Summary
The bacterial RNA polymerase (RNAP) holoenzyme consists of a catalytic core enzyme (α2ββ'ω) complexed with a σ factor that is required for promoter-specific transcription initiation. During early elongation, the stability of interactions between σ70 (the primary sigma factor in Escherichia coli) and core decreases, due to an ordered displacement of segments of σ70 from core triggered by growth of the nascent RNA. Here we demonstrate that the nascent RNA-mediated destabilization of an interaction between σ 70 region 4 and the flap domain of the β subunit is required for the bacteriophage λ Q antiterminator protein to contact holoenzyme during early elongation. We demonstrate further that the requirement for nascent RNA in the process by which Q engages RNAP can be bypassed if σ70 region 4 is removed. Our findings illustrate how a regulator can exploit the nascent RNA-mediated reconfiguration of the holoenzyme to gain access to the enzyme during early elongation.
Introduction
The bacterial RNA polymerase (RNAP) holoenzyme consists of a catalytic core enzyme (α2ββ'ω) complexed with a σ factor that confers on the core enzyme the ability to initiate promoter-specific transcription. The primary σ factor in Escherichia coli is σ70, and a typical σ70-dependent promoter bears two conserved sequence elements, the −10 and the −35 hexamers, which are separated by a spacer of ∼17 base pairs (bp) (reviewed in Gross et al., 1998). All primary σ factors share four regions of conserved sequence (regions 1-4) (Lonetto et al., 1992). Regions 2, 3, and 4 contain DNA-binding domains responsible for recognition of the promoter −10 element, extended −10 element (Bown et al., 1997), and −35 element, respectively, and a flexible linker (σ region 3.2) connects the other portions of region 3 to region 4 (Murakami et al., 2002a and 2002b; Vassylyev et al., 2002). Structures of the RNAP holoenzyme (Murakami et al., 2002a and 2002b; Vassylyev et al., 2002) reveal that two domains of σ lie along the predicted path of the nascent RNA: 1) region 3.2, which is positioned within the RNA exit channel, and 2) region 4, which, by virtue of its interaction with the flap domain of the β subunit (β flap), is positioned immediately adjacent to the end of the RNA exit channel. Thus, during early elongation, the nascent RNA first must displace σ region 3.2 from the RNA exit channel when the nascent RNA enters the channel (Murakami et al., 2002a; Mekler et al., 2002), at a length of ∼10-11 nucleotides (nt) (Borukhov and Nudler, 2003), and second, must displace σ region 4 from the β flap (or otherwise perturb the interaction) when the nascent RNA emerges from the RNA exit channel (Vassylyev et al., 2002), at a length of ∼16 nt (Komissarova and Kashlev, 1998; Korzheva et al. 2000; Nickels et al., 2005). It has been proposed that this ordered displacement of segments of σ from the RNAP core by the nascent RNA could facilitate gene regulation and specifically, that these rearrangements of the holoenzyme may allow elongation factors to access RNAP (Murakami and Darst, 2003).
Previous work has demonstrated that σ70 can play functional roles during transcription elongation (reviewed in Mooney et al., 2005). The most well characterized example involves the regulation of late gene transcription from the bacteriophage λ promoter PR' (Roberts et al., 1998), where σ70 mediates an early elongation pause that is essential for the function of the Q antiterminator protein (λQ). λQ specifically engages RNAP that has initiated transcription at PR' and this engagement process depends on two DNA sequence elements, a Q binding element (QBE) that is located between the promoter −10 and −35 elements (Yarnell and Roberts, 1992) and a pause-inducing element that is located in the initial transcribed region (Figure 1A). The pause-inducing element resembles a promoter −10 element and pausing, which manifests itself in complexes containing nascent RNAs of 16 or 17 nt, is mediated by protein-DNA interaction between σ70 region 2 and the −10-like element (Ring et al., 1996). Thus, the paused early elongation complex has properties characteristic of both an initiation complex (RNAP contains σ70and is bound to a sequence that resembles a promoter −10 element) and an elongation complex (RNAP has escaped the promoter and contains a stably associated RNA transcript). DNA-bound λQ interacts with this paused complex and becomes a stable component of the elongation complex (Yarnell and Roberts, 1999; P. Deighan and A. H. unpublished results), enabling RNAP to read through downstream terminators and transcribe the phage's late genes.
Figure 1.
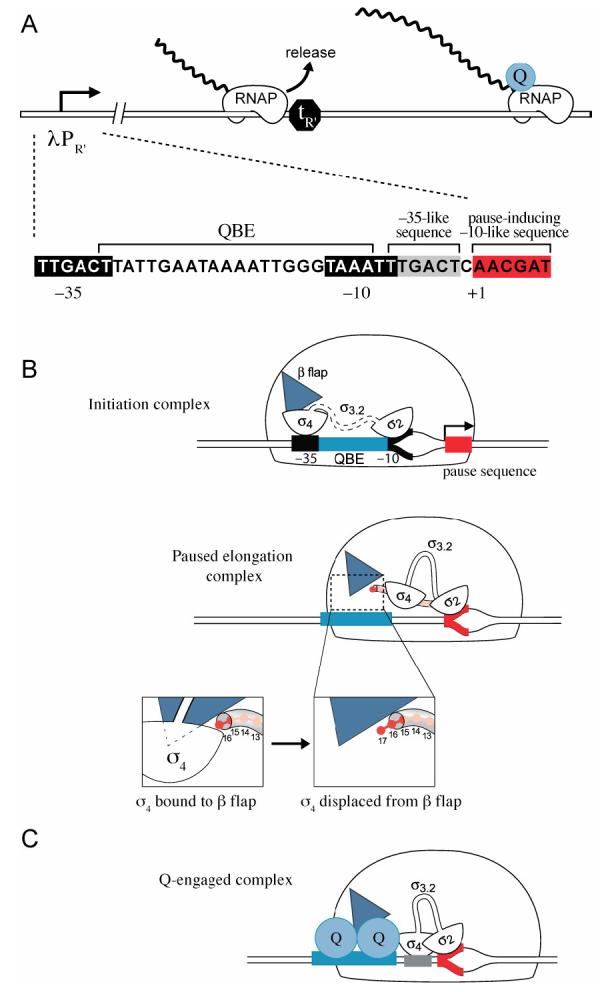
- Presence of λQ (shown in blue) allows RNAP that has initiated transcription from λPR' to read through terminator tR'. Blow-up shows the functionally important elements at λPR' including: the promoter −10 and −35 elements, the λQ-binding element (QBE), the pause-inducing −10-like element, and the −35-like element (TTGACT motif) positioned between the QBE and the pause-inducing element. Note that the TTGACT motif is separated by one base pair from the pause-inducing −10-like element.
- Cartoons depict sequence of events during initiation and early elongation at λPR'. Shown first is the initiation complex; σ70 region 3.2 (σ3.2) is shown dashed to indicate that it is located within the RNA exit channel, and σ70 region 4 (σ4) is shown bound to the β flap (blue triangle). The σ70 region 4/β flap interaction positions σ70 region 4 for interaction with the promoter −35 element when σ70 region 2 is bound to the promoter −10 element (where the −10 and −35 elements are separated by ∼17 base pairs) (Kuznedelov et al., 2002). Shown just below the initiation complex is the paused early elongation complex at λPR' with σ70 region 2 (σ2) bound to the pause-inducing −10-like element (shown in red). The nascent RNA (depicted as red beads) has displaced σ70 region 3.2 from the RNA exit channel and, as illustrated in the blow-ups, displaced σ70 region 4 from the β flap upon addition of the 17th nt.
- λQ (shown as a dimer) binds to the QBE and engages the paused elongation complex. λQ contacts σ70 region 4 and stabilizes its binding to the TTGACT motif (grey rectangle), causing RNAP holoenzyme to adopt a conformation in which σ70 region 4 and σ70 region 2 are simultaneously bound to DNA elements that are separated by 1 base pair (Nickels et al., 2002). Formation of this RNAP holoenzyme-DNA complex requires that σ70 region 4 be displaced from the β flap. λQ is depicted as contacting a surface of RNAP core that is occluded when σ70 region 4 is bound to the β flap.
Here we investigate whether the RNA-mediated destabilization of the σ70 region 4/β flap interaction facilitates the engagement of RNAP by Q, a hypothesis that is suggested by two lines of indirect evidence. First, the pause associated with λPR' manifests itself in early elongation complexes containing a 16- or 17-nt transcript (Roberts et al., 1998). Thus, in the context of this early elongation complex, the nascent RNA is of a length at which σ region 3.2 has been displaced from the RNA exit channel (Marr et al. 2001) and the interaction between σ region 4 and the β flap is destabilized (Nickels et al., 2005) (Figure 1B). Second, λQ's engagement of the paused elongation complex causes the RNAP holoenzyme to adopt a conformation that requires release of σ70 region 4 from the β flap (Marr et al., 2001; Nickels et al., 2002) (Figure 1C). In this study we provide direct evidence that the RNA-mediated destabilization of the σ70 region 4/β flap interaction is required for efficient engagement of the early elongation complex by λQ. Furthermore, we demonstrate that the requirement for nascent RNA in this engagement process can be bypassed by weakening or eliminating the σ70 region 4/β flap interaction. Thus, our results illustrate how the nascent RNA-mediated destabilization of the σ70 region 4/β flap interaction during the transition from initiation to elongation can facilitate the engagement of a regulatory factor with RNAP.
Results
Effects of altering the σ region 4/β flap interaction on λQ-dependent antitermination in vitro
We hypothesized that engagement of the paused elongation complex by λQ is facilitated by the nascent RNA-mediated destabilization of the σ70 region 4/β flap interaction. Predictions arising from this hypothesis are: 1) inhibiting the displacement of σ70 from the β flap should inhibit the engagement of the paused elongation complex by λQ (decreasing the amount of λQ-dependent antitermination), and 2) facilitating the displacement of σ70 from the β flap should facilitate engagement of the paused elongation complex by λQ (increasing the amount of λQ-dependent antitermination). To inhibit or facilitate displacement of σ70 region 4 from the β flap we took advantage of amino acid substitutions in σ70 region 4 that either increase (T544I/D581G) or decrease (L607P) the strength of the σ70 region 4/β flap interaction (Nickels et al., 2005). Previous work suggests that substitutions T544I and D581G, which strengthen the σ70 region 4/β flap interaction, inhibit the RNA-mediated displacement of σ70 region 4 from the β flap and substitution L607P, which weakens the σ70 region 4/β flap interaction, facilitates displacement of σ70 region 4 from the β flap (Nickels et al., 2005). Therefore, we used these amino acid substitutions to test the effect of inhibiting or facilitating the displacement of σ70 region 4 from the β flap on λQ antitermination function.
We performed single-round in vitro transcription assays using a λPR' template bearing the natural λ late terminator tR' (see Figure 1A). In the absence of λQ, nearly all (∼99%) of the RNAP molecules terminate at tR' (data not shown), while in the presence of λQ, modified RNAP molecules read through the terminator to produce the full length run-off transcript. Therefore, λQ antitermination function can be measured by calculating the percentage of full-length transcripts (% readthrough) emanating from λPR' in the presence of λQ. Assays were performed at low or high concentrations of λQ using holoenzyme reconstituted with wild-type σ70 or mutant σ's bearing substitutions that either strengthen (T544I/D581G) or weaken (L607P) the σ70 region 4/β flap interaction (Figure 2, panels A and B). At low concentrations of λQ (2.5 nM), strengthening the σ70 region 4/β flap interaction (T544I/D581G) reduced λQ-dependent antitermination (∼3-fold; Figure 2A) and weakening the σ70 region 4/β flap interaction (L607P) increased λQ-dependent antitermination (∼2-fold; Figure 2A). Furthermore, the effects of altering the σ70 region 4/β flap interaction were overcome when the reactions were performed at saturating concentrations of λQ (500nM); under these conditions, ∼50% readthrough was observed regardless of whether the reactions were performed with wild-type or a mutant holoenzyme (Figure 2B).
Figure 2.
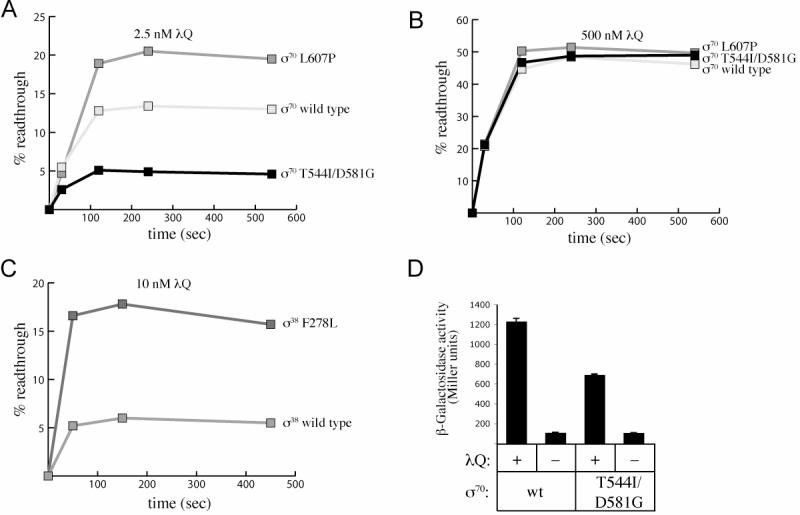
- A) and B) Effects of strengthening (T544I/D581G) or weakening (L607P) the σ70 region 4/β flap interaction in vitro. Shown are the results of single-round in vitro transcription assays performed using a linear template that contains sequence extending from −109 to +238 of λPR' that includes the natural terminator tR' (Figure 1A). Assays were done either at a low concentration of λQ (2.5 nM, panel A) or at a high concentration of λQ (500 nM, panel B). Graphs show the percentage of transcripts derived from terminator readthrough (readthrough/[readthrough + terminated]) at the indicated times after transcription was initiated. Reactions were performed using holoenzyme reconstituted with wild-type σ70, σ70 L607P or σ70 T544I/D581G, as indicated. Assays were performed three times on separate occasions with similar results. Plotted on the graphs are the data obtained from a single representative experiment.
- C) Effects of weakening (F278L) the σ38 region 4/β flap interaction in vitro. Shown are the results of single-round in vitro transcription assays performed at a low concentration of λQ (10 nM). Graphs show the percentage of transcripts derived from terminator readthrough (readthrough/[readthrough + terminated]) at the indicated times after transcription was initiated. Reactions were performed using holoenzyme reconstituted with either wild-type σ38 or σ38 F278L, as indicated. Assays were performed three times on separate occasions with similar results. Shown on the graph are the data obtained from a single representative experiment.
- D) Effects of strengthening (T544I/D581G) the σ70 region 4/β flap interaction in vivo. Reporter strain cells containing either wild-type σ70 or σ70 T544I/D581G and harboring a λPR'-lacZ reporter were transformed with a plasmid that did or did not encode λQ. The cells were grown in the presence of 100 μM IPTG and assayed for β-galactosidase activity. The bar graph shows the averages of four independent measurements (and standard deviations).
To extend these findings, we performed a set of parallel experiments using holoenzyme reconstituted with the stationary phase sigma factor, σ38. (Holoenzyme reconstituted with σ38 can be modified by λQ in vitro, although not as efficiently as holoenzyme reconstituted with σ70; Figure 2C and data not shown.) Although σ 38 shares a high degree of sequence homology with σ70, previous work suggests that the σ38 region 4/β flap interaction is stronger than the σ70 region 4/β flap interaction (Kuznedelov et al., 2002). Therefore, we tested the effect of weakening the σ region 4/β flap interaction in the context of the σ38-containing holoenzyme on λQ antitermination function. To do this, we took advantage of a single amino acid substitution in σ38, F278L, isolated in a screen for substitutions that specifically weakened the σ38 region 4/β flap interaction (Figure S1). We performed in vitro transcription assays using holoenzyme reconstituted with either wild-type σ38 or σ38 F278L. At low concentrations of λQ (10 nM), weakening the σ38 region 4/β flap interaction increased λQ-dependent antitermination (∼3-fold; Figure 2C). Furthermore, the effect of weakening the σ38 region 4/β flap interaction was reduced (but not eliminated) when the reactions were performed at saturating concentrations of λQ (data not shown).
Taken together, the data presented in Figure 2 (panels A and C) suggest that altering the strength of the σ region 4/β flap interaction can affect λQ-dependent antitermination. In particular, strengthening the σ region 4/β flap interaction decreases the amount of λQ-dependent antitermination, and weakening the σ region 4/β flap interaction increases the amount of λQ-dependent antitermination. Furthermore, the effects of altering the σ region 4/β flap interaction are most pronounced when the concentration of λQ is limiting.
Effects of altering the σ70 region 4/β flap interaction on λQ-dependent antitermination in vivo
We next tested the effect of altering the σ70 region 4/β flap interaction on λQ-dependent antitermination in vivo. To do this, we introduced the mutations specifying substitutions T544I and D581G into the chromosomal copy of the rpoD gene (encoding σ70). We found that E. coli strains bearing these mutations were viable and exhibited no apparent growth defect. (We note that similar attempts to introduce the L607P mutation into the chromosomal copy of rpoD were unsuccessful.) To test the effect of the rpoD-T544I/D581G mutations on λQ-dependent antitermination in vivo, we used a λPR'-lacZ fusion that consists of λPR' sequence extending from position –109 through position +238; this sequence includes the natural terminator tR'. The level of lacZ expression from this fusion construct can therefore report on the ability of plasmid-encoded λQ to function as an antiterminator for transcripts initiating from λPR'. We introduced the λPR'-lacZ reporter construct in single copy into strains containing either wild-type rpoD or rpoD with the T544I/D581G mutations. We then assayed the ability of plasmid-encoded λQ to induce the expression of the lacZ reporter gene in each strain (Figure 2D). Consistent with our in vitro observations (Figure 2B), at low concentrations of λQ, the T544I/D581G mutations in rpoD caused an ∼2-fold reduction in λQ function (Figure 2D). Furthermore, the effect of the T544I/D581G mutations was overcome at high concentrations of λQ (data not shown). Western analysis confirmed that the levels of λQ were identical in strains carrying the wild-type and mutant rpoD genes (data not shown). We conclude that strengthening the σ70 region 4/β flap interaction disrupts λQ-dependent antitermination in vivo.
Effects of altering the σ70 region 4/β flap interaction on the stability of the λQ-engaged paused elongation complex
The results presented in Figure 2 demonstrate that the effects of altering the σ region 4/β flap interaction are most pronounced when the concentration of λQ is limiting. Thus, we infer that altering the σ region 4/β flap interaction affects a concentration-dependent step in the λQ-antitermination process. The only plausible concentration-dependent step in the λQ-antitermination process is the initial binding of λQ to the paused early elongation complex. Therefore, we directly tested the effects of altering the σ70 region 4/β flap interaction on the stability of the complex that forms when λQ initially engages RNAP, using an exonuclease challenge assay (Yarnell and Roberts, 1992). In this assay, paused elongation complexes are formed by artificially stalling RNAP that has initiated transcription at λPR'. These artificially stalled complexes, which are halted after the synthesis of a 15-nt nascent RNA, are competent for modification by λQ (Yarnell and Roberts, 1992), indicating they closely resemble the natural substrate. After the stalled complexes are formed they are incubated with λQ; the binding of λQ to the QBE is stabilized by its interaction with RNAP. The λQ-bound complexes are challenged with exonuclease III, which digests the DNA in a 3′ to 5′ direction until its progress is blocked by the presence of a DNA-bound protein (Figure 3A). Thus, the stability of the complex that forms when λQ initially engages RNAP can be assessed by monitoring the half-life of the λQ-dependent barriers to exonuclease III digestion (the barriers between −31 and −27; see Figure 3, panels B and C).
Figure 3.

- Schematic of template used for exonuclease III challenge assays. Depicted is the λQ-engaged paused elongation complex. The λPR' template used in the assays is end-labeled at the 5′ end of the template (bottom) strand as indicated. Also indicated are the positions (relative to the transcription start site) at which the progress of exonuclease III digestion is blocked by λQ (−31) and RNAP (−22 and −12). The red DNA segment is the pause-inducing sequence; σ70 region 2 is shown bound to the non-template strand (Ring et al., 1996). The RNAP outline is dashed to indicate that it does not depict a barrier to exonuclease digestion.
- Exonuclease challenge assays. Stalled elongation complexes were formed with RNAP reconstituted with wild-type σ70, σ70 L607P, or σ70 T544I/D581G. These complexes were then incubated with 500 nM λQ and challenged with exonuclease III for the indicated times. Lanes 9 and 18 contain A+G sequencing ladders.
- Effects of substitutions that strengthen (T544I/D581G) or weaken (L607P) the σ70 region 4/β flap interaction. The exonuclease barriers shown in panel B were quantified with Imagequant, and label in the λQ-dependent band at −31 was plotted as a fraction of the sum of label in the bands at −31, −22 and −12; the barriers at −22 and −12 are specific for the σ70-dependent paused elongation complex, and are produced after exonuclease digests past the λQ barrier. We note that no differences in pause half-life were observed with these mutant holoenzymes (Nickels et al., 2005), suggesting that the effects of these σ70 substitutions on the half-life of the λQ barrier are not indirect manifestations of changes in the stability of the paused elongation complexes.
Exonuclease challenge assays were performed using a wild-type λPR′ template and holoenzyme reconstituted with wild-type σ70, σ70 T544I/D581G, or σ70 L607P. The assays indicate that strengthening the σ70 region 4/β flap interaction (T544I/D581G) destabilizes the complex that forms when λQ initially engages RNAP at λPR′ (Figure 3B; compare lanes 1-8 with lanes 19-26) and weakening the σ70 region 4/β flap interaction (L607P) stabilizes the complex that forms when λQ initially engages RNAP (Figure 3B; compare lanes 1-8 with lanes 10-17). Thus, λQ's association with the paused early elongation complex at λPR′ is inhibited by strengthening the σ70 region 4/β flap interaction and facilitated by weakening the σ70 region/β flap interaction (Figure 3C). Furthermore, we infer that the effects of altering the σ70 region 4/β flap interaction on λQ-mediated antitermination (Figure 2) reflect effects on the stability of the complex that forms when λQ initially engages RNAP.
In principle, the effects of substitutions T544I/D581G and L607P on λQ-dependent antitermination (Figure 2) could merely be an indirect consequence of their effects on the ratio of paused elongation complexes containing a 17-nt nascent transcript to paused complexes containing a 16-nt transcript (Nickels et al., 2005). The results of the exonuclease challenge assays presented in Figure 3 rule out this possibility (see Discussion).
Engagement of the paused elongation complex by λQ involves an interaction between λQ and σ70 region 4 that stabilizes the binding of region 4 to a DNA sequence element (TTGACT) that resembles a promoter −35 element (Figure 1) (Nickels et al., 2002). Because the binding of σ70 region 4 to this TTGACT motif requires RNAP holoenzyme to adopt a conformation in which σ70 region 4 has been displaced from the β flap, we considered the possibility that the displacement of σ70 region 4 from the β flap is required for λQ function solely because it permits σ70 region 4 to bind the TTGACT motif. The experiments shown in Figures S2 and S3 argue against this possibility and suggest that there is a mechanistic requirement for displacement of σ70 region 4 from the β flap during the λQ engagement process even under circumstances that do not permit λQ to stabilize the binding of σ70 region 4 to the TTGACT motif.
The function of the nascent RNA in the Q engagement process can be bypassed by weakening or eliminating the σ70 region 4/β flap interaction
The exonuclease challenge assays suggest that displacement of σ70 region 4 from the β flap is required for λQ to make productive contact with the paused early elongation complex. During early elongation, the nascent RNA destabilizes the σ70 region 4/β flap interaction (Vassylyev et al., 2002; Murakami and Darst, 2003; Borukhov and Nudler, 2003; Nickels et al., 2005). We therefore hypothesized that the requirement for nascent RNA in the Q engagement process could be bypassed by weakening or eliminating the σ70 region 4/β flap interaction. Specifically, we wished to test whether Q, which normally engages a paused early elongation complex, could engage an initiation complex if the σ70 region 4/β flap interaction was genetically disrupted or σ70 region 4 was removed altogether.
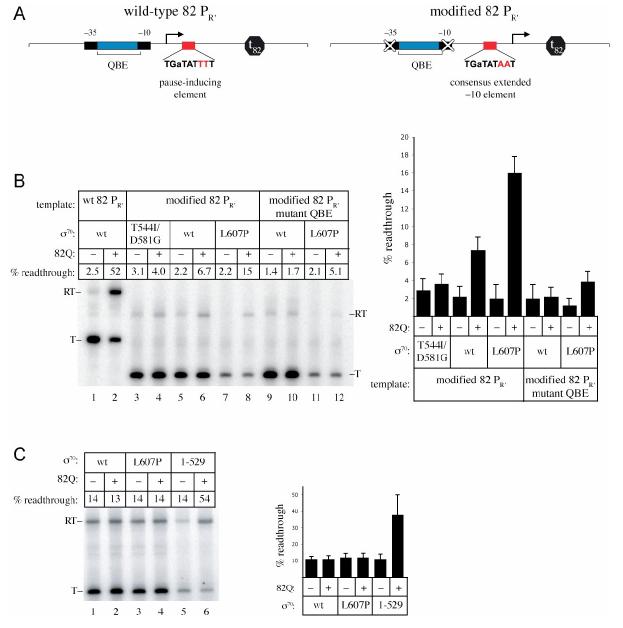
The ability of Q to functionally engage RNAP depends upon a precise spatial relationship between QBE-bound Q and RNAP bound at the pause site (Ring et al., 1996). Therefore, in order to maintain the correct spatial relationship between Q and the RNAP holoenzyme, we sought to convert the pause-inducing sequence to a bona fide promoter. Furthermore, in order to test whether Q could engage an initiation complex if σ70 region 4 was removed altogether, we needed to convert the pause-inducing sequence to a promoter that would support transcription by a mutant holoenzyme lacking σ70 region 4. Because σ70 region 4 is dispensable for transcription initiating at a consensus extended −10 promoter (Kumar et al., 1993) (consensus sequence: TGnTATAAT), our plan was to convert the pause-inducing sequence to a consensus extended −10 promoter. However, the pause-inducing sequence associated with λPR′ matches a consensus extended −10 promoter element at only 3 out of 8 positions (Figure 1A), and furthermore, introduction of a TG dinucleotide one base pair upstream of the λPR′ pause-inducing element disrupts λQ binding to the paused early elongation complex (data not shown). For these reasons we took advantage of the pause-inducing sequence associated with the PR′ promoter of a bacteriophage that is closely related to λ, phage 82. The phage 82 PR′ pause-inducing sequence, TGnTATTTT, differs at only two positions from the consensus extended −10 element (TGnTATAAT) (Figure 4A) (Ring et al., 1996). Thus, we converted the phage 82 PR′ pause-inducing sequence to a consensus extended −10 element and used the Q protein of phage 82 (82Q) to ask whether we could bypass the requirement for nascent RNA in the Q engagement process.
Figure 4.
- Left panel shows a diagram of the wild-type phage 82 PR' promoter. Indicated are the functionally important elements at phage 82 PR' including the promoter −10 and −35 elements, the 82Q-binding element (QBE), the pause-inducing extended −10-like element (located between +6 and +14), and terminator t82. Right panel shows a diagram of the modified template on which the 82 PR' promoter was inactivated with base pair substitutions in the promoter −10 and −35 elements and the pause-inducing element was converted to a consensus extended −10 promoter element. The transcription start site on each template is indicated (bent arrow).
- Effects of strengthening (T544I/D581G) or weakening (L607P) the σ70 region 4/β flap interaction on 82Q's engagement of a transcription initiation complex. Shown are the results of representative single-round in vitro transcription assays performed in the presence or absence of 100 nM 82Q using the indicated template and holoenzyme reconstituted with wild-type σ70, σ70 T544I/D581G, or σ70 L607P. The percentage of transcripts derived from terminator readthrough (readthrough/ [readthrough + terminated]) is indicated above each lane. RNA was end labeled using γ-32P-ATP (lanes 1 and 2) or γ-32P-GTP (lanes 3-12). Also indicated are the 81- (lanes 1 and 2) or 62- (lanes 3-12) nt terminated transcript (T) and the 116- (lanes 1 and 2) or 97- (lanes 3-12) nt readthrough transcript (RT). Plotted in the bar graph on the right are the averages of 3-6 independent measurements (and standard deviations).
- Effect of removing σ70 region 4 on 82Q's engagement of a transcription initiation complex. Shown are the results of representative single-round in vitro transcription assays performed in the presence or absence of 100 nM 82Q using the modified phage 82 PR' template and holoenzyme reconstituted with wild-type σ70, σ70 L607P, or σ70 lacking region 4 (σ70 1-529). The percentage of transcripts derived from terminator readthrough (readthrough/[readthrough + terminated]) is indicated above each lane. RNA was internally labeled using α-32P-CTP. Also indicated are the 62-nt terminated transcript (T) and the 97-nt readthrough transcript (RT). Plotted in the bar graph on the right are the averages of 4-6 independent measurements (and standard deviations).
We modified a template that contained phage 82 PR′ along with a downstream terminator by converting the wild-type pause-inducing sequence to a consensus extended −10 element and introducing substitutions into the phage 82 PR′ promoter elements (Figure 4A), thereby insuring that transcription would initiate exclusively under the control of the mutated pause-inducing sequence. Using this modified template, we then asked what effect the addition of 82Q had on the ability of wild-type RNAP or various RNAP mutants to read through the downstream terminator. We first performed assays using holoenzyme reconstituted with wild-type σ70, σ70 T544I/D581G, or σ70 L607P. As a control, we also tested 82Q function using a wild-type (unmodified) phage 82 PR′ template and wild-type holoenzyme; in this control assay, 82Q increased terminator readthrough from ∼2% to ∼50% (Figure 4B, lanes 1 and 2). Surprisingly, we found that when the assays were performed with the modified phage 82 PR′ template and wild-type holoenzyme, the addition of 82Q increased terminator readthrough from the ∼2% observed in the absence of 82Q to ∼7% (Figure 4B, lanes 5 and 6). This suggests that 82Q can, in fact, modify an initiation complex, albeit weakly compared to its ability to modify the early elongation complex at wild-type 82 PR′. In addition, we found that strengthening the σ70 region 4/β flap interaction (reactions performed with holoenzyme reconstituted with σ70 T544I/D581G) eliminated the ability of 82Q to modify the transcription initiation complex (Figure 4B, lanes 3 and 4), whereas weakening the σ70 region 4/β flap interaction (reactions performed with holoenzyme reconstituted with σ70 L607P) enhanced the ability of 82Q to modify the transcription initiation complex by a factor of ∼2 (terminator readthrough increased to ∼15%; Figure 4B, lanes 7 and 8). To control for the specificity of these effects, we tested whether the ability of 82Q to modify the transcription initiation complex depended on the functional integrity of the Q-binding element. To do this, we introduced base pair substitutions into the Q-binding element predicted to weaken 82Q binding (see Experimental Procedures). Introduction of these base pair substitutions into the converted template inhibited the ability of 82Q to modify transcription initiation complexes containing wild-type σ70, or σ70 L607P (Figure 4B, lanes 9-12).
Next, we performed transcription assays to test the effect of the complete removal of σ70 region 4 on the ability of 82Q to modify an initiation complex. To do this, we performed assays using the converted 82 PR′ template and holoenzyme reconstituted with wild-type σ70, σ70 L607P, or a truncated σ70 that lacked region 4, σ70 1-529 (Figure 4C). We found that in order to observe efficient transcription initiation using holoenzyme lacking σ70 region 4, it was necessary to alter the conditions of the in vitro assay (see Experimental Procedures). When the assays were performed using these different reaction conditions, terminator readthrough was ∼14% in the absence of 82Q (Figure 4C) (compared with ∼2% for the experiments shown in Figure 4B). Furthermore, in contrast to what was observed under the previous experimental conditions, neither wild-type holoenzyme nor holoenzyme reconstituted with σ70 L607P supported 82Q-dependent antitermination (Figure 4C lanes 1-4). However, when the reactions were performed with holoenzyme lacking σ70 region 4 (Figure 4C, lanes 5 and 6), the addition of 82Q increased terminator readthrough from ∼14% to ∼54% (an effect that depended on the functional integrity of the Q-binding element; data not shown).
Because of the indirect nature of the antitermination assay, we wished to show directly that removal of σ70 region 4 allows 82Q to bind efficiently to transcription initiation complexes lacking any nascent RNA products. To do this, we again used the exonuclease challenge assay. We formed promoter complexes on the modified 82 PR′ template using holoenzyme lacking σ70 region 4 (σ70 1-529). We incubated these complexes in the presence or absence of 82Q, challenged the resultant complexes with exonuclease III, and looked for evidence of an 82Q-dependent barrier to exonuclease digestion (Figure 5). As a control, we also incubated the modified 82 PR′ template with 82Q in the absence of the RNAP holoenzyme. As shown in Figure 5B, the appearance of a strong 82Q-dependent barrier to exonuclease digestion specifically in the presence of the mutant holoenzyme indicates that 82Q can bind efficiently to promoter complexes formed by holoenzyme lacking σ70 region 4 (compare lanes 1-6 with lanes 8-13).
Figure 5.
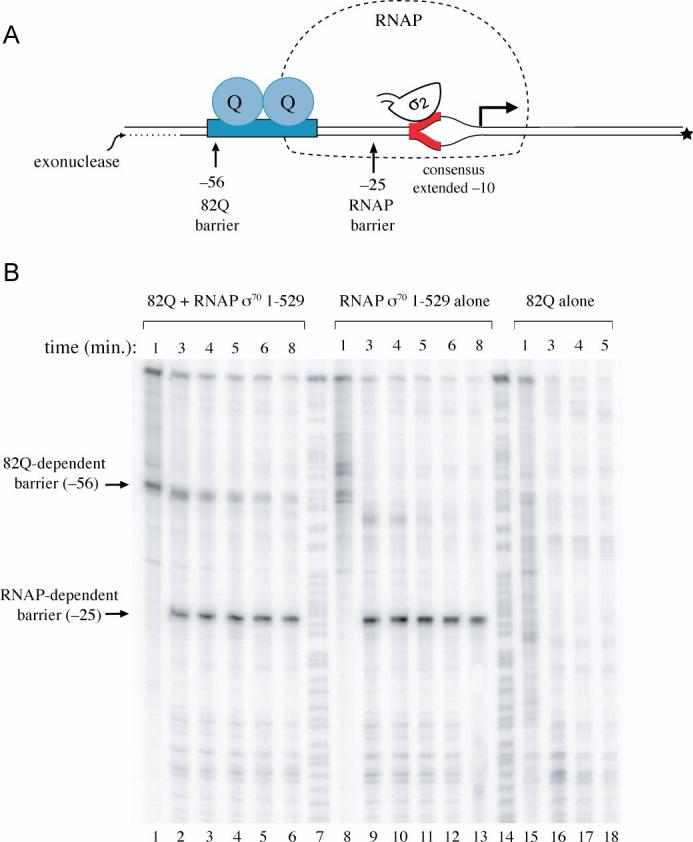
- Schematic of template used for exonuclease III challenge assay. Depicted is the 82Q-engaged transcription initiation complex lacking σ70 region 4. The modified phage 82 PR' template used in the assays is end labeled at the 5′ end of the template (bottom) strand, as indicated. Also indicated are the positions (relative to the transcription start site) at which the progress of exonuclease III digestion is blocked by 82Q (−56) and by RNAP (−25). The red DNA segment is the consensus extended −10 element; σ70 region 2 is shown bound to the non-template strand. The RNAP outline is dashed to indicate that it does not depict a barrier to exonuclease digestion. We note that a barrier corresponding to the RNAP-dependent barrier at −25 also is seen in paused complexes formed on the wild-type 82 PR' template (where it occurs at position −4 relative to the 82 PR' transcription start site) (data not shown), and likely is caused by σ70 regions 2 and 3.
- Effect of removing σ70 region 4. Initiation complexes were formed with RNAP reconstituted with σ70 lacking region 4, σ70 1-529 (lanes 1-6 and 8-13). These complexes were then incubated with 20 nM 82Q (lanes 1-6) or no 82Q (lanes 8-13) and challenged with exonuclease III for the indicated times. Control assays were performed using template DNA incubated with 20 nM 82Q only (lanes 15-18). Lanes 7 and 14 contain A+G sequencing ladders.
We also formed promoter complexes on the modified 82 PR′ template using wild-type holoenzyme and performed exonuclease challenge assays in the presence or absence of 82Q (Figure S4). Comparison of the assays performed with holoenzyme lacking σ70 region 4 (Figure 5B) and those performed with wild-type holoenzyme (Figure S4, panel B) indicates that removal of σ70 region 4 stabilizes the binding of 82Q to the transcription initiation complex, consistent with the results of the antitermination assays (Figure 4C).
Taken together, the results presented in Figures 4, 5 and S4 demonstrate that: 1) 82Q can weakly engage a transcription initiation complex, 2) strengthening the σ70 region 4/β flap interaction prevents this engagement, and 3) weakening the σ70 region 4/β flap interaction or removing σ70 region 4 facilitates this engagement.
Discussion
We examined the effects of altering the σ70 region 4/β flap interaction on Q-dependent antitermination. There are two main conclusions that we draw from our results. First, displacement of σ70 region 4 from the β flap is required for efficient modification of RNAP by λQ. Second, the requirement for nascent RNA in the Q engagement process can be bypassed by removing σ70 region 4 from the RNAP holoenzyme. Our results illustrate how the nascent RNA-mediated destabilization of the σ70 region 4/β flap interaction during the transition from initiation to elongation can facilitate the engagement of a regulatory factor with RNAP.
Displacement of σ70 region 4 from the β flap is required for efficient modification of RNAP by λQ
Using amino acid substitutions that strengthen or weaken the σ70 region 4/β flap interaction we demonstrated that strengthening the σ70 region 4/β flap interaction (T544I/D581G) reduced λQ-dependent antitermination and weakening the σ70 region 4/β flap interaction (L607P) increased λQ-dependent antitermination (Figure 2). The use of an exonuclease challenge assay allowed us to show that altering the σ70 region 4/β flap interaction affects the stability of the complex that forms when λQ initially engages the paused early elongation complex at λPR′ (Figure 3).
At λPR′ the early elongation pause manifests itself in transcription complexes containing a 16- or 17-nt nascent transcript. In previous work (Nickels et al., 2005), using the same amino acid substitutions in σ70 that we used here (T544I/D581G and L607P), we showed that steric clash between the nascent RNA and σ70 region 4 bound to the β flap affects the distribution of paused elongation complexes at λPR′ (i.e. the ratio of paused complexes containing a 17-nt transcript to paused complexes containing a 16-nt transcript). In particular, we found that strengthening the σ70 region 4/β flap interaction (using holoenzyme reconstituted with σ70 T544I/D581G) decreased the fraction of paused elongation complexes containing a 17-nt transcript, whereas weakening the σ70 region 4/β flap interaction (using holoenzyme reconstituted with σ70 L607P) increased the fraction of paused elongation complexes containing a 17-nt transcript (Nickels et al., 2005). In principle, therefore, the effects of amino acid substitutions T544I/D581G and L607P on λQ-dependent antitermination could merely be an indirect consequence of their effects on the distribution of λPR′ paused elongation complexes. However, the results of the exonuclease challenge assays presented in Figure 3 rule out this possibility because these assays are performed with a homogeneous population of transcription complexes that are artificially halted after the synthesis of a 15-nt nascent transcript. We found that amino acid substitutions T544I/D581G and L607P affected the stability of the complex that forms when λQ engages these artificially halted transcription complexes. Therefore, we conclude that the effects of substitutions T544I/D581G and L607P on λQ-dependent antitermination are not merely an indirect consequence of their effects on the distribution of λPR′ paused complexes containing either a 17-nt transcript or a 16-nt transcript.
Bypassing the requirement for nascent RNA in the Q engagement process
Based on the idea that the nascent RNA-mediated destabilization of σ70 region 4/β flap interaction facilitates λQ's engagement of the paused early elongation complex, we hypothesized that the function of the nascent RNA in the Q engagement process could be bypassed by weakening or eliminating the σ70 region 4/β flap interaction. We took advantage of the properties of the pause-inducing sequence associated with the phage 82 PR′ to confirm this hypothesis. Specifically, we converted the phage 82 PR′ pause-inducing sequence to a consensus extended −10 promoter element and showed that 82Q could engage transcription initiation complexes bound at this converted pause element increasingly efficiently as the σ70 region 4/β flap interaction was weakened or eliminated (by the removal of σ70 region 4) (Figures 4, 5 and S4).
Our results suggest that one function of the early elongation pause in the process of Q-mediated antitermination is to present Q with a promoter-specific substrate in which the nascent RNA has destabilized the σ70 region 4/β flap interaction (Figure 6). We infer that Q's ability to functionally engage the RNAP holoenzyme requires displacement of σ70 region 4 from the β flap. Specifically, we propose that the nascent RNA-mediated destabilization of the σ70 region 4/β flap interaction allows Q to capture and stabilize a conformation of the RNAP holoenzyme in which σ70 region 4 is not bound to the β flap. Currently, it is unknown what surface(s) of RNAP core Q interacts with during the engagement process, and furthermore, what Q/core contacts are required for antitermination. Our findings are consistent with the proposal that Q function requires contact with a surface of RNAP core that is occluded by σ70 region 4 when it is bound to the β flap.
Figure 6.
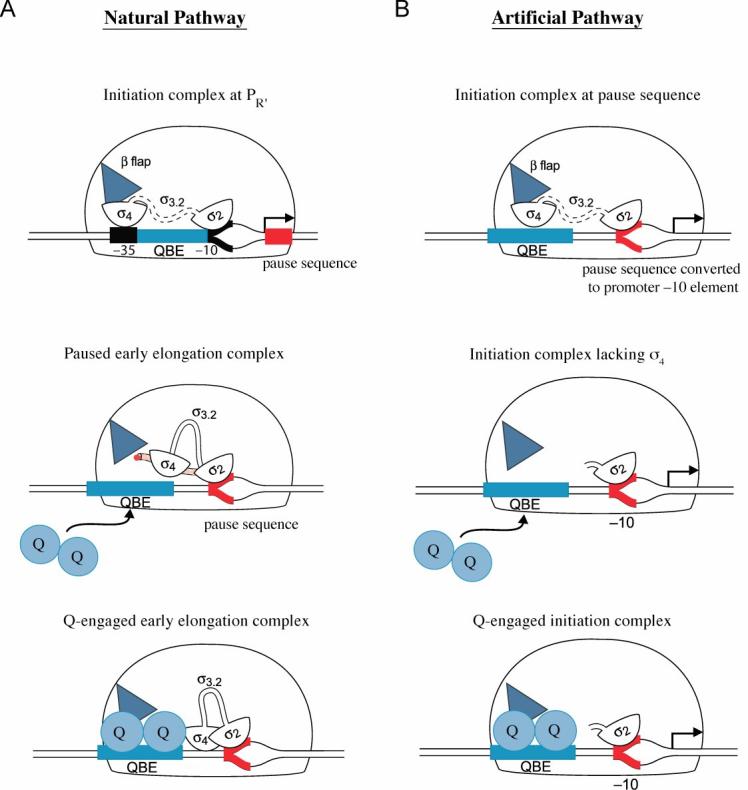
- Q engages a paused early elongation complex. Top panel depicts the PR' initiation complex in which σ region 3.2 (σ3.2) is located within the RNA exit channel and σ region 4 (σ4) is bound to the β flap (blue triangle). The pause-inducing sequence is shown in red. Middle panel depicts the paused early elongation complex in which the nascent RNA (shown as red beads emerging from the exit channel) has displaced σ region 3.2 (σ3.2) from the RNA exit channel and displaced σ region 4 (σ4) from the β flap. Bottom panel depicts Q-engaged early elongation complex.
- Q engages an initiation complex. Top panel depicts a wild-type initiation complex bound at a promoter created by converting the pause-inducing sequence to a consensus extended −10 element (shown in red). Middle panel depicts an initiation complex lacking σ region 4 bound at the converted pause-inducing sequence. Bottom panel depicts the Q-engaged initiation complex lacking σ region 4.
A transcription regulator that exploits the staged displacement of σ from core
Structural and biochemical evidence suggests that the transition from initiation to elongation involves a staged displacement of segments of σ70 from RNAP core (Marr et al., 2001; Murakami et al., 2002a and 2002b; Vassylyev et al., 2002; Mekler et al., 2002; Nickels et al., 2002; Nickels et al., 2005). In this work we provide evidence that the RNA-mediated destabilization of the σ region 4/β flap interaction facilitates interaction between a regulatory factor and RNAP during early elongation. High-resolution structures of other multi- and single subunit RNA polymerases reveal protein elements that, like σ region 3.2 and σ region 4, are positioned within the path of the elongating RNA transcript (Murakami and Darst, 2003 and references therein). Thus, the obligatory displacement of protein elements by the nascent RNA during the transition from initiation to elongation likely contributes to gene regulation in other organisms, as well.
Experimental Procedures
Proteins
His-tagged versions of wild-type σ70, σ70 T544I/D581G, σ70 L607P, σ70 1-529, σ38, and σ38 F278L were purified as described after overproduction from plasmid pLHN12-His (Panaghie et al., 2000). E. coli RNAP core was obtained from Epicentre, and holoenzyme was made by incubation with a five-fold excess of the appropriate σ. λQ protein and NusA were purified as described (Yarnell and Roberts, 1992). 82Q was purified as described (Goliger and Roberts, 1989). EcoRIGln111 was provided by P. Modrich and I. Artsimovitch.
Strains and Plasmids
A complete list of strains and plasmids is provided in Table 1 of the Supplemental Data. The Supplemental Data also include a detailed description of how the T544I/D581G mutations were introduced into the chromosomal copy of rpoD.
In Vitro Transcription
The λPR' template was obtained by the PCR from plasmid pFW11 Tet-λPR' (Nickels et al., 2002) and contains sequence extending from −109 to +238 of λPR' that includes the natural terminator tR'. The phage 82 PR' promoter was obtained by the PCR from plasmid p82a (Goliger and Roberts, 1989), which contains sequence extending from −100 to +116 of 82 PR' that includes the natural terminator t82. To modify the phage 82 PR' promoter for the experiments of Figures 4 and 5, base pair mutations were introduced into the template by site directed mutagenesis. The modified 82 PR' template contained the following substitutions: T−35C, T−34A, G−33C, T−7C, T+12A and T+13A.
In the context of the λPR' promoter, the base pair substitutions T−22G and G−25C disrupt the binding of λQ to the λQ-binding element (Guo and Roberts, 2004). Therefore, to disrupt the ability of 82Q to bind the 82Q-binding element, we introduced the corresponding base pair substitutions (T−22G and G−25C) into the modified 82 PR' template. We note that while these substitutions disrupted the ability of 82Q to engage the transcription initiation complex (Figure 4B and data not shown), they did not detectably disrupt the ability of 82Q to engage the paused early elongation complex when introduced into the wild-type phage 82 PR' template (data not shown).
λQ antitermination assays: Open complexes were formed by incubating 20 nM RNAP with 2 nM template and 150 nM NusA for 5 min at 37° in transcription buffer (20 mM Tris-HCl pH 8.0, 0.1 mM EDTA, 50 mM KCl, 100 μg/ml BSA) plus 200 μM GTP, UTP and CTP and 50 μM γ-32P-ATP at 1.5 mCi/ml. One tenth volume λQ or λQ dilution buffer (10 mM Tris pH 7.5, 500 μg/ml BSA, 100 mM DTT, 10% glycerol, 50 mM potassium glutamate) was added, and after 30 seconds transcription was initiated by adding 4 mM MgCl and 10 μg/ml rifampicin. Reactions were allowed to proceed for 8 minutes then stopped by addition of 5 reaction volumes of 1.2X stop solution (0.6M Tris-HCl [pH 8.0], 12 mM EDTA, 80 μg/ml tRNA). Samples were then extracted with phenol/chloroform (1:1), precipitated with ethanol, resuspended in 4μl of loading buffer (95% (v/v) formamide, 20 mM EDTA, 0.05% (w/v) bromophenol blue, and 0.05% (w/v) xylene cyanol), and electrophoresed on 6% polyacrylamide sequencing gels. Bands were visualized by phosphorimager and the data analyzed by Imagequant.
82Q antitermination assays: For the reactions shown in Figure 4B, 100 nM 82Q (or Q dilution buffer) was incubated with 20 nM template DNA and 150 nM NusA for 5 min at 37° in transcription buffer plus NTPs (200 μM GTP, UTP and CTP and 50 μM γ-32P-ATP at 1.5 mCi/ml for experiments performed with the wild-type phage 82 PR' template and 200 μM ATP, UTP and CTP and 50 μM γ-32P-GTP at 1.5 mCi/ml for experiments performed with the modified 82 PR' template). The indicated RNAP (at 20nM) was added and the reactions were incubated for 10 minutes. Next, transcription was initiated by adding 4 mM MgCl and 10 μg/ml rifampicin. Reactions were allowed to proceed for 10 minutes then stopped by addition of 5 reaction volumes of 1.2X stop solution and processed as described above.
The reactions shown in Figure 4C were performed as follows: 100 nM 82Q (or Q dilution buffer) was incubated with 5 nM template DNA and 150 nM NusA for 5 min at 37° in a modified transcription buffer (10 mM MgCl, 90 mM KCl, 40 mM Tris pH 8.0, 100 μg/ml BSA and 5% PEG 3350). The indicated RNAP (at 20 nM) was added and the reactions were incubated for 10 minutes. Next, transcription was initiated by adding NTPs (1 mM GTP, UTP and ATP and 50 μM α-32P-CTP at 1.0 mCi/ml) plus 100 μg/ml heparin. Reactions were allowed to proceed for 10 minutes then stopped by addition of 1 reaction volume of loading buffer. Samples were electrophoresed on 6% polyacrylamide sequencing gels. Bands were visualized by phosphorimager and the data analyzed by Imagequant.
Exonuclease III Challenge Assays
Assays performed with the λPR' template were done essentially as described (Yarnell and Roberts, 1992). In brief, open complexes were formed by incubating 20 nM RNAP and 2 nM DNA for 15 minutes at 37° in transcription buffer (but with 10 mM KCl) containing the initiating oligonucleotide ApApC at 50μM, and 25μM ATP, GTP, and UTP; MgCl2 was added to 5 mM and incubation continued 4 minutes to make +15 complexes. After addition of λQ to 500 nM (or buffer), EcoRIGln111 to 50 nM, and calf thymus DNA to 50 μg/ml, exoIII was added to 1.6 U/μl. Samples were removed, quenched as above, prepared as above, analyzed on a 7% sequencing gel, and bands were visualized by phosphorimager. The DNA template was 32P-labelled with T4 polynucleotide kinase on the bottom strand; the template contained both promoter sequences and a downstream EcoRI site, which provided a binding site for the catalytically inactive EcoRIGln111 to block exoIII digestion from downstream.
Assays with the modified 82 PR' template were performed identically, except that open complexes were formed by incubation for 10 minutes, no elongation step was used, and exoIII was added to 0.8U/μl. In addition, PEG3350 was added to 5% to stabilize the σ truncation.
β-galactosidase Assays
For the assays presented in Figure 2D, reporter strains cells were transformed with either plasmid pBRλQΔ-35, which directs the expression of low levels of λQ (Nickels et al., 2002) or plasmid pBRΔQ, which encodes no functional λQ (Nickels et al., 2002). Individual transformants were selected and grown in LB supplemented with carbenicillin (100 μg/ml), tetracycline (10 μg/ml), kanamycin (50 μg/ml) and 100μM Isopropyl-β-D-thiogalactoside (IPTG). β-galactosidase activity was assayed as described (Dove and Hochschild, 2004).
Supplementary Material
Acknowledgements
We thank Sean Garrity for construction of the σ70 T544I/D581G mutant strain, Mark Leibman for performing preliminary in vivo experiments with σ70 T544I/D581G, Padraig Deighan for sharing unpublished data, Tom Santangelo for providing purified λQ and Sergei Nechaev for advice. This work was supported by NIH grants GM44025 to A.H. and GM21941 to J.W.R.
References
- Borukhov S, Nudler E. RNA polymerase holoenzyme: structure, function and biological implications. Curr. Opin. Microbiol. 2003;6:93–100. doi: 10.1016/s1369-5274(03)00036-5. [DOI] [PubMed] [Google Scholar]
- Bown J, Barne K, Minchin S, Busby S. Extended –10 promoters. Nucleic Acids Mol. Biol. 1997;11:41–52. [Google Scholar]
- Dove SL, Hochschild A. A bacterial two-hybrid system based on transcription activation. Methods Mol. Biol. 2004;261:231–46. doi: 10.1385/1-59259-762-9:231. [DOI] [PubMed] [Google Scholar]
- Goliger JA, Roberts JW. Sequences required for antitermination by phage 82 Q protein. J. Mol. Biol. 1989;210:461–471. doi: 10.1016/0022-2836(89)90123-x. [DOI] [PubMed] [Google Scholar]
- Gross CA, Chan C, Dombroski A, Gruber T, Sharp M, Tupy J, Young B. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harbor Symp. Quant. Biol. 1998;63:141–155. doi: 10.1101/sqb.1998.63.141. [DOI] [PubMed] [Google Scholar]
- Guo J, Roberts JW. DNA binding regions of Q proteins of phages lambda and phi80. J. Bacteriol. 2004;186:3599–3608. doi: 10.1128/JB.186.11.3599-3608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komissarova N, Kashlev M. Functional topography of nascent RNA in elongation intermediates of RNA polymerase. Proc. Natl. Acad. Sci. USA. 1998;95:14699–14704. doi: 10.1073/pnas.95.25.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzheva N, Mustaev A, Kozlov M, Malhotra A, Nikiforov V, Goldfarb A, Darst SA. A structural model of transcription elongation. Science. 2000;289:619–625. doi: 10.1126/science.289.5479.619. [DOI] [PubMed] [Google Scholar]
- Kumar A, Malloch RA, Fujita N, Smillie DA, Ishihama A, Hayward RS. The minus 35-recognition region of Escherichia coli σ70 is inessential for initiation of transcription at an “extended minus 10” promoter. J. Mol. Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- Kuznedelov K, Minakhin L, Niedziela-Majka A, Dove SL, Rogulja D, Nickels BE, Hochschild A, Heyduk T, Severinov K. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science. 2002;295:855–857. doi: 10.1126/science.1066303. [DOI] [PubMed] [Google Scholar]
- Lonetto M, Gribskov M, Gross CA. The σ70 family: sequence conservation and evolutionary relationships. J. Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr MT, Datwyler SA, Meares CF, Roberts JW. Restructuring of an RNA polymerase holoenzyme elongation complex by lambdoid phage Q proteins. Proc. Natl. Acad. Sci. USA. 2001;98:8972–8978. doi: 10.1073/pnas.161253298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekler V, Kortkhonjia E, Mukhopadhyay J, Knight J, Revyakin A, Kapanidis AN, Niu W, Ebright YW, Levy R, Ebright RH. Structural Organization of Bacterial RNA Polymerase Holoenzyme and the RNA Polymerase-Promoter Open Complex. Cell. 2002;108:599–614. doi: 10.1016/s0092-8674(02)00667-0. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Darst SA, Landick R. Sigma and RNA polymerase: an on-again, off-again relationship? Mol Cell. 2005;20:335–345. doi: 10.1016/j.molcel.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: T. aquaticus RNA polymerase holoenzyme at 4 A resolution. Science. 2002a;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme-DNA complex. Science. 2002b;296:1285–1290. doi: 10.1126/science.1069595. [DOI] [PubMed] [Google Scholar]
- Murakami KS, Darst SA. Bacterial RNA polymerases: the wholo story. Curr. Opin. Struct. Biol. 2003;13:31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Nickels BE, Roberts CW, Sun H, Roberts JW, Hochschild A. The σ70 subunit of RNA polymerase is contacted by the λQ antiterminator during early elongation. Mol. Cell. 2002;10:611–622. doi: 10.1016/s1097-2765(02)00648-2. [DOI] [PubMed] [Google Scholar]
- Nickels BE, Garrity SJ, Mekler V, Minakhin L, Severinov K, Ebright RH, Hochschild A. The interaction between σ 70 and the β flap of Escherichia coli RNA polymerase inhibits extension of nascent RNA during early elongation. Proc. Natl. Acad. Sci. USA. 2005;102:4488–4493. doi: 10.1073/pnas.0409850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaghie G, Aiyar SE, Bobb KL, Hayward RS, de Haseth PL. Aromatic amino acids in region 2.3 of Escherichia coli σ70 participate collectively in the formation of an RNA polymerase-promoter open complex. J. Mol. Biol. 2000;299:1217–1230. doi: 10.1006/jmbi.2000.3808. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Yarnell WS, Roberts JW. Function of E. coli RNA polymerase σ factor σ70 in promoter-proximal pausing. Cell. 1996;86:485–493. doi: 10.1016/s0092-8674(00)80121-x. [DOI] [PubMed] [Google Scholar]
- Roberts JW, Yarnell W, Bartlett E, Guo J, Marr M, Ko DC, Sun H, Roberts CW. Antitermination by bacteriophage λ Q protein. Cold Spring Harbor Symp. Quant. Biol. 1998;63:319–325. doi: 10.1101/sqb.1998.63.319. [DOI] [PubMed] [Google Scholar]
- Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- Yarnell WS, Roberts JW. The Phage λ Gene Q Transcription Antiterminator Binds DNA in the Late Gene Promoter As It Modifies RNA-Polymerase. Cell. 1992;69:1181–1189. doi: 10.1016/0092-8674(92)90639-t. [DOI] [PubMed] [Google Scholar]
- Yarnell WS, Roberts JW. Mechanism of intrinsic transcription termination and antitermination. Science. 1999;284:611–615. doi: 10.1126/science.284.5414.611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.