (electronic supplementary material B).
4. Discussion
When human population densities were low prior to the 1970s, the Academy Bay population of G. fortis was strongly bimodal in beak size (figures 3 and 4; Ford et al. 1973). As human population densities then increased rapidly, strong bimodality was lost and has remained absent ever since. The change occurred abruptly between the 1968 and 1973 samples, suggesting that the conditions for adaptive radiation may be sensitive to particular thresholds. The observed loss of strong bimodality could reflect (i) a decrease in the relative abundance of large-beaked birds (which were initially less common); (ii) convergence in average beak size of the two modes; or (iii) an increase in the relative abundance of intermediate birds. We suggest that the last of these alternatives is most likely, given the temporal stability in coefficients of variation for beak size (electronic supplementary material A), and a filling of the former gap between large and small beak size modes (figure 5).
If we are correct in identifying intense human activities as the root cause of these changes at Academy Bay, beak size bimodality should persist at sites that have suitable resource distributions but are less impacted by humans. This appears to be the case at El Garrapatero, which is removed from human settlements (figure 2). Large samples collected by the same investigators at the same time clearly show that G. fortis bimodality is now much stronger at El Garrapatero than at Academy Bay (figures 3–5). Indeed, the degree of bimodality in recent El Garrapatero samples is matched only by historical Academy Bay samples. Historical samples are not available for El Garrapatero and so it remains possible that bimodality was different there in the past. Regardless, this spatial comparison of contemporary samples shows that bimodality is maintained when human impacts are low but not when they are high.
(a) Adaptive landscapes
Common forces probably drive the evolution of beak size modes in G. fortis and of greater beak size differences among ground finch species. One of these forces may be positive assortative mating. Another may be disruptive selection on rugged adaptive landscapes—the hypothesis we now advance further. Previous work has shown that Darwin's finch species have beak sizes and shapes that generally match distinct fitness peaks (Schluter & Grant 1984), with hybrids falling into low-fitness valleys between these peaks (Grant & Grant 1996). If the G. fortis beak size modes are similarly the result of disruptive selection on an adaptive landscape, bimodality should be absent at sites that lack appropriate resource distributions. At Borrero Bay, for example, the relative scarcity of medium and large seeds (Abbott et al. 1977) probably selects against birds with large beaks. Accordingly, large-beaked G. fortis were rare at this site both in the past and still are in the present (figure 5; electronic supplementary material A). The even larger-beaked G. magnirostris are also rare at Borrero Bay.
Extending this framework, we suggest that the formerly strong bimodality in Academy Bay G. fortis may have been lost owing to human activities that alter resource distributions and convert rugged adaptive landscapes into smooth ones. For example, humans might increase the relative abundance of intermediate-sized seeds by altering habitat, introducing non-native plants, and providing food for native and wild animals. Indeed, perturbations of this nature are prevalent in the Galápagos (Schofield 1989; Mauchamp 1997) and are particularly obvious at Academy Bay. As one striking example, we are aware of at least two, long-established finch ‘feeders’ within 500 m of our study site. We did not specifically collect birds that were using these feeders, but we did perform several qualitative observations. For about an hour each morning, approximately 100 ground finches were using each feeder at any given time. The rice at these feeders can be readily cracked by birds having a wide range of beak sizes, albeit with different efficiencies (Grant et al. 1976). We mention this active feeding of finches as just one example of how humans can materially change finch diets and presumably the adaptive landscape for beak size. We cannot, however, identify the specific changes that caused the loss of strong bimodality because food availability and diets were not quantified prior to the mid-1970s (Abbott et al. 1977). Our hypothesis regarding adaptive landscapes is therefore offered only as a plausible hypothesis.
If humans negatively impact intra-specific diversification, might such impacts also extend to inter-specific levels? Species integrity in a young adaptive radiation is usually attributed to some combination of low hybridization rates (formation of hybrids) and low hybrid fitness (survival and reproductive success; Schluter 2000). For ground finches, natural hybridization does occur and intrinsic genetic incompatibilities are lacking (Grant 1986; Grant & Grant 1996; Grant et al. 2004, 2005). Species integrity in this radiation thus depends at least in part on natural selection against hybrids that fall between ecological niches (Grant & Grant 1996). Factors that smooth adaptive landscapes should increase the relative fitness of hybrids and thereby increase the chance of species fusion. As a striking example, the formation and survival of hybrid Geospiza on the small island of Daphne Major increased when a prolonged El Niño event changed food resources (Grant & Grant 1996). Increased hybrid survival then contributed to the morphological and genetic convergence of the two species (Grant & Grant 2002; Grant et al. 2004, 2005). We suggest that similar effects could attend human activities, at least in principle. If humans also influence hybridization rates in Darwin's finches, as they do in other taxa (Seehausen et al. 1997; Streelman et al. 2004), the impacts on diversification could be even more pronounced.
On the flip side, humans can sometimes facilitate adaptive radiation by introducing resources that form new peaks on adaptive landscapes, as long as this does not substantially reduce the distinctiveness of old peaks. A particularly well-known example is the tendency of some native phytophagous insects to form locally adapted and reproductively isolated host races on newly introduced plants (e.g. Bush 1969; Carroll et al. 1997). Whether negatively or positively, changes to adaptive landscapes may be a particularly potent way that humans can influence adaptive radiation.
(b) Alternatives
We have advanced the hypothesis that humans can negatively impact evolutionary diversification by smoothing formerly rugged adaptive landscapes. Alternative scenarios for Academy Bay G. fortis are that humans are not the causal force, or that their specific effects are not mediated through adaptive landscapes. In the first case, the most obvious alternative candidate is climate, because droughts and El Niños are known to influence the evolution of beak size, as well as the survival of intermediate forms (Grant 1986; Grant & Grant 1995, 1996, 2002). Although climate effects are certainly possible for Academy Bay, rainfall data show no strong El Niños or prolonged droughts between 1968 and 1973, the period during which strong bimodality was lost (electronic supplementary material C). More critically, climate is a regional effect and should influence both Academy Bay and El Garrapatero, which are only about 10 km apart and at similar elevations (figure 2). How then could climate change cause the lost of strong bimodality at Academy Bay but not at El Garrapatero? The simplest plausible explanation for the difference between these two sites is the local effect of human population density.
Even if humans are the cause of changes in bimodality, the specific impacts could have little to do with adaptive landscapes. As noted above, humans might alter hybridization rates rather than hybrid survival. We cannot rule out this possibility, but it is not clear how increases in human population density would reduce the strength of assortative mating, which is strongly based on song variation (Grant 1986). A more plausible alternative is that humans alter the movement patterns of finches—perhaps intermediate birds from elsewhere are now more likely to use Academy Bay for foraging. This is an intriguing scenario, although it is not clear where these intermediate birds would be coming from, or whether they would be differentially attracted to human-dominated sites. One possibility is that humans provide an intermediate resource that attracts birds with intermediate-sized beaks. Information on the movement patterns of Darwin's finches on Santa Cruz Island, currently unavailable, would help distinguish among these alternatives. In summary, although we favour an hypothesis based on adaptive landscapes, we cannot unequivocally exclude several other potential human impacts.
(c) Synopsis
The evolutionary forces promoting bimodality within species probably mirror those driving adaptive radiation into multiple species. As such, bimodal populations afford exceptional opportunities to study diversification in action (Smith 1993; Smith & Skúlason 1996; Schluter 2000). For one such population, G. fortis at Academy Bay, human impacts appear to have reduced bimodality, thereby reversing the process of diversification. We have argued that the specific human impacts driving this change are plausibly related to altered food distributions. To generalize, we suggest that human activities can convert a rugged adaptive landscape, characterized by alternative resource peaks, into a smooth landscape where intermediate forms have increased relative fitness. Such impacts would be most likely in young adaptive radiations, where ecological niches overlap and reproductive isolation is incomplete. Examples of such radiations include African cichlids, three-spine sticklebacks and crossbills (McKinnon & Rundle 2002; Benkman 2003; Streelman et al. 2004; Gow et al. 2006). Although the most immediate goal of biological conservation should be to preserve species at self-sustaining population sizes, a long-term goal should be to preserve their ability to diversify. Only then can we reverse declines in biological diversity.
Acknowledgments
Fieldwork was coordinated through the Charles Darwin Research Station and the Galápagos National Park Service. Data collection for 1999–2005 was assisted by S. Huber, M. Rossi-Santos, D. Ruiz, A. Herrel, A. Gabela, M. Hendry, P. Kelley, L. Deleon and D. Delaney. Financial support for fieldwork in 1999 through 2005 was provided by the National Science Foundation (JP), with additional funding from the Natural Sciences and Engineering Research Council of Canada (APH). Mark Brewer was supported by the Scottish Executive Environment and Rural Affairs Department (MJB). The collection of data new to this study (1999–2005) was done in concordance with Animal Use Protocols approved by the University of Massachusetts Amherst.
Footnotes
These authors contributed equally.
Supplementary Material
Click here for additional data file
References
- Abbott I, Abbott L.K, Grant P.R. Comparative ecology of Galápagos ground finches (Geospiza Gould): evaluation of the importance of floristic diversity and interspecific competition. Ecol. Monogr. 1977;47:151–184. [Google Scholar]
- Benkman C.W. Divergent selection drives the adaptive radiation of crossbills. Evolution. 2003;57:1176–1181. doi: 10.1111/j.0014-3820.2003.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Bowman R.I. Morphological differentiation and adaptation in the Galápagos finches. Univ. Calif. Publ. Zool. 1961;58:1–302. [Google Scholar]
- Bradshaw W, Holzapfel C. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl Acad. Sci. USA. 2001;98:14 509–14 511. doi: 10.1073/pnas.241391498. 10.1073/pnas.241391498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer M.J. Discretisation for inference on normal mixture models. Stat. Comput. 2003;13:209–219. 10.1023/A:1024214615828 [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York, NY: 2002. Model selection and multimodel inference. [Google Scholar]
- Bush G.L. Sympatric host race formation and speciation in frugivorous flies of the genus Rhagoletis (Diptera: Tephritidae) Evolution. 1969;23:237–251. doi: 10.1111/j.1558-5646.1969.tb03508.x. [DOI] [PubMed] [Google Scholar]
- Carroll S.P, Dingle H, Klassen S.P. Genetic differentiation of fitness-associated traits among rapidly evolving populations of the soapberry bug. Evolution. 1997;51:1182–1188. doi: 10.1111/j.1558-5646.1997.tb03966.x. [DOI] [PubMed] [Google Scholar]
- Coltman D.W, O'Donoghue P, Jorgenson J.T, Hogg J.T, Strobeck C, Festa-Blanchet M. Undesirable evolutionary consequences of trophy hunting. Nature. 2003;426:655–658. doi: 10.1038/nature02177. 10.1038/nature02177 [DOI] [PubMed] [Google Scholar]
- Ford H.A, Parkin D.T, Ewing A.W. Divergence and evolution in Darwin's finches. Biol. J. Linn. Soc. 1973;5:289–295. [Google Scholar]
- Gow J.L, Peichel C.L, Taylor E.B. Contrasting hybridization rates between sympatric three-spined sticklebacks highlight the fragility of reproductive barriers between evolutionarily young species. Mol. Ecol. 2006;15:739–752. doi: 10.1111/j.1365-294X.2006.02825.x. 10.1111/j.1365-294X.2006.02825.x [DOI] [PubMed] [Google Scholar]
- Grant P.R. Princeton University Press; Princeton, NJ: 1986. Ecology and evolution of Darwin's finches. [Google Scholar]
- Grant B.R, Grant P.R. University of Chicago Press; Chicago, IL: 1989. Evolutionary dynamics of a natural population. [Google Scholar]
- Grant P.R, Grant B.R. Predicting microevolutionary responses to directional selection on heritable variation. Evolution. 1995;49:241–251. doi: 10.1111/j.1558-5646.1995.tb02236.x. [DOI] [PubMed] [Google Scholar]
- Grant B.R, Grant P.R. High survival of Darwin's finch hybrids: effects of beak morphology and diets. Ecology. 1996;77:500–509. [Google Scholar]
- Grant P.R, Grant B.R. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. 10.1126/science.1070315 [DOI] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R, Smith J.N.M, Abbott I.J, Abbott L.K. Darwin's finches: population variation and natural selection. Proc. Natl Acad. Sci. USA. 1976;73:257–261. doi: 10.1073/pnas.73.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R, Markert J.A, Keller L.F, Petren K. Convergent evolution of Darwin's finches caused by introgressive hybridization and selection. Evolution. 2004;58:1588–1599. doi: 10.1111/j.0014-3820.2004.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Grant P.R, Grant B.R, Petren K. Hybridization in the recent past. Am. Nat. 2005;166:56–67. doi: 10.1086/430331. 10.1086/430331 [DOI] [PubMed] [Google Scholar]
- Hendry A.P, Kinnison M.T. The pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Hendry A.P, Wenburg J.K, Bentzen P, Volk E.C, Quinn T.P. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. 10.1126/science.290.5491.516 [DOI] [PubMed] [Google Scholar]
- Herrel A, Podos J, Huber S.K, Hendry A.P. Bite performance and morphology in a population of Darwin's finches: implications for the evolution of beak shape. Funct. Ecol. 2005;19:43–48. 10.1111/j.0269-8463.2005.00923.x [Google Scholar]
- Keller L.F, Grant P.R, Grant B.R, Petren K. Heritability of morphological traits in Darwin's finches: misidentified paternity and maternal effects. Heredity. 2001;87:325–336. doi: 10.1046/j.1365-2540.2001.00900.x. 10.1046/j.1365-2540.2001.00900.x [DOI] [PubMed] [Google Scholar]
- Koskinen M.T, Haugen T.O, Primmer C.R. Contemporary fisherian life-history evolution in small salmonid populations. Nature. 2002;419:826–830. doi: 10.1038/nature01029. 10.1038/nature01029 [DOI] [PubMed] [Google Scholar]
- Lack D. Cambridge University Press; Cambridge, UK: 1947. Darwin's finches. [Google Scholar]
- Levinton J.S, Suatoni E, Wallace W, Junkins R, Kelaher B, Allen B.J. Rapid loss of genetically based resistance to metals after the cleanup of a Superfund site. Proc. Natl Acad. Sci. USA. 2003;100:9889–9891. doi: 10.1073/pnas.1731446100. 10.1073/pnas.1731446100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauchamp A. Threats from alien plant species in the Galápagos Islands. Conserv. Biol. 1997;11:260–263. 10.1046/j.1523-1739.1997.95356.x [Google Scholar]
- McKinnon J.S, Rundle H.D. Speciation in nature: the threespine stickleback model systems. Trends Ecol. Evol. 2002;17:480–488. 10.1016/S0169-5347(02)02579-X [Google Scholar]
- Olsen E.M, Heino M, Lilly G.R, Morgan M.J, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. 10.1038/nature02430 [DOI] [PubMed] [Google Scholar]
- Price T. Diet variation in a population of Darwin's finches. Ecology. 1987;68:1015–1028. [Google Scholar]
- Schluter D. Oxford University Press; Oxford, UK: 2000. The ecology of adaptive radiation. [Google Scholar]
- Schluter D, Grant P.R. Determinants of morphological patterns in communities of Darwin's finches. Am. Nat. 1984;123:175–196. 10.1086/284196 [Google Scholar]
- Schofield E.K. Effects of introduced plants and animals on island vegetation: examples from the Galápagos archipelago. Conserv. Biol. 1989;3:227–238. 10.1111/j.1523-1739.1989.tb00081.x [Google Scholar]
- Seehausen O, van Alphen J.J.M, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277:1808–1811. 10.1126/science.277.5333.1808 [Google Scholar]
- Smith T.B. Disruptive selection and the genetic basis of bill size polymorphism in the African finch Pyrenestes. Nature. 1993;363:618–620. 10.1038/363618a0 [Google Scholar]
- Smith T.B, Skúlason S. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 1996;27:111–133. 10.1146/annurev.ecolsys.27.1.111 [Google Scholar]
- Snow D.W. Moult and the breeding cycle in Darwin's finches. J. Ornithol. 1966;107:283–291. 10.1007/BF01677899 [Google Scholar]
- Stockwell C.A, Hendry A.P, Kinnison M.T. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003;18:94–101. 10.1016/S0169-5347(02)00044-7 [Google Scholar]
- Streelman J.T, Gmyrek S.M, Kidd M.R, Kidd C, Robinson R.L, Heret E, Ambali A.J, Kocher T.D. Hybridization and contemporary evolution in an introduced cichlid fish from Lake Malawi National Park. Mol. Ecol. 2004;13:2471–2479. doi: 10.1111/j.1365-294X.2004.02240.x. 10.1111/j.1365-294X.2004.02240.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Click here for additional data file