Abstract
Viral disease diagnosis has traditionally relied on the isolation of viral pathogens in cell cultures. Although this approach is often slow and requires considerable technical expertise, it has been regarded for decades as the “gold standard” for the laboratory diagnosis of viral disease. With the development of nonculture methods for the rapid detection of viral antigens and/or nucleic acids, the usefulness of viral culture has been questioned. This review describes advances in cell culture-based viral diagnostic products and techniques, including the use of newer cell culture formats, cryopreserved cell cultures, centrifugation-enhanced inoculation, precytopathogenic effect detection, cocultivated cell cultures, and transgenic cell lines. All of these contribute to more efficient and less technically demanding viral detection in cell culture. Although most laboratories combine various culture and nonculture approaches to optimize viral disease diagnosis, virus isolation in cell culture remains a useful approach, especially when a viable isolate is needed, if viable and nonviable virus must be differentiated, when infection is not characteristic of any single virus (i.e., when testing for only one virus is not sufficient), and when available culture-based methods can provide a result in a more timely fashion than molecular methods.
INTRODUCTION
The discovery in the early 1900s that human cells could be propagated in vitro provided virologists with an alternative to embryonated eggs and laboratory animals for in vitro isolation of viruses. Cell cultures, which are derived from dispersed cells taken from original tissue and disaggregated by enzymatic, mechanical, or chemical means, provided large numbers of cells suitable for virus isolation, facilitated control of contamination with antibiotics and clean-air equipment, and helped decrease the use of experimental animals (55). Viruses reach high titers when grown within susceptible cells, and culture tubes are convenient to manipulate.
Although virus isolation in cell cultures was employed by research laboratories by the early 1960s, diagnostic services were very limited, varying from laboratory to laboratory and often not available at all, except in major medical centers. However, by the early 1970s, diagnostic virology expanded dramatically, largely because of the availability of highly purified reagents and commercially prepared cell lines (71). The types of cells that can be grown in vitro in flasks and test tubes are many, providing living hosts that many human viruses can infect. Cell cultures are more convenient and less expensive than eggs and animals, are convenient to examine microscopically for evidence of viral proliferation, and, for many years, have provided a desirable environment for the detection and identification of many human viral pathogens. Virus isolation in cell cultures has long served as the “gold standard” for virus detection, and it is the method to which all others have been compared (71). However, in recent years, technological advances, ranging from the development of monoclonal antibodies to the introduction of molecular diagnostics, have provided powerful tools to use in attempting to detect the presence of viral infections. Molecular detection of viral DNAs and RNAs and molecular amplification by PCR and other techniques are now becoming more widely available in diagnostic laboratories. Sensitive and highly specific viral identification can be obtained with these techniques. Molecular methods, as well as others such as viral antigen detection, do not require the lengthy incubation period needed for viral isolation in cell cultures, may involve less technical expertise, and are useful for viruses that do not proliferate in standard cell cultures.
At this point, it is provocative to ask, “Is virus isolation in cell cultures still a useful approach in viral diagnostics?” and “What does the future hold for this approach in the diagnostic virology laboratory?” The purpose of this review is to present the current status of viral detection in cell cultures, describe developments in the field, and critically analyze situations in viral diagnosis to indicate when viral isolation methods are likely to yield the most desirable outcome.
VIRUS ISOLATION IN TRADITIONAL CELL CULTURES
As early as 1913 vaccinia virus (152) was grown in cell cultures, and in the 1930s both smallpox virus (133) and yellow fever virus (94) were propagated in cell cultures for the purpose of vaccine production. However, it was not until the 1950s that the interest in using cell cultures for virus isolation expanded, largely due to the discovery that polioviruses would proliferate in cell cultures that were not of neural origin (43, 134). The use of cultured cells to isolate viruses was advanced further by the addition of antibiotics to cell culture media, the development of chemically defined culture media, and the use of cell-dispensing equipment for preparing replicate cultures (142). Although, initially, flasks and tubes of cells for use in diagnostic laboratories were prepared in the laboratory, biological supply houses soon began to mass produce various cell strains and lines which could be purchased and delivered ready to use. Although many diagnostic virology laboratories purchase all of their cell cultures, some laboratories still prepare cell cultures in-house.
Although cell cultures can be purchased or prepared in a variety of containers, the 16- by 125-mm glass or plastic round-bottom screw-cap tube is standard, with the cell monolayer adhering from the midpoint to the bottom of one side of the tube. Typically, several different cell lines are inoculated with each clinical sample in an attempt to provide a suitable host for whichever virus might be present in the sample. Cell cultures of primary, diploid, and heteroploid cells are kept on hand in the virology laboratory. Examples of well-known cell types that are standard for most virology laboratories are primary rhesus monkey kidney (RhMK) cells, primary rabbit kidney cells, human lung fibroblasts (MRC-5), human foreskin fibroblasts, human epidermoid carcinoma cells (HEp-2), human lung carcinoma cells (A549), and others. The number and types of cell culture tubes inoculated for each clinical specimen depend on the specimen source and the viruses suspected of causing a given disease. The cost of cell culture tubes ranges from $1.50 to $6.50 per tube, depending on the cell line; primary cells are more expensive than nonprimary cells (Table 1). The cost per tube also depends on numbers of tubes purchased, shipping specifications, etc.
TABLE 1.
Cost, turnaround time, advantages, and disadvantages of various virus detection approaches
| Method | Costa/avg turnaround time | Advantages | Disadvantages |
|---|---|---|---|
| Cell culturesb | |||
| Traditional tubes | $1.50-$4.00 per tube for nonprimary cells and $2.15-$6.15 per tube for primary cells; use 2 to 6 tubes per culture/5-10 days | Isolate wide variety of viruses (including unanticipated agents, mixed cultures); provide isolate for additional studies: antiviral susceptibility testing, serotyping, and epidemiologic studies; increased sensitivity over rapid antigen tests | Technical expertise needed to read CPE; long incubation period for some viruses, need for purchasing/maintaining a variety of cell culture types in-house |
| Shell vials with centrifugation/ pre-CPE stain | Same as comparable cell culture tubes; use at least 2 vials of each cell line/24-48 h | Short turnaround time for detection; take up less space than tubes; some available as cryopreserved cells; may isolate viruses that replicate poorly or not at all in standard tube cell cultures; require less technical expertise than tube cultures if pre-CPE staining is used | Not as sensitive as traditional cultures for culturing blood samples for CMV; reading stained preparations is time-consuming and labor-intensive; unanticipated agents may be missed when pre-CPE staining targets only one or a few viruses; isolates not available from fixed/stained vials |
| Cocultivated cells | Approx. $1.25 more per vial than standard shell vials; use 3 vials for each culture/ 24-48 h | Same as for shell vials plus decreased need for maintaining wide variety of cell cultures, support growth of a wider range of viruses, most results finalized in 2-3 days when pre-CPE staining is used, may be more sensitive than tube cultures for some viruses | Same as for shell vials |
| Transgenic cells (ELVIS) | $2.35-$3.00 more per vial than standard shell vials; use 2 vials for each culture/ 24-48 h | Same as for shell vials plus detection by color change rather than application of MAbs, simplify identification because of specificity for a single virus, can be used to type HSV-1 and HSV-2 | Targeted for detection of only a single virus group (HSV) |
| Nonculturec | |||
| Antigen detection by IF | $2-$7.00 for MAbs for each sample/40 min | Generally good sensitivity (which varies with virus detected); excellent specificity; CMV antigenemia is more sensitive than traditional or shell vial cultures for CMV in blood | Generally not as sensitive as cell cultures; requires expertise in reading; not useful for all viruses; adenovirus sensitivity especially poor |
| Antigen detection, non-IF | $10-$22 for each sample/30 min | Generally good specificity for RSV and influenza A and B viruses; no special technical expertise required; results available very rapidly; most cleared for point-of-care testing | Generally poor sensitivity compared to cell culture; currently available for RSV and influenza A and B viruses only; additional testing of negative samples by cell culture is recommended |
| Nucleic acid detection (molecular) | $35-$125 for each sample tested with ASR or FDA-cleared kits; $10-$35 for each in-house developed assay (may require patent royalties)/2 h for real-time PCR; 8 h for traditional PCR | Excellent sensitivity and specificity; short turnaround with real-time PCR; useful for viruses that cannot be cultured in traditional cell cultures | FDA-cleared kits and standardized protocols not widely available for most viruses; technical expertise required in-house for developing and standardizing methods; expensive due to costs of instrumentation (especially for low-vol testing); probes and primers extremely specific (may miss mutated virus); detects only viruses sought (will miss unanticipated agents and mixed infections in most cases); many assays available at reference/research laboratories only |
Cost includes reagents only.
All types of cell cultures require viable virus in order to produce a positive result. This allows these methods to differentiate viable from nonviable virus. Because viable virus is required, specimens must be handled carefully to preserve viral infectivity.
None of the nonculture methods requires viable virus in order to produce a positive result; therefore, these methods cannot differentiate viable and nonviable virus. Because viability is not required, specimen handling is not as critical. No viral isolate is available upon completion of testing.
The appropriate selection, collection, transport, and processing of clinical samples are important for successful virus isolation. Collection of samples that contain the highest titer of virus is most desirable. Preservation of the viral titer and viral infectivity until cell cultures can be inoculated is essential. Body sites and collection methods vary according to the type of infection and viral etiology. In general, clinical samples collected from body sites such as skin and the genital tract, which are usually contaminated with microbial flora, are collected with a Dacron or polyester swab and placed in viral transport medium (VTM), most types of which contain antibiotics, a buffered salt solution, a proteinaceous substance (such as albumin, gelatin, or serum), and a pH indicator. Respiratory tract samples include sputum, bronchial alveolar lavage specimens, nasopharyngeal (NP) washes, NP aspirates (NPA), NP swabs (NPS) in VTM, oropharyngeal swabs in VTM or a combination of NP and oropharyngeal swabs in a single VTM tube. Specimens such as cerebrospinal fluid (CSF) and body fluids, which are expected to be free of microbial contamination, are collected in sterile containers and are not placed in transport medium. Keeping the samples cool (2 to 8°C or on wet ice) until cell culture inoculation helps preserve viral infectivity and increases the virus recovery rate, particularly for labile viruses such as respiratory syncytial virus (RSV). Information concerning selecting, collecting, and transporting clinical samples for viral culture is provided in several reference texts (52, 146).
Although specimen processing guidelines differ from laboratory to laboratory, many laboratories clarify certain sample types (e.g., respiratory samples) as follows prior to inoculation into cell cultures. The transport medium tube is vortexed, the swab is discarded, the liquid medium is centrifuged, and the supernatant fluid is used to inoculate the cell cultures. Thus, bacteria, fungi, cells, blood, mucus, fibers, etc., are pelleted into the bottom of the spun tube, while the viruses, which will not be spun down by the g-force generated by most general laboratory centrifuges, remain dispersed throughout the liquid. The sample pellet can be used for various antigen detection assays. Extensively contaminated clinical material such as stool may be liquefied in antibiotic-containing medium and filtered through a 0.45-μm filter prior to inoculation into cell cultures. Samples from sites expected to be free of microbial contamination may be used for cell culture inoculation without any treatment or processing. Suggestions for processing clinical samples for viral culturing are available in several reference texts (52, 146).
As with specimen collection and processing, procedures for inoculation of cell cultures and the number and types of cell cultures inoculated for each specimen may vary among laboratories and according to specimen type, virus suspected, and patient population. The processed inoculum may be added to the cell culture tube, either by simply adding 0.2 ml or 0.3 ml of the sample to each tube or by adsorption inoculation. Adsorption inoculation involves decanting the cell culture medium from the cell culture monolayer and applying the inoculum directly to the monolayer. After a 30- to 90-min (88) incubation of the inoculated tube in a horizontal position at 35 to 37°C, excess inoculum is discarded and fresh cell culture medium is added (90). Adsorption inoculation is thought to allow more efficient adsorption of viral particles to the cells and to enhance rates of recovery of some viruses (52, 88, 146). Inoculated cell culture tubes can be incubated in stationary slanted racks or, alternatively, in rotating/rolling racks at 35 to 37°C, which may enhance the speed and sensitivity of virus recovery (102). Viral tube cultures are incubated for days to weeks depending on the specimen source and the suspected virus(es). Cell monolayers are screened by microscopic examination daily for the first week of incubation to maximize the detection of viral growth and on alternate days for the remainder of the incubation period (88). The microscopic examination involves placing the tube on the stage of a standard light microscope and viewing the cells through the glass wall of the tube with the low-power (10×) objective.
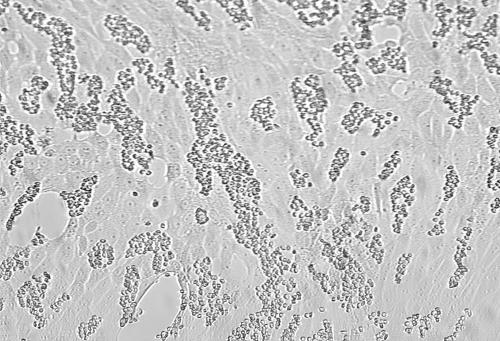
The microscopic examination of the unstained cell culture monolayer has long been the standard approach for detecting viral proliferation. Degenerative changes in monolayer cells provide evidence of viral presence. The spectrum of change is broad, ranging from swelling, shrinking, and rounding of cells to clustering, syncytium formation, and, in some cases, complete destruction of the monolayer. These changes are collectively called the cytopathogenic or cytopathic effect (CPE) of the virus. The typical CPEs of several common viruses are shown in Fig. 1. Dramatic CPE may be easily detected, but the subtle CPE of many viruses, early CPE, or CPE that is not typical may go unrecognized unless the observer has considerable expertise. Although herpes simplex virus (HSV) may produce easily visible CPE within the first 24 h of incubation, most viruses demonstrate CPE only after 5 to 10 days of incubation, with some, such as cytomegalovirus (CMV), averaging 10 to 30 days for CPE production (Table 2). The experienced observer may be able to predict which virus is present based on the characteristics of the CPE, the cell line involved, the length of incubation, and the type of clinical specimen, but confirmatory testing is needed to make a definitive viral identification.
FIG. 1.
Uninfected cell cultures and cell cultures showing CPE of viruses commonly isolated. (A) Uninfected A549 cells; (B) HSV-2 in A549 cells; (C) adenovirus in A549 cells; (D) uninfected MRC-5 fibroblasts; (E) CMV in MRC-5 fibroblasts; (F) rhinovirus in MRC-5 fibroblasts; (G) uninfected RhMK cells; (H) enterovirus in RhMk cells; (I) influenza A virus in RhMk cells; (J) uninfected HEp-2 cells; (K) RSV in HEp-2 cells; (L) monkey virus contaminant in RhMk cells. Magnification, ×85.
TABLE 2.
Cytopathogenic effect in standard cell cultures of human viral pathogens common in the United Statesa
| Virus | Cytopathogenic effect in:
|
Final identification of isolates | |||
|---|---|---|---|---|---|
| Fibroblasts | A549 cellsb | RhMK cells | Otherc | ||
| Adenovirus | Some produce clusters | Grape-like clusters or “lacy” pattern; 5-8 days | Some produce clusters | HNK: grape-like clusters; 5-7 days | IF for group, neutralization for type |
| CMV | Foci of contiguous rounded cells; 10-30 days | None | None | Use shell vials for rapid detection | CPE alonee |
| Enteroviruses | Some produce CPE, same as in RhMK cells; 2-5 days | Infrequent, degenerative | Small, round cells with cytoplasmic tails; 2-5 days | IF for groups, neutralization for type | |
| HSV | Rounded large cells; 2-6 days | Rounded large cells; 1-4 days | Some produce CPE, same as in A549 cells, 4-8 days | RK or HNK: rounded large cells; 1-4 days | IF |
| Influenza virus | None | None | Undifferentiated CPE, cellular granulation; 4-8 days | HAD-positive with GP | IF |
| Parainfluenza virus | None | None | Rounded cells, some syncytia; 4-8 days | HAD-positive with GP | IF |
| Rhinovirus | Degeneration, rounding; 7-10 days | None | None | Incubate fibroblasts at 33°C | CPE onlyf (difficult to differentiate from enteroviruses) |
| RSV | Infrequent, granular degeneration | Infrequent | Syncytia; 4-10 days | HEp-2d: syncytia; 4-10 days | IF |
| VZV | Some CPE; small, round cells; 6-8 days | Small, round cells; 6-8 days | None | HNK: small, round cells; 6-8 days | IF |
Measles, mumps, and rubella viruses are seldom encountered in the United States at present. Measles virus produces large syncytia in RhMK cells in 7 to 10 days and is hemadsorption positive with Rh cells. Virus identification may be confirmed by IF. Mumps virus produces rounded cells with large syncytia in RhMK cells in 6 to 8 days and is hemadsorption positive with guinea pig erythrocytes, and its identification may be confirmed by IF. Rubella virus requires special cultures such as African green monkey kidney, rabbit kidney, or BSC-1 cells and does not produce CPE; special detection by interference challenge or another method is needed.
Human lung carcinoma.
GP, guinea pig erythrocytes; HAD, hemadsorption; HNK, human neonatal kidney cells, RK, rabbit kidney cells.
Human laryngeal carcinoma.
Some laboratories may base final identification of CMV on characteristic CPE alone. Others may inoculate shell vials and stain for CMV early antigen to confirm the identification.
Some laboratories may base final identification of rhinovirus on CPE, which is similar to that of the enteroviruses but appears in fibroblast lines rather than RhMk cells. The term “rhino-like virus” is sometimes used for reporting when the identification is based on CPE alone. Others may test for acid lability to differentiate rhinoviruses from the enteroviruses.
An alternative approach for detecting viruses that produce CPE slowly or not at all in primary culture of clinical specimens is to perform a hemadsorption (HAD) test. HAD is useful only for viruses such as influenza virus, parainfluenza virus, and mumps virus that express their hemagglutinating proteins on the plasma membranes of virus-infected cells. These proteins are not visible with the light microscope but can be detected by their affinity for erythrocytes. HAD testing is routinely performed at the end of the incubation period for cell cultures that fail to produce CPE or earlier in the incubation period, at days 3 and 7 of incubation (105). Hemadsorbing foci have been found in human fetal lung diploid cell cultures within 12 h after inoculation with influenza viruses A and B (122). In HAD testing the cell culture medium is removed and replaced with a dilute suspension of erythrocytes, usually guinea pig erythrocytes, and the cell culture tubes are incubated at 4°C for 30 min (90). Tubes are then examined microscopically. If a hemadsorbing virus is present, erythrocytes will adhere in clumps to the infected areas of the cell monolayer (Fig. 2). Erythrocytes will not adhere to uninfected cells or to cells infected by nonhemadsorbing viruses. Nonadherent erythrocytes float free when the cell culture tube is tapped or rotated. Although only a few human viral pathogens produce a positive HAD result, confirmatory testing of all HAD-positive cell cultures is required to differentiate among the hemadsorbing viruses. Despite the availability of alternative methods for detecting viral presence in infected cell cultures, CPE and HAD are the most commonly used techniques in diagnostic virology laboratories today.
FIG. 2.
Positive hemadsorption result in parainfluenza virus-infected RhMk cells. Magnification, ×100.
Confirmatory testing of virus cultures positive by CPE or HAD has traditionally been based on the reaction of antibodies of known specificity with viral antigens expressed in the infected cells. Most of this testing is accomplished at present through immunofluorescence (IF) techniques that use fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies (MAbs). The cells are scraped from the infected monolayer and placed on a microscope slide. The preparation is fixed in acetone and then flooded with FITC-labeled MAbs of known specificity. Binding of MAbs to viral proteins is signaled by the presence of fluorescence when the preparation is viewed using the fluorescence microscope. The type of fluorescence (e.g., speckled versus confluent) and the location of the fluorescence in the cell (e.g., nuclear versus cytoplasmic) are also useful in differentiating certain viruses. This process takes only 1 to 2 h and overall gives a sensitive and specific viral identification.
Unfortunately, IF staining cannot be used to definitively identify all viruses. Examples of this are the coxsackieviruses, polioviruses, and echoviruses of the “enterovirus” group, which are closely related and have numerous serotypes. In some cases, these may be identified as to their family by IF. However, the MAbs for enteroviral identification have been shown to lack sensitivity (77), cross-react with rhinoviruses (77), and lack reactivity with enterovirus 71 (155). Identification of enteroviral serotypes within the families requires confirmation by the neutralization method (132). In neutralization testing, the virus-infected cells are incubated with antibodies of known viral specificity; an aliquot of the mixture is then inoculated into susceptible cell cultures, and the cell cultures are observed for evidence of viral proliferation. CPE production indicates that the antibodies did not bind, inactivate, or neutralize the virus. Conversely, a lack of CPE production indicates that the antibodies bound to the virus and inactivated or neutralized it, allowing the identity of the virus to be established according to the specificity of the antibody used. This is a cumbersome procedure that requires determining the titer of the virus prior to the start of the procedure and a lengthy incubation after inoculation of cell culture tubes with the mixture of antibody and virus-infected cells. Although neutralization testing may be used in identifying all types of viruses, it is used only when less cumbersome, more rapid methods are not available. Neutralization testing is not routinely performed in most clinical laboratories and is generally reserved for reference laboratories.
The main advantage of the traditional cell culture approach (Table 1) is the capacity to isolate a wide variety of viruses. At this writing, the following familiar viral pathogens are the ones that can be isolated in traditional tube cultures: adenovirus, CMV, many of the enteroviruses (i.e., polioviruses, coxsackieviruses, and echoviruses), HSV, influenza A and B viruses, measles virus, mumps virus, parainfluenza virus types 1 to 4, RSV, rhinoviruses, and varicella-zoster virus (VZV). Other viruses, such as Ebola virus, severe acute respiratory syndrome coronavirus (SARS-CoV), and human metapneumovirus (hMPV), also proliferate in traditional tube cultures. By inoculating clinical samples into several types of cell cultures, a suitable environment is provided for most of these viruses. Using a broad range of cultured cells may allow the detection of unanticipated agents, rather than focusing on the detection of only one or a few specific viruses. This approach also facilitates detection of more than one virus from the same sample. In a review of mixed viral infections, Waner (158) noted that mixed infections are reported to occur in respiratory samples of immunocompetent patients in an average of 5 to 10% of cultures. The medical significance of this is not established, but it has been suggested that dual infections, particularly in young children, may increase the severity of respiratory disease (11, 54). An increased incidence of mixed infections was seen in immunocompromised patients, especially with latent viruses such as HSV and CMV. Accurate detection of all viruses present is especially important with these patients to ensure timely treatment with proper antiviral therapy. Replication of viruses in cell cultures also provides an isolate that can be used for additional studies such as antiviral susceptibility testing, serotyping, and epidemiologic evaluations. Viral proliferation also confirms the viability of the virus and differentiates viable from nonviable virus, a differentiation that is not made by antigen and most nucleic acid detection methods. This information may be important for medical decision-making, such as in differentiating disease from latent infection (92); deciding when to implement, discontinue, or change antiviral therapy (93, 97); or making other decisions concerning patient management. The cell culture approach also offers increased sensitivity over most rapid antigen detection methods.
However, the need for technical expertise in evaluating cell culture monolayers microscopically, the generally long incubation period required for some viruses to produce CPE, the inability of some viruses to proliferate in traditional cell cultures, the expense involved in purchasing and maintaining cell cultures, and the availability and constant improvement of alternate technologies are all factors to consider when evaluating the long-standing gold standard of virus isolation in cell culture. Other advanced technologies, such as nucleic acid amplification tests (NAATs), have been applied to speed viral detection and decrease the level of technical expertise required for the process of viral isolation and identification. However, cell culture technology has also experienced innovative modifications, allowing virus isolation in cell culture to continue to make a significant contribution in viral disease diagnosis.
NEWER CELL CULTURE FORMATS
The traditional 16- by 125-mm screw-cap cell culture tube, which has been the standard for many years, is now only one of several different configurations in which cell cultures are used in viral diagnostic laboratories. A small vial, called a 1-dram vial or a shell vial, has become popular for containment of cell culture monolayers. In this configuration, the cell monolayer is grown on a coverslip that resides in the bottom of the vial. The small vials fit easily into a centrifuge for use in viral detection assays that involve centrifugation-enhanced inoculation of the monolayer. These assays, which facilitate rapid detection of viruses (especially those that grow slowly in traditional tube cultures) is described in “Centrifugation-Enhanced Inoculation and Pre-CPE Detection of Viruses in Cell Cultures” below. The inoculated vials take up much less space in incubators and are not usually incubated in rotating or rolling racks, as is done with traditional cell culture tubes. The cost of cell cultures in shell vials is the same as that of cell cultures prepared in traditional cell culture tubes (Table 1).
Cell monolayers are also available in microwell plates, sometimes called “cluster plates.” The plates feature flat-bottomed wells. The number of wells in a cluster plate may vary, ranging from 24 to 96, depending on the purpose for which it is used. A 24-well configuration is very popular, but even with the 24-well configuration, the user can determine how many of the wells will actually contain cell monolayers; anywhere between 6 and 24 wells may actually contain cells. The microwell plate arrangement is convenient to use because the entire plate may be positioned on the stage of the microscope for the purpose of observing the stained cell preparations in the wells. In addition, the microwell plate format facilitates centrifugation if desired. Both the shell vial and cluster plate configurations have taken on a greater role in the laboratory with the advent of the centrifugation-enhanced inoculation and pre-CPE assays as well as the development of transgenic and cocultivated cell lines (see “CENTRIFUGATION-ENHANCED INOCULATION AND PRE-CPE DETECTION OF VIRUSES IN CELL CULTURES,” “VIRUS ISOLATION IN COCULTURED CELLS,” and “VIRUS ISOLATION IN TRANSGENIC CELL LINES” below). The extensive use of cell cultures contained in shell vials and microwell plates allows some virologists to boast of “totally tubeless” virus isolation. Cross contamination of cultures is rare but can occur when using cluster plates (via splashing from well to well) and with shell vials (from cap to cap) (140). Therefore, technical procedures should be monitored and enforced to prevent carryover during both sample inoculation and detection manipulation.
CRYOPRESERVED CELL CULTURES
Although some diagnostic laboratories continue to prepare their own cell cultures in-house, many buy all cell cultures from commercial sources. Multiple cell lines are kept on hand in the virology laboratory to accommodate isolation of the common human viral pathogens. Purchased cell cultures are routinely shipped to the virology laboratory once or twice each week, with the actual transport process involving a variety of delivery services. Regardless of the delivery service used, including “express” couriers, the cell cultures may be stressed during transport by extremes in temperature; may be mishandled as they are packed, stacked, and loaded; or may be compromised by delays in delivery due to bad weather, holiday closures, and many other uncontrollable circumstances. All of these factors may contribute to suboptimal performance of the cell cultures (74). In addition, the virology laboratory must determine in advance how many culture tubes will be needed. If a circumstance such as an outbreak of a viral illness in the hospital or community occurs, the virology laboratory may not have sufficient numbers of culture tubes on hand to deal with the increased specimen volume.
The use of cryopreserved cells may help to minimize some of the issues involved with the in-house preparation of shell vials, tubes, or cluster plates. Virology laboratories that prepare cell culture material in-house could prepare cryopreserved cells, if desired. However, many clinical virology laboratories today rely solely on commercial vendors for all their cell culture products. In response to the need for cryopreseved cells, Diagnostic Hybrids, Inc. (Athens, OH) offers frozen preparations of a number of types of cells. These are named Frozen FreshCells. The suspensions for clinical virology applications are produced with cells at densities suitable for making monolayers that grow to confluence within 4 days from planting. The virology laboratory maintains these cell mixtures in the frozen state; the cells have an extended shelf life when stored according to the manufacturer's instructions. When there is a need for additional cell cultures or for a type of cell culture that is not routinely kept on hand in the laboratory, the frozen cell suspensions can be thawed and aliquoted into culture tubes or shell vials. Instructions and feeding medium are provided along with the frozen cell mixtures.
Another application of cryopreservation technology has made the routine use and maintenance of prepared cell lines even easier. This technology involves cryopreserved ready-to-use cell monolayers grown in shell vials that are shipped on dry ice and stored at −70°C (ReadyCells; Diagnostic Hybrids, Inc.). Prior to using these cells, the desired number of frozen vials is removed from the freezer and incubated in a 35 to 37°C water bath for 4 min. The freeze medium is removed and replaced with cell culture medium supplied by the manufacturer. The clinical sample is then added. Currently, cryopreserved monolayers of cells that are highly susceptible to chlamydiae (McCoy ReadyCells), HSV and CMV (Hs27 ReadyCells), and the various viral respiratory pathogens (R-Mix ReadyCells) are available. In comparison studies, these frozen monolayers performed with sensitivity comparable to that of standard cell cultures for the detection of HSV and influenza A and B viruses (74). In addition, cryopreserved cells were shown to have the following benefits (74): (i) they retain the same level of sensitivity and are stable for up to 4 months when stored at −70°C under proper storage conditions, (ii) they are always on hand for variable volumes of test requested, (iii) the purchase of a large number at the same time reduces interlot variations in testing and eliminates the costs and stresses involved with shipping, (iv) a new lot of cells can be subjected to quality control procedures prior to being put into use, and (v) tighter inventory control is possible because cell cultures are used only when needed.
CENTRIFUGATION-ENHANCED INOCULATION AND PRE-CPE DETECTION OF VIRUSES IN CELL CULTURES
Incubation of tube cell cultures in rotating or rolling racks has been shown to enhance viral replication (102). HSV tube cultures rolled at 2 rpm or 96 rpm showed CPE faster than tube cultures incubated in stationary racks. Tube cultures rolled at 2 rpm had a 2.4-fold increase in HSV foci over stationary tube cultures, and those rolled at 96 rpm had a 6.8-fold increase in foci over stationary tube cultures (102). The knowledge that movement enhances viral proliferation may have spurred interest in investigating the use of centrifugation to enhance infectivity in cell culture systems.
Originally used to enhance the isolation of Chlamydia trachomatis, a technique featuring cell monolayers grown on 12-mm round coverslips in 1-dram shell vials was adapted for use in virus isolation. Inoculation of a shell vial involves decanting the medium and placing the processed clinical sample directly on the monolayer. The entire inoculated vial is spun in the centrifuge at low speed (700 × g) for an hour, fresh culture medium is then added, and the vials are incubated at 35 to 37°C for the desired time period in an upright position with the cell monolayer covered by the cell culture medium Although the shell vial monolayer may be examined microscopically for CPE with the inverted microscope, detection of viral infection is usually performed at a designated time interval, and the detection method does not rely on CPE production by the virus. This pre-CPE detection routinely involves staining of the infected monolayer with horseradish peroxidase (HRP)- or FITC-labeled MAbs of the desired specificity to detect viral antigen in the infected cells. The coverslip may be removed from the vial and stained (32), or staining may be carried out while the coverslip remains in the vial (90). Stained coverslips are mounted on a microscope slide. Examination with the light (for HRP-labeled MAbs) or fluorescence (for FITC-labeled MAbs) microscope follows. This system typically speeds virus detection dramatically, compared with virus isolation in the traditional tube cell culture system. The exact mechanism by which centrifugation enhances the rate of viral infectivity is not known. Although it was initially assumed that accelerated infectivity resulted from forcing bits of virus-infected material against the monolayer, it is reported that the stressing of the monolayer cells by centrifugal force is the important factor (75). This has been shown to increase cell proliferation, decrease cell generation times, activate genes, alter cell metabolism, and increase cell longevity.
The shell vial system was initially adapted for use in virology in an attempt to speed CMV isolation. In traditional tube cell culture tubes, CMV is slow to produce CPE, typically requiring 10 to 30 days of incubation before CPE is detectable. This extremely slow proliferation of CMV in cell cultures, coupled with the increasing interest in CMV disease in the rapidly growing population of immunocompromised patients, was the catalyst that spurred investigation of approaches to speed CMV detection. Gleaves and colleagues (62) used MRC-5 cells grown on coverslips in shell vials and pioneered a method that involved low-speed centrifugation at the time of inoculation of the vials and ended, after a brief incubation period, with detection of viral antigen in the monolayer cells by staining of the monolayer with MAbs to early CMV proteins. They reported detection in 16 to 24 h of 90% of CMV-positive urine cultures. Others further investigated the technique for CMV detection in various types of specimens (91, 120, 129) and for CMV quantitation in peripheral blood granulocytes (19), generally showing significantly more positive samples detected in shell vials than in cell cultures. Sensitive and rapid CMV detection in shell vials was seen in specimens from most body sites; however, of concern was the 25% of positive blood samples detected by traditional tube culture alone (120). This study and others have prompted investigators to suggest that if culture alone is to be used, both shell vial and conventional tube culture systems must be used for optimal CMV recovery (103, 104, 120).
Centrifugation inoculation and pre-CPE detection by IF staining were rapidly adapted for use with viruses other than CMV. By changing the cell line grown on the coverslip and the specificity of the MAbs used for staining, detection of other viruses was easily facilitated. HSV (63), influenza virus (45, 130), mumps virus (56), various respiratory viruses (108, 117, 126), enteroviruses (155), adenoviruses (155), dengue virus (136), and VZV (15, 161) have been isolated in shell vials. Recent studies by Landry et al. (85) demonstrated the ability to detect hMPV by day 2 postinoculation in A549, HEp-2, and LLC-MK2 shell vials when stained with a MAb (MAb 8510; Chemicon International, Temecula, CA) to hMPV matrix protein.
Human diploid foreskin fibroblast cells inoculated with centrifugation-enhanced inoculation, incubated overnight, and stained with HRP-labeled MAbs against HSV type 1 (HSV-1) and HSV-2 produced stained plaques of infected cells that were large enough to be detected with the naked eye (168). This method of detecting HSV-positive results was as sensitive as that of observing for CPE for 10 days and typing by enzyme immunoassay (EIA). The HRP staining method often yielded results within less than 24 h of inoculation, whereas standard isolation based on CPE required an average of 3 to 4 days after inoculation for detection of a positive result. Centrifugation cultures of MRC-5 and primary rabbit kidney cells stained after 16 to 24 h of incubation with a direct IF stain or an indirect HRP stain were compared with standard tube cell cultures of MRC-5 and primary rabbit kidney incubated for 7 days for detection of HSV (121). The tube cell cultures had the best isolation rate, detecting more positive samples than any of the centrifugation culture-stain combinations. However, the primary rabbit kidney cells stained with HRP stain were the most sensitive combination, and the indirect HRP stain was more sensitive than the direct IF stain for both types of the centrifugation-enhanced cell cultures.
Through the use of centrifugation inoculation and pre-CPE detection methods, virus isolation in cell cultures has been accelerated and enhanced. Some advantages of this cell culture approach include the following: (i) viruses that replicate poorly and may require subsequent passages in tube cell cultures before detection can be definitely identified at the end of the initial incubation period in the shell vial, (ii) the time to positivity is shorter than that required for most traditional tube cultures, (iii) this approach may detect viruses that would not replicate in tube cell cultures, and (iv) the assays are relatively easy to perform and less subjective (require less expertise than reading for CPE).
As with other cell culture systems, the shell vial system is effective in virus isolation only when specimens are collected, transported, and stored properly to maintain the viability of the viruses. Although processing and reading of shell vials are time-consuming and labor-intensive, results for the detection of the most common viruses are available within 24 to 48 h. Hence, this adaptation of cell culture technology has enabled viral isolation in cell culture to provide a timely diagnosis that in many cases is useful for effective patient management. This approach has been applied with cocultured cells (see “VIRUS ISOLATION IN COCULTURED CELLS” below) and with transgenic cell lines (see “VIRUS ISOLATION IN TRANSGENIC CELL LINES” below). Because centrifugation cultures are routinely blind stained for a specific virus or viruses at a designated time interval rather than evaluated for CPE, only the viruses sought will be detected, and unanticipated viruses will be missed.
VIRUS ISOLATION IN COCULTURED CELLS
Techniques involving combinations of different cell types grown together as a single monolayer in a vial and the application of various MAbs, each labeled with a different fluorochrome, have been applied for the detection of several viruses in the same vial. Culturing for the simultaneous detection of adenovirus, CMV, and HSV in the same shell vial has been approached using a mixture of MRC-5 and A549 cells in the cell monolayer and staining with a cocktail of adenovirus, CMV, and HSV antibodies, each raised in a different species (17). A second antibody cocktail with labeled antispecies antibodies, each with a different label, was added next. Labels included FITC, Cy3, and 7-amino-4-methylcoumarin-4-acetate. Stained coverslips were examined first with an FITC filter in place on the fluorescence microscope and then again with a UV filter. This assay produced sensitivities of 93.8% for adenovirus, 88.9% for CMV, and 100% for HSV compared to individual tube cell cultures performed in parallel (17).
Currently, several commercially produced cocultivated cell lines are available (Diagnostic Hybrids, Inc.) for the rapid identification of a variety of viruses. The R-Mix rapid cell culture technique uses patented cell monolayers of mixed cells selected for their ability to isolate a variety of viruses that cause respiratory infections. R-Mix is comprised of A549 and mink lung (Mv1Lu) cells and is available as ready-to-use fresh cells in shell vials or cluster plates or as frozen cell suspensions that can be aliquoted by the laboratory (R-Mix Frozen FreshCells) or purchased frozen as monolayers in shell vials (R-Mix ReadyCells) (see “CRYOPRESERVED CELL CULTURES” above). Three R-Mix vials are inoculated for each clinical specimen in combination with a proprietary Refeed medium (Diagnostic Hybrids, Inc.). The vials are then centrifuged and incubated at 37°C in 5% CO2. After 18 to 24 h of incubation, one R-Mix monolayer is stained for the presence of viral antigens by using a pool of fluorescein-labeled MAbs directed against influenza A virus; influenza B virus; parainfluenza virus types 1, 2, and 3; adenovirus; and RSV (Fig. 3). If a specimen is positive, a second R-Mix monolayer is scrapped and cell spots made. The cell spots are then stained with individual fluorescent MAbs to identify the infecting virus(es). The third monolayer can be used to freeze down the isolate for use in strain typing or other studies if desired at a later date.
FIG. 3.
Immunofluorescence detection of respiratory pathogens in R-Mix cells. (A) Uninoculated R-Mix cells; (B) adenovirus; (C) influenza A; (D) influenza B virus; (E) parainfluenza virus type 1; (F) parainfluenza virus type 2; (G) parainfluenza virus type 3; (H) RSV. Magnification, ×170. Photos courtesy of Diagnostic Hybrids, Inc.
If the first R-Mix monolayer is negative at 24 h, a second R-Mix monolayer is stained with the MAb pool at 48 h. If positive, cell spots are made from the third monolayer and the virus(es) identified. Another option for follow-up if the 24-h monolayer is negative is examining a second R-Mix monolayer for CPE prior to blind staining for the respiratory viruses. Experienced virologists can also detect the CPE of other viruses such as HSV, CMV, and enterovirus in R-Mix (94a). If the second R-Mix monolayer is negative, the laboratory can elect to discard the culture, including the third monolayer, at 48 h, since the majority (approximately 98%) of viruses are recovered within this time period (51). Alternatively, the third R-Mix monolayer can be incubated after the 48-h period for an additional 5-day period and screened periodically for CPE. Slow-growing viruses, viruses of very low titer, and mixed infections can be recovered (94a).
Several investigators who compared the rate of detection of influenza A virus in R-Mix to that in tube cell culture, direct fluorescent-antibody assay (DFA) for antigen detection, or non-IF direct antigen testing demonstrated superior sensitivity of R-Mix. Sensitivities of influenza A virus detection were as follows: R-Mix, 96%; cell culture, 85%; and DFA, 67% (51) and R-Mix, 100%; cell culture, 67%; and non-IF direct antigen test, 66% (150). Fader (47) demonstrated that the detection of influenza A virus by using R-Mix cells was significantly more sensitive than detection by a rapid nonculture immunochromatographic antigen detection method (Binax NOW Flu A; Binax, Portland, ME). In comparison to R-Mix, the overall sensitivity, specificity, positive predictive value, and negative predictive value of the Binax NOW FluA assay were 64.9%, 98.4%, 89.3%, and 93.2%, respectively.
Comparisons of R-Mix to cell culture for detection of the respiratory viruses have yielded some conflicting results. Robinson et al. (134a) demonstrated equal detection sensitivity for R-Mix compared to A549 and RMK shell vials for the isolation of influenza A virus and parainfluenza virus types 1 and 3 but a lower sensitivity for R-Mix for the detection of both RSV (94% versus 100%) and adenovirus (88% versus 100%). Weinberg et al. (159) found R-Mix harvested at 48 h to have sensitivities and diagnostic accuracies comparable to those of traditional tube cultures for the isolation of influenza A virus, influenza B virus, RSV, and parainfluenza virus types 1 to 3. However, R-Mix yielded poor results for the detection of adenovirus. Lotikar et al. (94a) demonstrated equal sensitivity for the detection of influenza A and B viruses, parainfluenza virus, and adenovirus and enhanced sensitivity for RSV detection by R-Mix (63%) over cell culture (42%) when results were compared to those obtained by DFA (98%).
Dunn et al. (42) compared the sensitivity of R-Mix, screened only at 18 to 24 h, to those of both DFA and shell vial cultures that were observed for CPE for up to 10 days and tested by HAD if CPE was not detected. Respiratory viruses were identified in 152 of 711 specimens. DFA alone was positive for 37.5% of the specimens, and shell vial culture alone was positive for 20.4% of the specimens. Overall, in 18 to 24 h, R-Mix detected 87.1% of all respiratory viruses that were DFA negative and 96.7% of samples positive by both cell culture and DFA. The sensitivity for influenza viruses was 96.7% for R-Mix and 70.5% for DFA. DFA was more sensitive (94%) for the detection of RSV than R-Mix, which recovered RSV in only 27% of the DFA-positive samples. However, R-Mix detected an additional five RSV-positive samples identified as negative by DFA and was more sensitive than a combination of shell vial cultures of buffalo green monkey kidney (BGMK), A549, MRC-5, and RhMK shell vial cultures. R-Mix showed good sensitivity compared to 10-day shell vial cultures despite the fact that R-Mix was tested at 18 to 24 h only. Extending the R-Mix culture to at least 48 to 72 h may have significantly improved the overall detection rate for all viruses, including both RSV and adenovirus. The increased sensitivity of DFA over R-Mix and of R-Mix over cell culture for the detection of RSV was also demonstrated by Fong et al. (51) in testing seven RSV DFA-positive samples; only three were detected in R-Mix, and only one was detected in traditional cell culture. Espy et al. (46a) compared virus isolation in R-Mix to the Binax NOW Flu A/B (Binax, Inc.) antigen assay and a LightCycler (Roche Diagnostics Corp., Indianapolis, IN) reverse transcriptase PCR (RT-PCR) assay for the detection of influenza A virus in 617 respiratory tract specimens. In total, 92 specimens were positive by RT-PCR, of which 76 (83%) were confirmed by a secondary RT-PCR; 49 specimens were positive by R-Mix, and only 24 specimens were positive by rapid antigen testing.
A feature common to all studies was the significant improvement in time to positive results when using R-Mix versus traditional tube culture methods. Barenfanger et al. (7) determined that the turnaround time for R-Mix for positive specimens was 1.4 days, versus 5.2 days for tube cell cultures. Fong et al. (51) showed that 95% of positive samples were detected by 48 h in R-Mix, compared to 6 days for 98% of samples to be positive by cell culture. The rapid detection of respiratory viruses is essential for the prompt initiation of appropriate antiviral therapy, to reduce the unnecessary use of antibiotics, and to ensure appropriate infection control measures.
R-Mix cells have also been evaluated for the growth of viruses that are not typically isolated in cell cultures. Gillim-Ross et al. (57) evaluated multiple human and animal cell lines by using a multiplex RT-PCR for the detection of SARS-CoV. The RT-PCR assay targets (i) glyceraldehyde 3-phosphate dehydrogenase, as an internal control for RNA integrity and cDNA production; (ii) SARS-CoV genomic RNA, for the detection of input virus; and (iii) the SARS-CoV 3′-coterminal specific subgenomic RNAs, which are indicative of virus entry and specific for initiation of viral replication. Vero E6 cells produced the highest titer of SARS-CoV, and this was the only cell line that demonstrated CPE. R-Mix (Mv1Lu component), primary RhMK cells, primary cynomolgous monkey kidney cells, and the human cell lines HEK-293T and Huh-7 also supported the growth of SARS-CoV, although CPE was absent.
Due to the highly pathogenic nature of SARS-CoV, most laboratories do not want to isolate this virus in culture. To reduce the risk of potentially growing this virus to high titers in routine cell cultures or R-Mix, clinical laboratories can select an alternative cocultured cell line called R-Mix Too (Diagnostic Hybrids, Inc.). This cell line is comprised of Madin-Darby canine kidney (MDCK) cells and A549 cells, both of which have been shown to be unable to support the growth of SARS-CoV (57) or other coronaviruses but to be very sensitive for isolation of respiratory viruses. The performances of R-Mix and R-Mix Too have been compared. By testing 100 nasopharyngeal aspirate supernatants stored at −70°C, Eskey et al. (44a) found that R-Mix Too shell vials were more sensitive than R-Mix for the detection of respiratory viruses (76% versus 62%, respectively), particularly adenoviruses and influenza B viruses. Wilkey et al. (161a) found almost comparable results for R-Mix (detected 67/67 positive samples) and R-Mix Too (detected 64/67 positive samples). Observations by users of R-Mix have suggested that R-Mix cells do not always permit efficient passage of the virus to RhMK cell lines. To address this issue, viruses detected in both cell lines were passed to RhMK cell cultures (161a). R-Mix permitted the successful propagation of 16/44 influenza viruses and 12/15 parainfluenza viruses and adenoviruses. R-Mix Too permitted the successful propagation of 24/44 influenza viruses and 12/15 parainfluenza viruses and adenoviruses. In addition, the R-Mix Too cells demonstrated enhanced propagation in 10 of the subcultured influenza virus-positive samples, showing increased fluorescent staining compared to R-Mix-passed RhMK cultures. No overall difference in fluorescent staining was observed for the parainfluenza virus- and adenovirus-positive samples passed from R-Mix or R-Mix Too into RhMK cultures. Karchava et al. (76b) demonstrated that the sensitivities of R-Mix and R-Mix Too were comparable for the detection of influenza A virus (all at day 1). However, for 2 of 13 influenza A virus-positive cultures, the genomic copy titers in R-Mix Too were more than 1 log lower by plaque titration than those for R-Mix. The remaining 11/13 influenza A virus-positive cultures had comparable genomic copy titers, i.e., within 1 log. Additional studies are necessary to accurately determine the level of propagation of influenza A virus in these two cell lines. The ability to successfully propagate respiratory viruses, in particular influenza A and B viruses, from R-Mix and/or R-Mix Too into standard cell lines is important when a sufficient virus titer is required for susceptibility testing or strain typing, which is needed for epidemiologic studies and vaccine strain selection. Currently, no studies that evaluated R-Mix and R-Mix Too for the growth of avian influenza virus strains (H5 and H9) have been published. However, avian influenza virus has been shown to propagate in A549 and MDCK cells, suggesting that both R-Mix and R-Mix Too cultures should support the growth of the avian influenza virus strains (39, 48, 165).
Setterquist and Gray (143a) compared LLC-MK2 shell vials versus R-Mix by using three low-passage hMPV clinical isolates with identical low-level inocula. RT-PCR, targeting the F gene, was performed from the culture supernatants of each cell line at days 3, 6, 10, and 13, and the resulting DNA band intensities were compared over time to the intensity of the PCR band derived from testing the initial inoculum. For two isolates, the RT-PCR band generated from both cell lines was generally of the same intensity as that from the inoculum on day 3. The R-Mix band intensity increased on day 6 for both isolates; the signal waned by day 10 and then increased again on day 13. This is in contrast to the LLC-MK2 bands, which increased in intensity beginning at days 10 and 13, respectively, for two isolates. For the third isolate, weak bands were detected through day 6 for both cell lines; however, by days 10 and 13, the R-Mix band disappeared while the LLC-MK2 band increased in intensity. Although a molecular technique was used for hMPV detection in this study, it was shown that R-Mix cells will support the growth of hMPV. Petrich et al. (121a) compared hMPV detection in respiratory samples by three methods: DFA using hMPV MAb DFA reagents (Diagnostic Hybrids, Inc.), R-Mix culture with hMPV MAb blind staining at 48 h, and an in-house RT-PCR assay targeting the hMPV nucleoprotein. The sensitivities of the RT-PCR, DFA, and R-Mix cultures for the detection of hMPV were 100%, 91.8%, and 85.7%, respectively. Two additional studies (13a, 67a) demonstrated that hMPV can be detected in R-Mix culture after 24 to 48 h of incubation and subsequent IF staining with the Diagnostic Hybrids hMPV MAb reagent.
Yang et al. (163) compared rates of CMV recovery in R-Mix, Mv1Lu, and MRC-5 cells when CMV TurboTreat (Diagnostic Hybrids, Inc.) was used. TurboTreat pretreatment includes Earle's minimum essential medium with Earle's balanced salt solution without phenol red, 10% fetal bovine serum, 25 mM HEPES, and 50 mg/ml gentamicin. Monolayers of the three cell lines were plated in 48-well plates and treated with CMV TurboTreat, either overnight or for 4 h, or left untreated and then inoculated with a variety of frozen, previously tested CMV-positive clinical samples (respiratory, urine, biopsy, etc.). TurboTreat enhanced detection two- to threefold after 4 h of treatment and four- to sixfold after overnight treatment in Mv1Lu and R-Mix cells and to a lesser extent in MRC-5 cells. Mv1Lu cells treated overnight, treated for 4 h, or untreated detected 23, 21, and 15 positive specimens, respectively. R-Mix cells detected 19, 18, and 14 positive specimens, respectively, and MRC-5 cells detected 16, 15, and 15 positive specimens, respectively.
In summary, R-Mix and R-Mix Too cells provide a rapid and sensitive method for the identification of the most common respiratory viruses, without requiring the expertise associated with traditional cell culture. Detection of RSV and adenovirus may require DFA and/or supplemental cell lines to be added to optimize recovery. In addition, other viruses such as CMV, HSV, and enterovirus can be recovered in R-Mix, although these cells may not be best for optimal recovery. Finally, R-Mix and R-Mix Too, although not as sensitive as molecular-based methods, provide a reasonable and efficient method for laboratories that currently do not perform molecular testing.
Another cocultivated cell line, H & V Mix FreshCells (Diagnostic Hybrids, Inc.), is comprised of a mixed monolayer of African green monkey (Cercopithecus aethiops) kidney cells (strain CV1) and MRC-5 cells. In combination, these cells support the detection of many viruses, in particular HSV-1, HSV-2, and VZV (72). Specimens are inoculated into H & V shell vials and centrifuged at 700 × g for 1 h at 24°C before incubation at 36°C in a CO2 incubator. Cultures are examined daily for 5 days for CPE, and if CPE is detected, infected cells are typed using HSV-1- and HSV-2- or VZV-specific MAbs (Fig. 4). Alternatively, shell vials can be pre-CPE stained at day 2 for VZV to shorten the time to detection. The advantages of H & V cells for VZV include more isolates detected, faster recovery, and, for HSV, a final result in 2 days (72). Huang et al. (72) concluded that H & V cells were as sensitive for the detection of HSV as were shell vial cultures of primary rabbit kidney cells and Mv1Lu cells pre-CPE stained at 2 days of incubation. VZV CPE in H&V cells was apparent by day 3 to 4, with many large foci; MRC-5 and A-549 shell vial cultures demonstrated fewer and smaller foci that were difficult to see at day 5. Sensitivity for VZV detection by CPE was 100% for H & V cells, 82% for MRC-5 cells, and 70% for A-549 cells when atypical vesicular lesions were cultured. In addition, the time to detection could also be reduced by pre-CPE staining for VZV at 48 h. In the same study, Huang et al. (72) also evaluated the sensitivity of H & V cells versus DFA, since several previous studies have demonstrated that DFA is more sensitive than cell culture for the detection of VZV (33, 61). The detection of VZV CPE from 21 fresh specimens in H & V cells was found to be at least as sensitive as antigen detection by DFA (100% for H & V cells versus 90.5% for DFA). Based on these findings, the authors concluded that since DFA is not 100% sensitive, negative samples should be backed up by culture. Of further interest, in this study 1% of the specimens submitted for HSV testing were found to be VZV positive in H & V cells.
FIG. 4.
Immunofluorescence detection of Herpesviridae family viruses in H & V cells. (A) Uninoculated H & V cells; (B) CMV; (C) VZV; (D) HSV-1; (E) HSV-2. Magnification, ×170. Photos courtesy of Diagnostic Hybrids, Inc.
The ability to detect both HSV and VZV in a single culture system is advantageous for several reasons. The appearances of HSV and VZV lesions are often similar, leading to inaccurate differentiation based on clinical presentation alone. Physicians caring for immunocompetent patients in dermatology settings must be aware of lesions that resemble shingles but are really HSV lesions and vice versa. Inaccurate discrimination of disease caused by HSV or VZV in immunosuppressed patients can result in poor infection control, can compromise patient therapy, and may result in extended hospitalization.
H & V cells are also well suited to detect other viruses. Diagnostic Hybrids, Inc., indicates that more CMV inclusions (Fig. 4) are detected in H & V Mix than in MRC-5 cells alone (Diagnostic Hybrids, Inc., personal communication). Because both cell lines in H & V Mix, when cultured singly, will support the propagation or detection of mumps virus, it is assumed that H & V Mix should be useful in mumps virus isolation. Likewise, viruses such as measles virus, rotavirus, poliovirus type 1, simian virus 40, and some encephalitis viruses, which proliferate in CV1 cells cultured singly, and adenovirus, CMV, echovirus, influenza virus, poliovirus, rhinovirus, mumps virus, and RSV, which grow in MRC-5 cells cultured singly, can be expected to grow in H & V cells.
The cocultivated cell preparations offer many advantages over traditional individual cell lines. Their use eliminates the need to keep a large variety of different cell lines on hand in the virology laboratory. Since these cocultivated cells are available in various configurations, they are useful for the techniques of centrifugation-enhanced inoculation and pre-CPE detection that have been so very important in speeding virus detection and identification. In addition, because some of these cocultured cells are also available as cryopreserved cells, inventory control is simplified. Lotlikar et al. (94b) compared costs of virus isolation from 3,864 respiratory samples in R-Mix cultures in cluster plates (tested in triplicate and held for 7 days) and in traditional tube cultures (four cell lines held for 14 days). Although R-Mix cells were more expensive than traditional tube cultures, the overall cost savings for the laboratory was significant (approximately $29,000), because less technical time was required for the R-Mix cultures than for reading, refeeding, HAD testing, and staining of traditional tube cultures. In contrast, Barenfanger et al. (7) found that the overall cost was 11% more for R-Mix than for traditional tube cultures. However, they concluded that the added cost of R-Mix is offset by enhanced time to detection. The price of cocultured cells is only somewhat higher (approximately $1.25 more per vial than for nonprimary cells and $0.75 more per vial than for primary cells) than that of other cell lines cultured singly in shell vials (Table 1).
VIRUS ISOLATION IN TRANSGENIC CELL LINES
In order to improve both the speed and accuracy of virus detection in cell cultures, the powerful tool of transgenic technology was tapped. Transgenic technology, together with increasing knowledge of the molecular pathways of virus replication, offered the possibility of using genetically modified cell lines to improve virus growth in cell culture and to facilitate the detection of virus-infected cells (116). Transgenic technology has long been explored in the research arena but has been used in the clinical virology laboratory for only the past 10 years or so. The application of transgenic cells in cell cultures involves the stable introduction of genetic elements into a cell such that when a virus, and only a particular virus, enters this cell, a virus-specific event is triggered that results in the production of an easily measurable enzyme. This strategy provides a simple and virus-specific detection system. The genetic elements can be derived from viral, bacterial, and cellular sources (116) and are called virus-inducible reporter gene segments. Variables in transgenic technology are the viral promoter used, the cell type transformed, and the reporter gene used. For transgenic cells to be useful in the diagnostic laboratory, the desirable features include a promoter that is “quiet” in uninfected cells, is sufficiently up-regulated by the viral transactivator proteins, and has a specificity that does not allow heterologous viral transactivating proteins to activate the promoter (116). For a transgenic system to work, the virus being detected must be able to bind to the cell, enter the cell, and initiate its replicative cycle, which does not need to go to completion but must be sufficient to trigger the reporter gene via the viral promoter. An extensive explanation of the process and progress of transgenic cell development and applications for detecting viruses was published previously (116).
Various attempts during the late 1980s to develop transgenic cell lines susceptible to human immunodeficiency virus type 1 (HIV-1) met with limited success. A CD4-positive lymphoid cell line transformed with a retrovirus vector containing an HIV-1 long terminal repeat (LTR) promoter linked to a chloramphenicol acetyltransferase gene (49) and HeLa (HT4 cells) transformed through insertion of the CD4 gene and a lacZ gene behind the HIV-1 LTRE promoter (31) were developed. Unfortunately, transactivation was not specific for HIV-1; simian immunodeficiency virus and human T-cell lymphotropic virus type 4 could both transactivate, although at a much lower level than HIV-1. Although not useful for initial HIV-1 diagnosis, both transgenic lines were proposed as being potentially useful in screening anti-HIV agents (49, 141).
Transgenic technology was successfully applied in the identification of a poliovirus receptor through the use of mouse L cells transformed with a cDNA library from poliovirus-susceptible HeLa cells (123). The poliovirus receptor, once identified, was then expressed in mouse L cells (L20B), making the previously nonsusceptible mouse cells susceptible to poliovirus infection in vitro. When these cells were tested in parallel with human HEp-2C cells for detection of poliovirus, the transgenic L20B cells were only slightly less sensitive than the HEp-2C cells but had the advantage of being nonpermissive for other enteric human picornaviruses.
A transgenic cell line that has been successfully incorporated for routine use in diagnostic virology laboratories was designed for sensitive and specific detection of both HSV-1 and HSV-2. HSV rapidly produces CPE in many types of cell cultures, and identification/typing is typically completed by the use of staining with MAbs. HSV can be detected, often within 16 to 24 h of inoculation, when techniques involving centrifugation-enhanced inoculation in shell vials and pre-CPE staining are used. However, these types of detection are relatively labor-intensive and may require substantial expertise on the part of the virologist. In addition, some HSV isolates will not be detectable until 48 h of incubation. To more rapidly and more easily detect HSV, a transgenic system was developed that would allow HSV detection within 24 h but not require significant technical expertise or expensive MAbs. The transgenic system features the use of an HSV promoter sequence derived from the UL39 gene, which encodes the large subunit of ribonucleotide reductase. The UL39 promoter and the Escherichia coli lacZ gene were used to stably transform a baby hamster kidney (BHK) cell line, resulting in the BHKICP6LacZ cell line (147). lacZ gene expression is low in uninfected cells. Upon infection with either HSV-1 or HSV-2, the promoter is strongly transactivated by the virion-associated transactivator protein VP16 and other HSV transcriptional transactivators such as ICP0 (116). This activation of the lacZ gene appears to be highly specific for HSV-1 and HSV-2 only, and expression from this promoter occurs within hours after infection. The activation of the UL39 promoter in turn activates the lacZ gene, resulting in the production of β-galactosidase. Detection of β-galactosidase is accomplished by the addition of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), a chromogenic β-galactosidase substrate that turns from colorless to blue in the presence of β-galactosidase. HSV-infected cells are stained blue by this reaction. (Fig. 5) This color change is easily evaluated, and infected cells/plaques may be conveniently counted.
FIG. 5.
Detection of HSV-1 and HSV-2 in ELVIS cells. (A) Blue cells positive for HSV with X-Gal stain; (B) immunofluorescence of uninoculated ELVIS cells; (C) HSV-1-positive ELVIS immunofluorescence (note nuclear pattern); (D) HSV-2-positive ELVIS immunofluorescence (note cytoplasmic pattern). Magnification, ×170. Photos courtesy of Diagnostic Hybrids, Inc.
In initial comparisons of BHKICP6 transgenic cell cultures and traditional cell cultures for isolation of HSV from clinical samples, 31 of 31 (100%) of HSV-positive samples were detected by both culture systems (148). However, in 15 of the 31 positive specimens, CPE was not evident for 2 days or more in traditional tube cell cultures; in contrast, the transgenic system was positive in all cases by 16 to 24 h. There was one possibly false-positive result with the transgenic system among the 64 samples that showed no CPE in the traditional tube culture system; this sample showed one blue-stained cell. The transgenic system was shown to be both sensitive and specific in comparison to tube cell cultures. In addition, the technical expertise required for microscopic evaluation of CPE and the expensive MAbs needed to confirm virus identity in traditional cultures were not needed with the transgenic system. The BHKICP6 transgenic system received clearance from the U.S. Food and Drug Administration (FDA) and is currently marketed in shell vials by Diagnostic Hybrids, Inc., under the acronym ELVIS (for enzyme-linked virus-inducible system).
Comparisons of the HSV ELVIS with CPE detection in traditional tube cell cultures (89) and with shell vial cultures either observed for the appearance of CPE (125) or tested by pre-CPE staining with MAbs (36) have all shown ELVIS to be both sensitive and specific. ELVIS detected HSV in 1 day in positive cultures, 40% of which were CPE negative at the same time interval (125), and was as sensitive as shell vial culture and pre-CPE staining with FITC-labeled MAbs (36). ELVIS testing of ocular samples showed 85 to 86% sensitivity compared to cell culture in both retrospective and prospective assessments (79). ELVIS cells have also been used for determining neutralizing serum antibody titers to HSV (5). The blue color of the infected ELVIS cells was evaluated by an objective colorimetric readout that provided a value that could be compared to a calibration curve.
The original ELVIS for HSV detection provided identification of HSV but did not facilitate typing of the virus as HSV-1 or HSV-2. Because differentiation of the two HSV types was desired by many, the original ELVIS was modified to facilitate the differentiation and marketed as the ELVIS HSV ID/Typing system (Diagnostic Hybrids, Inc.). In the new ELVIS, two MAbs, one directed against HSV-1 proteins and one directed against HSV-2 proteins, were added to the X-Gal detection reagent. These MAbs bind with the specific HSV proteins in the infected cells. The antibody directed against HSV-2 is fluorescein labeled. Evaluation of results in this system begins, as it does in the original ELVIS, with an examination of the shell vial for the presence of blue-stained cells. Their presence confirms that the sample is positive for HSV. The blue-stained specimen is then examined under the fluorescence microscope for the presence of cells with an apple-green cytoplasmic pattern. If apple-green specific staining is observed, the presence of HSV-2 is indicated (Fig. 5). Cultures with blue cells that do not fluoresce are considered negative for HSV-2. The specific HSV-1 MAb is unlabeled and must be identified by applying a fluorescein labeled goat anti-mouse immunoglobulin G antibody to the monolayer. This secondary antibody binds to any unlabeled HSV-1 mouse monoclonal antibodies that bound during the initial staining step. The HSV-1 cells stain with an apple-green nuclear pattern (Fig. 5).
This system has been shown to accurately and rapidly detect and type HSV-1 and HSV-2 (154). A very small percentage (i.e., less than 1%) of low-titer samples may produce blue staining initially and negative results for specific typing of both HSV-1 and HSV-2 in the ELVIS HSV ID/Typing system. Subsequent evaluation of these samples, by repeating the ELVIS assay and/or by PCR testing of infected cells from the ELVIS monolayer, has shown that the original ELVIS result was correct (i.e., the samples are HSV positive). This issue with low-titer specimens occurs with equal frequency with HSV-1- and HSV-2-infected samples (119). Hill et al. (68a) compared the ELVIS HSV ID/Typing system to a LightCycler PCR HSV 1,2 detection kit (Roche Applied Science, Indianapolis, IN) and conventional shell vial by using primary rabbit kidney and MRC-5 cells. The sensitivity, specificity, positive predictive value, and negative predictive value for each method were as follows: for PCR, 93%, 99%, 99%, and 96%, respectively; for shell vial culture, 84%, 100%, 100%, and 91%, respectively; and for ELVIS, 79%, 100%, 100%, and 89%, respectively. Although ELVIS was less sensitive overall than PCR and shell vial cultures, results were available in 1 day for 13% more of the positive samples than for other shell vial cultures, which took up to 5 days to detect many positive samples. Bankowski et al. (5a) compared PCR and ELVIS and determined that PCR was more sensitive than ELVIS (100% versus 89.9%), but both methods were 100% specific for generic HSV detection. However, PCR was more specific for HSV-1 and HSV-2 typing (100%) than ELVIS (95.5%).
C. Ginocchio et al. (unpublished data) compared the costs of ELVIS and traditional tube culture for 71 specimens, of which 35 were HSV positive and 36 were HSV negative. Including cells, media, reagents, and technical time, the cost per test for ELVIS ($10.76) was significantly less than that for traditional tube culture ($29.86). The list price of ELVIS shell vials is approximately $3.00 more per vial than that of nonprimary cells and $2.25 more per vial than that of primary cells cultured singly in shell vials (Table 1).
Although among the enterovirus group, which includes the polioviruses, the coxsackieviruses, the echoviruses, and others, there is much similarity in terms of structure and surface antigens, there is no single cell line that allows proliferation of all enterovirus strains. If enterovirus was the viral suspect in a clinical sample, multiple cell lines such as primary monkey kidney, A-549, BGMK, human embryonic lung fibroblasts, and other lines had to be inoculated (78). Production of detectable CPE in most of these cell lines generally requires 5 or more days. Although use of centrifugation-enhanced inoculation of shell vial cultures of enterovirus-susceptible lines sped detection, use of multiple vials was required (145). She et al. (145) found that primary monkey kidney and MRC-5 shell vials recovered the majority of enterovirus isolates, and the addition of BGMK and human rhabdomyosarcoma (RD) shell vials increased the recovery rate by 13%. Primary monkey kidney and BGMK shell vials were effective in isolating coxsackieviruses, and RD and MRC-5 shell vials were useful particularly in isolating echoviruses. CPE was found in only a single cell line for 52.6% of the enteroviral isolates.
Several of the enteroviruses, as well as all of the types of echovirus that can hemagglutinate human eyrthrocytes, can bind to a particular receptor in human cells. The receptor is human decay-accelerating factor (hDAF), which is also known as CD55. This is a 70-kDa glycosylphosphatidylinositol-anchored glycoprotein involved in the regulation of complement activation and in cell signaling (95). Echoviruses 3, 6, 7, 11, 12, 13, 19, 21, 24, 25, 29, 30, and 33; coxsackieviruses A21, B1, B3, and B5; and enterovirus 70 all interact with hDAF during the process of entry into a cell. BGMK cells, which are sensitive for isolation of coxsackieviruses B but relatively insensitive for echovirus detection, were transfected with hDAF to produce BGMK-hDAF, an engineered cell line with expanded host range and increased sensitivity in the detection of enteroviruses compared to wild-type BGMK cells. When 17 enterovirus-positive samples were cultured in both BGMK and BGMK-hDAF cells, the BGMK-hDAF cells were far superior, isolating more than double the number of enteroviruses (73).
The coculturing of the transgenic BGMK-hDAF cells with CaCo-2, a human colon adenocarcinoma cell line, produced a cell culture combination that was more sensitive than other cell lines in isolating enteroviruses from 34 known-positive frozen clinical samples. The BGMK-hDAF/CaCo-2 cultures detected 97% of the positive samples, compared to MRC-5 (76.5%), RhMK (76.5%), CaCo-2/BGMK (85.3%), A-549/BGMK (82.4%), and H292/RD (82.4%) cells (73). This cocultured combination was commercially marketed as Super E-Mix (Diagnostic Hybrids, Inc.). Buck et al. (18) compared these Super E-Mix cells with two RT-PCR methods and conventional cell culture for the diagnosis of enteroviral meningitis. As expected, the detection of enterovirus in CSF was most sensitive by the two different RT-PCR methods (93% and 88%). Although the sensitivity of the Super E-Mix cells (76%) was lower than that of RT-PCR, it was still significantly better than that of routine cell culture (51%).
The Super E-Mix cell line has been modified to increase the breadth of enterovirus type recovery and now contains A549 cells in place of CaCo2 cells. This new combination has replaced the old Super E-Mix product, which is no longer on the market. The combination of Super BGMK and A549 cells in Diagnostic Hybrid's Super E-Mix potentially provides the capability to detect all known culturable enteroviruses in a single vial. Evaluations of the capacity of Super E-Mix to isolate the parechoviruses have not been published. According to the manufacturer's procedure, each specimen is inoculated onto triplicate Super E-Mix monolayers, centrifuged, and incubated for up to 5 days. One shell vial is fixed and stained at 24 and another at 72 h postinoculation, using class-specific (enterovirus) antibodies for the early detection of the virus by fluorescence immunoassay. The third monolayer is used for confirmatory staining, if required, and examined for 5 days for CPE. If CPE is found on or before day 5 or if on day 5 no CPE is found, then the monolayer is fixed, stained using fluorescence immunoassay, and examined for fluorescence (Fig. 6).
FIG. 6.
Detection of coxsackievirus B in Super E-Mix cells. (A) Unstained, uninoculated cells; (B) unstained coxsackievirus B CPE; (C and D) immunofluorescence staining with a pan-enterovirus antibody pool of uninoculated Super E-Mix cells (C) and coxsackievirus B-infected cells (D). Magnification, ×170. Photos courtesy of Diagnostic Hybrids, Inc.
Vestal et al. (156a) compared Super E-Mix and conventional tube culture using MRC-5 and RhMK cells for the detection of enterovirus in 1,650 clinical samples. Super E-Mix had a sensitivity of 92% and specificity of 100%, compared to a sensitivity of 63% and a specificity of 100% for tube culture. Lotlikar et al. (94b) compared Super E-Mix, R-Mix, and routine cell culture, using RhMK, A-549, and MRC-5 cells for the recovery of enterovirus from CSF, rectal, and respiratory samples. For all sample types the sensitivities of R-Mix and Super E-Mix were equivalent (CSF samples, 81.3%; rectal samples, 85.3%; respiratory samples, 100%) but were significantly higher than those for routine cell culture (CSF samples, 37.5%; rectal samples, 41.2%; respiratory samples, 50%). The benefit of Super E-Mix over R-Mix was a shorter time to detection; 8 of 50 positive samples were detected 1 to 5 days sooner in Super E-Mix than in R-Mix. Additional studies by the laboratory of C. C. Ginocchio (unpublished data) found that the sensitivities of traditional cell culture, R-Mix, Super E-Mix, and a NucliSens nucleic acid sequence-based amplification (NASBA) method (bioMérieux, Durham, NC) for enterovirus detection in CSF were 41.9%, 76.3%, 76.3%, and 93.5%, respectively. The NASBA method was the most sensitive; however, the increased sensitivity of enterovirus detection from CSF using Super E-Mix and R-Mix, relative to isolation in traditional tube cell culture, significantly improved the time to diagnosis. Despite the fact that it has been clearly shown in numerous studies that molecular methods are far superior to culture for the detection of enterovirus in CSF (60, 112, 128, 135, 157), many laboratories currently do not have the ability to perform such testing in-house. As molecular test methods become more routine and available on simple platforms such as the GeneExpert (Cepheid, Sunnyvale, CA), all laboratories should make every effort to convert from culture-based systems to molecular methods for the detection of viruses in CSF. However, in the interim period, the use of enhanced cell culture systems such as Super E-Mix cells can improve both the rate of detection and the time to detection over those with traditional culture methods. In addition, the detection of enterovirus in Super E-Mix in 1 day may provide a diagnosis in a time frame that could have an impact on patient treatment and length of hospitalization. A 1-day turnaround time for enterovirus detection in Super E-Mix can be comparable to the turnaround time of laboratories that perform molecular tests in-house only once per day, which is often the case. In these instances samples arriving in the laboratory late in the day may not have molecular testing performed until the next day, at which time a Super E-Mix culture may already be positive. In addition, enterovirus detection in Super E-Mix within 24 h represents a significantly improved turnaround time for hospitals that must refer all molecular testing to a distant reference laboratory.
A genetically engineered human embryonic kidney 293T cell line with a reporter gene inducible by influenza A virus has been developed (96). An RNA polymerase I promoter/terminator cassette was used to express RNA transcripts encoding green fluorescence protein or firefly luciferase flanked by the untranslated regions of the influenza A/WSN/33 virus nucleoprotein segment. Reporter gene activity is expressed within 6 h after the cells are infected with influenza A virus, and the expressed luciferase is detected using a chemiluminescence detection system. This system was tested by varying the quantity of viral inoculum and by adding hemagglutinin-specific antibodies or amantidine to the transgenic cells, demonstrating that the system could be useful in identifying, quantitating, subtyping, and determining antiviral drug susceptibility of influenza A virus (96).
The infectivities of multiple strains of influenza A virus in the engineered 293T cells, nonengineered 293T cells, RD cells, and Mv1Lu cells were compared (76a). The engineered 293T cells and RD cells were shown to be comparable to Mv1Lu cells in permissiveness in the rapid detection of multiple strains of influenza A virus and to express luciferase only when infected by influenza A virus and not with a panel of other respiratory viruses, including influenza B virus.
In addition to the rapid and sensitive detection provided by centrifugation-enhanced pre-CPE detection of virus that is facilitated by shell vial cultures in general, transgenic cells provide the advantage of simplified and cost-effective final virus identification. Transgenic lines produce a unique detectable product only when infected by a particular virus. The substance is detectable by color change reactivity, rather than by relying on reactivity with expensive monoclonal antibodies. The ELVIS HSV system can be used in laboratories that do not offer any viral culture testing but wish to provide virus detection of a single virus, HSV. The equipment and reagents needed are supplied by the manufacturer, and because no special technical expertise is needed, the system can be incorporated with ease into the routine of a diagnostic microbiology laboratory.
Because infectivity is required for positivity in the transgenic systems, this culture system, like others already described, can differentiate infectious from noninfectious virus. This is in contrast to most molecular and antigen assays, which show positivity for both viable and nonviable virus. This differentiation can be useful when trying to assess a response to antiviral therapy and to differentiate active versus latent infection. Of course, this benefit is accompanied by the necessity to ensure proper specimen selection, collection, transport, and storage that preserve viral infectivity. With transgenic cells, if any virus other than the one sought is present, it will remain undetected.
NONCULTURE METHODS COMPARED TO VIRUS ISOLATION IN CELL CULTURES
IF Methods
Detection of viral antigens by IF methods performed directly on clinical samples was used as early as the 1970s. These methods provided the virology laboratory with an approach for virus detection that did not rely on proliferation and isolation of the virus in cell culture. The 1980s saw the development of a variety of high-quality MAbs directed against HSV-1, HSV-2, and VZV and against seven common respiratory pathogens: adenovirus; influenza A and B viruses; parainfluenza virus types 1, 2, and 3; and RSV. Initially, the rapid methods for viral antigen detection were based on IF staining involving a two-step sandwich “indirect” fluorescent-antibody assay (IFA) methodology that required 2 h or so for completion. Many of these original two-step IF methods have evolved into one-step direct (DFA) methods in which the primary (and only) antibody is labeled with a fluorescent dye. These staining protocols require only 20 to 30 min. Compared with virus isolation in cell cultures, the rapid IF methods for viral antigen detection generally show excellent specificity and very good sensitivity. Performance characteristics will depend on the virus detected, specimen type, age of the patient, time of sample collection after onset of symptoms, MAb reagents, and level of expertise of the performing laboratory (127). Overall, IF is somewhat less sensitive (with the exception of RSV and VZV detection) and less specific than cell culture.
HSV antigen detection by IF in all types of samples is less sensitive than virus isolation in cell culture. Reported sensitivities range from 44% (90) to 95% (86) but can be 70 to 95% when testing is performed by experienced technologists on early genital or oral lesions (50). VZV antigen can also be detected by IF techniques (29). This has been shown to be a more sensitive method (92 to 97.5%) than traditional tube (49 to 64%) and centrifugation (59 to 79%) cultures (33, 61). The IF method is very useful in rapidly distinguishing HSV and VZV lesions, a determination which may be critical for patients with atypical lesions.
Within the past decade, IF methods, called CMV antigenemia assays, have been applied for direct detection and quantification of CMV antigens in peripheral blood leukocytes (65, 103, 104). A thorough review of CMV antigenemia methods and clinical applications has been published previously (10). These methods involve separation of the leukocytes from anticoagulated peripheral blood, quantitation of the leukocytes by manual or automated counting, preparation of smears of quantitated leukocytes on microscope slides via cytocentrifugation (usually 300,000 leukocytes are used), and IFA staining of the smears with MAbs against pp65 early matrix proteins of CMV. The assay can be completed in 3 to 4 h. The stained smears are examined for fluorescence, and the number of fluorescing cells is determined. Because the actual number of leukocytes that are transferred onto the slide during centrifugation is not monitored, the assay is semiquantitative rather than quantitative. The original methods used HRP stains, but FITC quickly replaced HRP. CMV detection in 3 to 4 h via antigenemia assays was a major advance in dealing with detection of CMV, which can require 10 to 30 days to produce CPE in traditional tube cell cultures (65, 103, 104). An added advantage is the semiquantitative numerical value (number of fluorescent cells per number of leukocytes per slide) obtained. The significance of the number of fluorescent cells varies, depending on the nature of the patient's immunosuppression. Numerical CMV antigenemia values are useful in confirming infection, suggesting prognosis, and determining response to therapy (151). CMV antigenemia assays, the reagents for which are commercially available from several sources, have been shown to be specific and more sensitive than CMV isolation in conventional or shell vial cell cultures (16, 44, 149). However, the antigenemia assay is particularly labor-intensive and time-consuming, especially when large numbers of samples are tested. Other disadvantages of the assay include the high level of technical expertise required to read the slides, difficulties encountered in testing patients who are neutropenic, and the need to process cells within 24 h after collection due to poor stability of the antigens in blood specimens. Alternatively, molecular-based CMV assays have been developed both for detection of CMV disease and for monitoring responses to antiviral therapy (21). PCR and hybrid capture assays (Digene, Gaithersburg, MD) provide either quantitative or qualitative results. In contrast, the NucliSens pp67 NASBA assay (bioMérieux, Durham, NC) detects pp67 mRNA which is present only after a round of viral replication. Similar to the case for culture-based assays, this qualitative assay can therefore differentiate between active and latent infection, and the assay has been shown to be a better predictor than PCR of active CMV central nervous system (CNS) disease in AIDS patients (58, 167).
Although most of the respiratory viruses produce CPE in traditional cell cultures, some do so slowly or produce little if any CPE. Therefore, the rapid detection of respiratory virus antigens (adenovirus; influenza A virus; influenza B virus; parainfluenza virus types 1, 2, and 3; and RSV) by IF has received considerable attention and has been shown to be very helpful in identifying this group of viruses. This is especially true with samples from pediatric patients, who shed virus longer and in higher titer than adults. Sensitivity for RSV detection by IF compared to conventional cell culture is higher than that for detection of any of the respiratory viruses, ranging from 84% (68) to 93% (115) to 99% (84). Two studies comparing RSV detection by DFA and by rapid cell culture showed DFA to be more sensitive than cell culture (42, 51). The excellent sensitivity of RSV IF compared to viral culture is mainly due to the lability of the virus. RSV is quickly inactivated in samples that are not kept refrigerated and are not inoculated into cell culture within a short time after specimen collection.
The reported sensitivities of IF testing for the other respiratory viruses compared to virus isolation in cell culture are lower that those reported for RSV and vary considerably from report to report. Representatives sensitivities are as follows: for influenza A virus, 62% (68, 90), 83% (84), 99% (66), and 100% (30); for influenza B virus, 66% (30), 83% (84), and 87% (90); and for parainfluenza virus (types 1 to 3), 63 to 72% (90) and 95% (84). The sensitivity of adenovirus antigen detection by IF compared to virus isolation in cell culture has been shown to be much lower than that for the other respiratory viruses, with reported sensitivities ranging from 0% (when none of the adenovirus-positive samples were detected by IF) (68) to 51% (90) and 58% (84).
MAbs to hMPV are now available for use in direct testing of respiratory sample cell pellets (Chemicon, Temecula, CA, and Diagnostic Hybrids, Inc.) or for the detection of hMPV in R-Mix and R-Mix Too cultures. Performance of the Diagnostic Hybrids, Inc., MAbs is discussed in “Virus Isolation in Cocultured Cells” above. Landry et al. (85) evaluated the Chemicon hMPV MAbs, developed by the Centers for Disease Control and Prevention (CDC), for direct specimen testing and for culture identification from LLC-MK2, A549, and HEp-2 shell vials. They found that hMPV was detected with equal efficiency in all three types of shell vials and that hMPV staining was optimal at 2 days postinoculation. However, direct staining of clinical specimens was not successful due to nonspecific background staining that made the reading of slides tedious and interpretation difficult.
IF screening for the seven respiratory viruses (adenovirus; influenza A and B viruses; parainfluenza virus types 1, 2, and 3; and RSV) simultaneously with pooled MAbs is now common. A positive result of apple-green fluorescence indicates that antigens of one or more of the seven viruses have been detected. For experienced virologists, the degree, cellular localization, and pattern of fluorescence are excellent indicators as to which respiratory virus may be present. Further testing with MAbs specific for the suspected virus(es) is necessary for confirmation. Overall sensitivity of pooled respiratory viral antigen screening has been reported as 81% (90, 166).
Although MAb pools detect multiple viral antigens simultaneously, the additional testing required to determine which one of the viral targets was detected slows the process of viral identification. Through the use of different fluorescent dyes used to label MAbs in pooled mixtures, it has become possible to detect and differentiate viral antigens simultaneously. This differentiation is currently limited to making only two differentiations at the same time, but this speeds antigen detection. Currently, several of these mixtures are marketed commercially (Light Diagnostics SimulFluor reagents; Chemicon International, Temecula, CA). The reagents are cleared by the FDA for direct specimen testing and for culture confirmation. Two fluorochromes with overlapping spectra are used to label the antibodies. When visualized with an FITC filter set on the fluorescence microscope, one antibody will fluoresce apple-green and the second will appear gold or golden orange. The following dual-labeled mixtures are available currently: CMV and adenovirus; HSV and VZV; HSV-1 and HSV-2; influenza A virus and influenza B virus; parainfluenza virus types 1, 2, and 3 and adenovirus; parainfluenza virus types 1 and 2 and parainfluenza virus type 3; RSV and influenza A virus; RSV and parainfluenza virus type 3; and a respiratory screen reagent in which RSV appears golden and the other six respiratory viruses appear green. These antibody mixtures have shown excellent sensitivity and specificity, comparable to that with individual stains (29, 84).
IF staining has been described as “a demanding technique available only in advanced laboratories” and as “rarely available at community hospitals” (153). The lack of the technical expertise required for interpretation of IF test results prevents many laboratories from offering this testing. This can be a significant problem during evening, overnight, and weekend shifts when technologists are busy staffing high-volume areas of the laboratory. It is difficult to justify dedicating a skilled technologist to rapid viral antigen testing by IF, despite the good sensitivity provided by this approach, when the demand for testing is unpredictable and the technologist is needed in other areas of the laboratory. In addition to the skill required of the technologist, limitations of viral antigen IF testing include the following: poor sensitivity of some assays (e.g., adenovirus antigen), lack of availability of IF reagents for direct specimen testing for some viruses, and the inability of the assays to differentiate infectious from noninfectious virus. Regardless of the reported ranges of sensitivity and specificity of the IF methods for direct viral antigen detection, culture confirmation for samples with negative IF results is recommended by investigators (refer to studies described above) and by reagent manufacturers (Trinity Biotech, Wicklow, Ireland, for Bartels Viral Respiratory Screening and Identification Kit [package insert 10/03] and Chemicon International, Temecula, CA, for Light Diagnostics HSV DFA [package insert 6/2001]), since these methods may yield falsely negative results. Despite these limitations, IF remains the most sensitive, compared to virus isolation in cell culture, of the rapid nonculture, nonmolecular tests. IF testing is available for direct detection of a wider range of viral antigens (e.g., HSV, VZV, seven respiratory viruses, etc) than the rapid membrane EIAs, which are available for only influenza A and B viruses and RSV. The advantages and disadvantages of IF testing are listed in Table 1.
The cost of labor for IF testing is difficult to estimate, but reagent costs are relatively low, at $2 to $7 for each MAb used. Barenfanger et al. (6) assessed the benefits of rapid reporting of respiratory viruses by comparing patients whose samples were processed using standard techniques such as antigen EIAs, shell vial assays, and tube cell culture assays (year 1 group) to patients whose samples were processed with the same standard techniques in addition to IF for RSV; influenza A and B viruses; parainfluenza virus types 1, 2, 3; and adenovirus directly on cytocentrifuged samples (year 2 group). IF results were available within hours. The specificity of the cytospin IF for all viruses was 100%. The IF sensitivities for influenza A virus and RSV were 90 and 98%, respectively, but the sensitivities for influenza B virus and adenovirus were unacceptable (14.3 and 0%, respectively). The mean turnaround time for detection of all positive viruses was 4.5 days for the year 1 group and 0.9 day for the year 2 group (P = 0.001). The mean length of hospitalization for patients with respiratory viral isolates was 10.6 days for the year 1 group versus 5.3 days for the year 2 group. Mean variable costs for these patients were $7,893 in the year 1 group and $2,177 in the year 2 group. After subtracting reagent costs and technological time, the savings in variable costs was $144,332/year. The cytospin IF markedly decreased turnaround time, resulted in physicians having access to information sooner, and was associated with decreased mortality, length of stay, and costs and with better antibiotic stewardship.
Non-IF Methods
Although IF continues to be a mainstay for viral antigen detection, new methods have evolved. Beginning in the late 1980s, a wide variety of technologies, including membrane-based EIAs in cassette format and optical immunoassays (OIAs) (Thermo Electron Corp. [formerly Thermo BioStar], Boulder, CO), have been introduced for rapid and less technically demanding viral antigen detection. OIAs feature a mirror-like silicon wafer coated with an optical molecular thin film and antiviral antibodies and involve steps in testing that are similar to those of a membrane EIA. However, a positive OIA result, which is binding of antibody, antigen, and detecting antibody, produces mass enhancement on the optical surface of the silicon wafer, resulting in a purple color. Immunochromatographic/lateral-flow systems, which involve the migration of viral antigen and antibodies along a test strip, have also been introduced for viral antigen detection. Numerous products, in a variety of formats and from multiple vendors, are currently available. Some of the newer assays detect and differentiate more than one virus (e.g., influenza A and influenza B viruses), and some contain an internal control to monitor the performance of both the assay and the user. Many of the lateral-flow assays have been granted waived status according to the Clinical Laboratory Improvement Act guidelines, which facilitates performance in physicians' offices and clinics. FDA-cleared non-IF rapid antigen tests are available for the following culturable viruses: influenza A virus, influenza B virus, and RSV. FDA-cleared non-IF rapid antigen testing is not commercially available for detection of adenovirus, HSV, the parainfluenza viruses, VZV, or CMV. The rapid assays cost $10 to $22 per test and generally require 15 to 30 min to perform. External controls used to monitor kit performance and detect lot-to-lot variations must be purchased as well. Total costs are higher than those for IF methods or for cell cultures (Table 1).
Although all of these techniques speed the turnaround time for results and simplify the technical component to allow rapid detection of RSV, influenza A virus, and influenza B virus, sensitivities compared to virus isolation in cell culture tend to be low (Table 3). It has been shown that at least 100,000 viral particles must be present for the OIA rapid antigen systems to yield a positive result, and other non-IF antigen systems may require as many as 1,000,000 viral particles; this is in contrast to viral culture, which may require as few as 10 infectious virus particles for successful virus isolation (150). Similar to the IF methods, the performance characteristics of the non-IF antigen tests depends on the virus detected, specimen type, age of the patient, time of sample collection after onset of symptoms, and level of expertise of testing personnel.
TABLE 3.
Rapid nonculture, non-IF methods for RSV or influenza A and B virus antigen detection: comparison to cell culture, 2000 to 2005-2006
| Virus | Manufacturer/test name/principle/time (min) | Sensitivity (%)a | Specificity (%)a | PPV (%)b | NPV (%)b | Reference |
|---|---|---|---|---|---|---|
| Influenza virus | Becton Dickinson (Sparks, MD)/Directigen Flu A and B/EIA, membrane-based cassette; viral antigen in specimen is bound nonspecifically on membrane, labeled antiviral antibodies bind to bound antigens and produce color change when substrate is added; detects and differentiates influenza A and B virus antigens; multistep procedure/15-20 | 44 | 100 | 91 | 97 | 23 |
| 72 | 98 | 89 | 95 | 25 | ||
| 93 | 95 | 83 | 98 | 30 | ||
| 55 | 100 | 100 | 92 | 41 | ||
| 56 | 100 | 100 | 54 | 83 | ||
| 86 | 94 | 89 | 92 | 139 | ||
| 56 | 98 | 93 | 85 | 160 | ||
| Binax (Portland, ME)/Binax NOW Flu A and B/lateral flow; | 62 | 96 | NAc | NA | 37 | |
| one-step application of specimen to test strips; as specimen | 65 | 98 | 89 | 93 | 47 | |
| migrates, viral antigens in specimen are bound by | 53 | 93 | 94 | 52 | 83 | |
| membrane-bound, conjugated (with colored particles) | 76 | 94 | 93 | 81 | 160 | |
| antiviral antibodies; antigen-conjugate complexes are captured by immobilized anti-influenza virus antibody, forming a colored line; detects and differentiates influenza A and B virus antigens; 1-step procedure/15 | ||||||
| Biostar (Thermo Electron Corp. [formerly Thermo BioStar, | 55 | 74 | 73 | 56 | 12 | |
| Inc.], Boulder, CO)/BioStar Flu AB OIA/OIA; viral | 48 | 97 | NA | NA | 69 | |
| antibody on silicon wafer binds viral antigen in specimen; | 93 | 82 | 84 | 92 | 137 | |
| enzyme-labeled antiviral antibodies bind to bound antigen, | 64 | 95 | NA | NA | 143 | |
| producing optical changes in thickness and color change when substrate is added; detects and differentiates influenza A and B virus antigens; multistep procedure/15-20 | ||||||
| Quidel (San Diego, CA)/QuickVue Flu A and B/lateral flow; | 70 | 98 | 85 | 95 | 25 | |
| extraction of influenza virus antigens, then application of | 77 | 98 | 74 | 98 | 124 | |
| specimen to test strip; antiviral antibodies on test strip | 95 | 76 | 81 | 93 | 137 | |
| capture viral antigen in sample, causing a colored band to | 91 | 86 | 78 | 95 | 139 | |
| form; detects influenza A and B viruses (does not differentiate); 2 steps/10 | ||||||
| Remel (Lenexa, KS)/Xpect Flu A and B/lateral flow; dilute specimen, apply to strip; labeled (with colored particles) antiviral antibodies on strip bind viral antigen in sample and produce colored band; detects and differentiates influenza A and B virus antigens; 2 steps/15-20 | 94 | 100 | 100 | 98 | 24 | |
| ZymeTx (Oklahoma City, OK)/ZstatFlu/viral neuraminidase | 65 | 83 | NA | NA | 76 | |
| acts on chromogenic substrate to produce colored | 70 | 92 | 76 | 90 | 113 | |
| precipitate; detects influenza A and B viruses (does not | 72 | 83 | 80 | 75 | 137 | |
| differentiate)/5 “hands on,” 30 total | ||||||
| RSVd | Becton Dickinson/Directigen EZ RSV/lateral flow | 59 | 98 | 93 | 88 | 115 |
| Becton Dickinson/Directigen RSV/EIA | 77 | 96 | 88 | 92 | 115 | |
| 86 | 93 | 95 | 91 | 131 | ||
| Binax/NOW RSV/lateral flow | 89 | 100 | 100 | 95 | 3 | |
| 74 | 100 | 100 | 90 | 14 | ||
| 87 | 94 | 80 | 92 | 99 | ||
| 89 | 100 | 100 | 95 | 115 | ||
| Biostar/RSV OIA/OIA | 88 | 99.6 | 99 | 95 | 2 | |
| Remel/Xpect RSV/lateral flow | 75 | 98 | 95 | 90 | 14 |
Sensitivity and specificity compared to virus isolation in cell culture.
NPV, predictive value of a negative result; PPV, predictive value of a positive result.
NA, not available.
See corresponding influenza virus test for method, etc.
For the non-IF RSV antigen detection methods, as with IF methods for RSV antigen, the sensitivity of antigen detection compared to cell culture is higher than that of similar tests that detect influenza virus antigens (Table 3). In general, the specificities (compared to virus isolation in cell culture) reported for rapid non-IF assays for RSV and influenza virus antigen detection tend to be high (Table 3), and the predictive value of a positive result is high, especially during respiratory virus season (162). However, some report a lack of sensitivity and specificity for influenza B virus detection (25, 30, 83) in these antigen detection systems, while others (23, 24) found that the sensitivity of influenza B virus detection was equivalent to that of influenza A virus detection.
The substantial variations in the findings of studies (Table 3) may reflect, in part, the many variables involved in the study designs, and laboratories must critically examine the results in consideration of the parameters that affect test results. The type of specimen tested can be important. For example, Ahluwalia et al. (1) compared RSV recovery from paired NPA and NPS samples collected from hospitalized infants. RSV was recovered in cell culture more often from NPA specimens (72%) then from NPS specimens (47%) and was detected equally by IF in NPA specimens and NPS specimens. However, the number of fluorescing cells was greater for NPA specimens. The composition of the patient population is also significant. Upper respiratory tract secretions from adults contain smaller amounts of virus than those from children. Landry et al. (82) determined that cytospin-enhanced DFA detected 92.5% and a rapid antigen detection membrane EIA detected 75.5% of all positive influenza A virus samples collected from both adults and children. All positive NPA specimens from children were detected with both assays; this is in contrast to the results found with specimens from adults, in which 89.9% of the positives were detected by DFA and only 66% by EIA. The level and type of virus circulating in the particular season, skill of testing personnel, and format of the reference cell culture method (rapid culture versus conventional tube culture) are additional factors that must also be considered before these nonculture, non-IF assays—or any assay—are implemented in clinical testing. The sensitivity and specificity obtained with the rapid antigen tests when used in the routine diagnostic virology laboratory are often lower than those stated by the manufacturer and lower than those reported in studies that were conducted under tightly controlled circumstances targeting a particular group of patients (109).
Although the need for technical expertise is advertised as being minimal for performance of these non-IF rapid tests, testing that is carried out by technicians or other personnel who are less experienced with test kits, especially in reading results that are weakly positive, yields lower sensitivity and specificity relative to cell culture; this finding is unexpected in view of the touted simplicity of some assays (113). There are a number of concerns for FDA-cleared Clinical Laboratory Improvement Act-waived testing that is performed outside the laboratory at point-of-care or “near-patient” waived sites in physicians' offices and clinics. Mackie et al. (99) found that 16 of 27 discordant observations reported by point-of-care staff performing Binax NOW testing (Binax, Portland, ME) were reversed when testing was repeated on the same sample by laboratory staff. The authors concluded that a potential disadvantage of the “simple” tests is that less emphasis is placed on training and fewer restrictions are placed on the number of health care workers allowed to carry out testing. It is likely that test sensitivity and specificity suffer if those who perform waived testing are not properly trained and provided with adequate oversight. These issues and others involved with waived testing have been reviewed (28) and should be considered if results of waived-status viral antigen tests performed outside the laboratory are to be used in patient management.
Because the specificity of these systems is usually high during respiratory virus season when virus has been documented in the community by virus isolation in cell culture, positive results should be considered true positives; however, negative results should be confirmed by IF testing, culture, or another secondary test (3, 69). Investigators also caution users that the non-IF rapid tests, when used for screening purposes in large populations, may miss infected patients and are not the most reliable laboratory tests for influenza virus detection (23). In addition, during “off” seasons, i.e., when influenza virus and RSV are not documented to be prevalent in the population, the predictive value of a positive result decreases. Because the non-IF viral antigen detection methods are available for the detection of only RSV and influenza A and B viruses, in a setting where the target virus may be other than these three, IF and/or virus isolation in cell culture is the logical alternative, particularly for laboratories that do not perform molecular testing. In addition, a positive rapid non-IF test does not eliminate the possibility that patients may be coinfected with another virus that may be contributing to their symptoms (24). This is of particular significance when testing persons with impaired immune function and children with severe respiratory illness (11, 54). Therefore, if dual infections are of interest, virus isolation used as an adjunct to the rapid test may yield the desired information.
In summary, the pairing of rapid antigen testing and virus isolation in cell culture is useful in many ways, including for the continuous monitoring of the quality of the rapid antigen test, to increase sensitivity of virus detection, to obtain viral isolates for further testing, and to detect viruses other than the few types that are targeted by the rapid non-IF antigen methods (109). Many investigators conclude that the rapid non-IF methods may be useful in screening but should not be used without backup with cell culture for negative samples and as an adjunct for testing samples from patients with potentially mixed infections (3, 23, 69, 162).
Molecular Methods
Since the early 2000s, detection of viruses in clinical samples through the use of molecular methods has become more widely available. The recent literature on NAAT applications in viral diagnostics is far too extensive to be effectively reviewed here, but the comparisons to virus detection in cell culture or by IF and/or non-IF antigen methods are invariably made with each study, many of which have been discussed in previous sections. Excellent reviews and texts describing molecular technologies, instrumentation, applications, and comparisons to more traditional test methods such as viral culture have been published previously (20, 34, 38, 40, 46, 67, 98, 100, 110, 111, 156). Readers should refer to these articles and texts for specific details and references.
Viruses can be detected directly in clinical samples and in cell culture supernatants by using highly specific nucleic acid probes that are complementary to the target viral RNA or DNA sequences or using by NAATs. NAATs allow for the detection of viral pathogens before viral antigens are present in sufficient quantities to be detected and do not require viable virus. In addition, nucleic acids are easily purified and separated from antibodies, which is not always the case for viral antigens, whose detection may be inhibited by antigen/antibody complexes (164). Although virus growth in cell culture has been referred to as “nature's PCR,” this amplification is slow compared to the rapid amplification by NAATs, which require only a few hours in order to logarithmically increase the amount of viral nucleic acid. Many amplification technologies have been used to detect viruses in clinical samples, including PCR, RT-PCR, NASBA, strand displacement amplification, and transcription-mediated amplification. In more traditional methods, amplification is performed first and is then followed by amplicon detection using gel electrophoresis, colorimetric methods such as EIA, or electrochemiluminescence-based formats. Alternatively, applications may be “real time,” where amplification and detection are performed simultaneously and detection of the target nucleic acid is monitored continuously using fluorescent probes such as molecular beacons, TaqMan probes, or fluorescent resonance energy transfer probes.
NAATs have been applied widely in research applications for almost all known viruses and with expanding applications for clinical diagnostics. The thrust towards clinical molecular virology was greatly affected by the development of applications involving viruses that do not proliferate in standard cell cultures (e.g., HIV-1; hepatitis C virus [HCV]; HBV; parvovirus B-19; human herpesviruses 6 [HHV-6], 7, and 8; Epstein-Barr virus [EBV]; human papillomavirus; BK virus; and JC virus). Quantitative NAATs have provided medically useful tools in assessing patient prognosis, treatment response, and antiviral resistance, especially for CMV (22), HIV-1 (35), HCV (53), HBV (106), and other retroviruses and hepatitis viruses that are not isolated in standard cell cultures. In addition, the high sensitivity of molecular assays and the poor performance of culture-based methods for testing CSF have made molecular testing essential for the diagnosis of viral CNS disease (60, 70, 80, 138).
Overall, well-developed NAATs demonstrate superior analytical and clinical sensitivity over cell culture and IF and non-IF antigen methods and better specificity than IF and non-IF antigen methods. However, the performance of molecular assays can vary significantly due to nucleic acid extraction methods, primer and probe design, amplification and detection technologies, instrumentation, and technical expertise. All these factors must be considered in assay selection. Thorough assay verification studies should be performed prior to clinical testing, and ongoing competency and assay validation protocols must be established. As with culture techniques, NAAT performance is subject to variability related to specimen types, timing of specimen collection and handling, and the nature of the virus itself.
The impact of NAAT results on viral disease diagnosis varies with the virus in question and according to the clinical situation. NAAT results will always be key in decision-making when the NAAT (i) is the only approach available (i.e., for nonculturable viruses such as HCV or for viruses such as SARS-CoV or variola virus that laboratories do not want to grow in culture), (ii) is the most sensitive detection method (i.e., for certain sample types such as CSF, from which the isolation of HSV, VZV, and enterovirus [60, 70, 80, 138] is known to be suboptimal), (iii) can provide quantitative results (i.e., for HIV, HCV, BK virus, and EBV), and (iv) provides a result in a time frame that can affect patient management.
However, the use of NAATs in other situations may not be as important or useful in patient management. Lanciotti et al. (81) demonstrated that due to the transient nature of the viremic stage of arboviral infections and the often low levels of virus in the CSF and serum, molecular methods are more sensitive than cell culture, and a positive result with a NAAT is diagnostic. However, up to 55% of CSF samples and only approximately 10% of serum samples were positive by NAAT in patients with serologically confirmed cases of West Nile virus infection. Therefore, a negative NAAT result does not preclude the possibility of an arboviral infection, and serology remains the gold standard for diagnosis and/or confirmation.
Oosterheert et al. (118) tested lower respiratory tract samples by real-time PCR for common respiratory viruses (influenza A and B viruses, RSV, parainfluenza virus types 1 to 4, adenoviruses, coronavirusese OC43 and 229E, and rhinoviruses), Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila, but the PCR results were made available within 48 h only to the attending clinicians of patients in an intervention group. The main findings of this study were that real-time PCR significantly increased the diagnostic yield compared with conventional diagnostic tests alone but did not reduce antibiotic use, duration of hospital stay, or treatment costs. Treatment cost were much higher in the intervention group, and the reporting of real-time PCR results led to partial or total cessation of antibiotic therapy for only 11% of the 55 patients in the intervention group. Part of the reason that therapy did not change in cases in which a viral pathogen was identified by real-time PCR was that clinicians were hesitant to discontinue antibiotic therapy because of the concern about coinfection with bacterial pathogens. The increases in costs were directly related to the additional costs of the NAAT testing. The argument is made that NAAT results must be made available within 24 h if they are to have more influence on early therapeutic decisions (107). However, Oosterheert et al. (118) concluded that clinicians are hesitant to discontinue antibiotic therapy based on a virus-positive PCR result because they are waiting for bacterial culture results, which are available later than the PCR results; therefore, it is doubtful that more rapid reporting of PCR results will lead to improved cost-effectiveness. This may change over time as clinicians become more familiar with evaluating the significance and clinical implication of these more rapid test results.
NAATs that must be “batched” or run infrequently due to cost concerns may not be especially useful in patient management and may hold little advantage over cell culture, especially in view of the rapid cultures that can yield a result within 24 h. Laboratories that are unable to perform NAATs in-house generally refer specimens to regional or national reference laboratories. While specimen transport is much improved over the 3- to 5-day transport period often required in the past, the transportation issue remains significant if a result is desired within 24 h. Usually, specimens are picked up at the referring laboratory by a courier at a designated time each day and transported either directly to the reference laboratory or to the airport where the sample will be flown to more distant reference laboratories. If the sample is picked up on schedule and transported directly to the reference laboratory, NAAT testing begins in the morning of the following day. If testing is by real-time PCR, it is possible that the NAAT result will be available and transmitted to the referring laboratory within 24 h of specimen collection. However, if the specimen arrives at the referring laboratory after the courier from the reference laboratory has completed the daily pickup, the specimen will remain at the referring laboratory for another day, until the courier's next pickup time. This adds 24 h to the turnaround time. The subsequent shipping time and the additional wait for initiation of NAAT testing at the reference laboratory on the following morning yields results no sooner than 48 h after specimen collection. If traditional PCR rather than real-time PCR is used at the reference laboratory, the actual testing period may extend to 8 h or even overnight, further delaying results. In such cases, it is likely that results of rapid (24 to 48 h) cell cultures performed in-house would be available before the NAAT result, and it is likely that the cell culture result would be the one that would be used in making decisions on patient management.
An advantage of cell culture is that it provides an approach for detecting a variety of viruses that might be present in a clinical sample. This is in contrast to most NAATs, which generally target only one or a few viruses; any other viruses in the sample will remain undetected unless multiple NAATs are performed. Likewise, if a positive NAAT result is obtained for one virus, this does not rule out that a second virus is present. However, more real-time and traditional NAATs are becoming available that offer multiplexing capabilities where numerous primer and probe combinations are present that allow the detection of a variety of viruses from a single amplification reaction (e.g., the Herpes Generic Consensus kit [Argene Biosoft, Varilhes, France], which detects HSV-1, HSV-2, CMV, EBV, HHV-6, and VZV, and the ProFlu-1 assay [Prodesse, Waukesha, WI], which detects influenza A virus, influenza B virus, and RSV). Newer detection technologies using arrays and bead detection formats (e.g., that from Luminex Technologies, Austin, TX) will greatly expand the potential for multiplex viral detection. An example is the ID-Tag Respiratory Virus Panel Assay (TM Bioscience, Toronto, Canada). Investigators are currently seeking FDA clearance for this system as an in vitro diagnostic device (100a). This internally controlled assay uses a combination of RT-PCR, target specific primer extension, and Luminex bead detection for the identification of 19 different viruses (influenza A virus [H1, H3, and H5]; influenza B virus; parainfluenza virus types 1, 2, 3, and 4; adenovirus; rhinovirus/enterovirus; RSV A; RSV B; hMPV; and coronavirus [SARS-CoV, NL63, 229E, OC44, and HKU1]) from respiratory samples. This type of information will provide excellent insight into the prevalence of various viruses and the clinical significance of mixed viral infections. However, one must always consider the new or unexpected viruses that would be missed even using these broader-range applications. All laboratories, whether using cell culture-based assays or molecular tests, must be ready to implement additional assays as new viral targets of clinical significance are identified.
One drawback of NAATs is the expense of this testing (Table 1). The investment required for equipment is significant, ranging from $5,000 to more than $100,000 for some instruments. Reagent prices per specimen tested may be as low as several dollars for in-house-developed assays or as high a $300 for some FDA-cleared assays (e.g., HIV-1 genotyping). In addition, often the laboratories are directly responsible for paying patent royalties on certain in-house-developed assays, analyte-specific reagents (ASRs), and/or non-FDA-cleared kits. Reimbursement for molecular tests also varies greatly depending on the test, the target, the insurance plan, and individual state payment schedules for Medicare/Medicaid patients. In general, costs of NAATS far exceed those of cell culture. However, the benefits of molecular testing may far supersede additional costs (e.g., alternative diagnostic procedures, increased length of stay, or inappropriate antibiotic therapy) incurred by the institution if such testing is not available. In addition, factors such as morbidity, mortality, and patient and physician satisfaction play a key role in selecting the best options.
Perhaps the greatest obstacle in incorporating NAATs in the diagnostic virology laboratory is simply the issue of availability or accessibility of testing. At this writing, there are few FDA-cleared NAAT “kits” for viral detection (available for HIV-1, HCV, and human papillomavirus). Many ASRs are now available, but the use of these requires extensive verification studies before the tests can be used clinically. This often requires personnel with the expertise and dedicated time to work with molecular assays and molecular testing equipment that may be able to support only one or a very few related assays. There may be insufficient test volumes to justify expensive in-house NAAT testing. However, as simpler technologies and more FDA-cleared tests become available, more laboratories will be afforded the opportunity to bring testing in-house. Recent developments in instrumentation have eliminated some of the technological roadblocks to adaptation of the technology into the diagnostic virology laboratory. Many different systems from a variety of manufacturers are available for nucleic acid isolation, amplification, and detection, including some systems, such as the GeneXpert (Cepheid, Sunnyvale, CA), Viper (Becton Dickinson Diagnostics, Sparks, MD), and Tigris (GenProbe, San Diego, CA), that can fully integrate the entire process.
Access to cell culture reagents and equipment is greater than access to NAAT testing supplies and instrumentation for most laboratories. This is especially true in laboratories that have used cell cultures in the past and in microbiology laboratories that use IF methods routinely for other purposes. With the rapid cell culture formats that use blind IF staining or color change reactions to identify viruses, the technologist does not need to be skilled in cell culture maintenance or in examining cell cultures for CPE. Also, for some viruses, such as HSV from vesicular lesions, that are easily culturable in tube cell culture, a sensitive and specific result can be produced with rapid or conventional tube cell culture within 24 to 48 h in a routine microbiology laboratory setting by technologists with moderate skill levels. Although the use of PCR may improve sensitivity for detection of HSV from lesions by 20% or more, the added expense and the lack of approved methods, standardized protocols, and access to instrumentation may make converting to this technology less of a priority. Although many may argue that virus isolation in cell culture requires much more technical expertise than NAAT techniques, the underestimation of the level of expertise required for NAAT performance is unfortunate. The need for technical skill remains in NAAT testing, both in specimen manipulation and in monitoring of instrument performance. Blind acceptance of NAAT instrument printouts without proper oversight and monitoring is poor practice.
Additional drawbacks of NAATs include (i) the inability of most NAATs to distinguish between viable and inactivated virus (9, 13) or between actual disease and latent infection (92, 93); (ii) the lack of standardization of some of the methods, causing laboratories to report discordant findings with both false-negative and false-positive reports; and (iii) the potential for cross contamination of samples, causing false-positive results.
As development, applications, standardization, and availability of FDA-cleared kits and products for real-time PCR and other NAAT-based assays progress, the usefulness of these assays in viral disease diagnosis will continue to increase (114). However, at this writing, existing NAAT technologies alone are not sufficient to meet the needs of all situations in viral disease diagnosis, and virology laboratories will continue to rely on virus isolation in cell culture in many situations. Laboratories have the option to offer combinations of different test options, including IF and non-IF rapid antigen tests, rapid cell culture, traditional tube cell culture, and molecular tests. Near the end of this review, some of the challenges in viral diagnosis are presented, and recommendations are made for applications of various viral diagnostic approaches, including NAATs, in the particular setting.
APPLICATIONS OF VIRAL DIAGNOSTIC TECHNIQUES
Thus far, this review has focused on analytical sensitivities and specificities of various virus detection methods in comparison to virus isolation in cell culture and on technical variables such as turnaround time, technical expertise, and cost-effectiveness. It is clear that no single approach is optimal for detecting all viruses in all clinical situations. Therefore, it is vital to combine culture and nonculture methods to optimize viral disease diagnosis, yielding medically useful, cost-effective, and labor-saving viral testing results. In determining appropriate testing algorithms for the laboratory, one must consider a broad range of factors, including the patient population (i.e., age, immune status, and comorbidities), clinical manifestations, physician's diagnosis, and time of year (i.e., many viral infections tend to be seasonal). However, with global travel and changing epidemiology of viral diseases, it is incumbent upon the virology laboratory to be prepared for the unexpected. For example, laboratories that cease influenza virus testing during the summer months are at risk for missing both imported cases and unexpected local outbreaks. The actual occurrence of this is illustrated by the North Shore University Hospital Virology Laboratory in Manhasset, NY, where at least one influenza A virus was isolated in every month beginning in November 2003 and continuing through August 2005 (C. C. Ginocchio, unpublished data).
Laboratorians must also evaluate the resources required and available at their facility to use in delivering viral diagnostic services. Issues to consider include the level of training and expertise required for performing assays, availability of sufficient staff, desired production schedules (i.e., when testing will be offered, necessity for immediate testing, and expected turnaround times), test volumes, capacity for full-service virology versus limited service (i.e., offer testing for a wide range of viruses by a number of approaches or focus on only a single virus or a few viruses and refer other testing to another laboratory), and equipment and space needs. Although certain viral assays may be known to produce optimal results, it may not be within the capacity of a given facility to offer all of these services.
The information that follows gives suggestions for approaching viral diagnostic testing of a variety of specimen types. Combinations of various viral diagnostic technologies are suggested, with explanations of why these approaches are useful, how methods can be paired to provide effective diagnoses, and what role virus isolation in conventional or rapid cell culture plays in each situation.
Testing for Respiratory Viruses
There are a variety of approaches for respiratory virus testing, and each laboratory must determine which is the most appropriate for the institution, laboratory, and patient population involved. The first line of testing for influenza A virus, influenza B virus, or RSV in populations in which immunocompromised patients do not predominate is often a nonculture, non-IF rapid antigen detection method. During periods of low virus activity, rapid influenza A and B virus or RSV membrane EIAs, OIAs, or lateral-flow antigen assays may be used, but it is recommended that all positive results be confirmed by IF, viral culture, or RT-PCR (162). This is to ensure that the positive results with the rapid tests are true positives, as the predictive value of a positive result is very low with the rapid tests during off-season periods (162). Once influenza virus or RSV has been isolated in a given geographic area, signaling the start of “the season,” positive results with non-IF rapid antigen tests need not necessarily be confirmed by virus isolation. Most of these assays have good specificity which should allow diagnosis to be made based on a positive result.
If rapid EIA or lateral-flow antigen testing is negative in a patient strongly suspected of having a respiratory viral infection, specimens should be tested further by a more sensitive assay. If a 1- to 2-h turnaround time is needed, DFA screening for the seven common respiratory viruses (influenza A and B viruses; RSV; parainfluenza virus types 1, 2, and 3; and adenovirus) can be done. In particular, for pediatric, geriatric, and immunocompromised patients, DFA for hMPV should be considered for incorporation into routine respiratory screening panels. However, at this writing, FDA-cleared monoclonal antibodies are not yet commercially available for performing DFA or rapid culture for hMPV. MAbs for research use only are available from Chemicon International (anti-MPV 75.1) and from Diagnostic Hybrids, Inc. Although additional testing by DFA of samples that are negative by rapid tests may not seem to be cost-effective, it can be useful when the rapid EIA or lateral-flow assays are performed initially at point-of-care sites (i.e., emergency departments or hospitals that refer virology samples to another site). In addition, DFA testing may include a full respiratory panel (seven or eight viruses), which could provide additional information before culture results would be available. DFA is also reported to be more sensitive than cell culture for RSV detection (42, 51), so RSV DFA alone may be considered if a full panel of DFA testing is not desired.
In many settings, the rapid non-IF antigen detection methods are not used due to their poor performance in certain patient populations and with certain specimen types (See “Non-IF Methods” above). In these settings DFA screening for the seven standard or eight (standard panel in addition to hMPV) respiratory viruses is likely to be the first testing performed, with further testing of negative samples by either rapid or traditional cell culture. In a setting where there is no need for immediate results and a 24- to 48-h turnaround time is acceptable, there may be no nonculture testing by IF and/or non-IF antigen detection methods. Isolation of the eight respiratory viruses in a rapid (24- to 48-h) culture system such as shell vials and R-Mix is an attractive alternative. Also, if turnaround time is not an issue or if viruses other than the eight listed are expected or are of interest, a full conventional culture can be performed. Culture also should be considered for samples positive by EIA and/or DFA that might contain another virus (4, 8, 158).
Another approach for respiratory virus testing involves NAATs. NAATs may be the only test, a supplemental test, or an additional test for specimens negative by other test methods. Presently, no FDA-cleared kits are commercially available for viral respiratory pathogens; however, single or multiplex ASRs for this purpose can be purchased from a variety of manufacturers. NAAT testing for a number of viral pathogens is available at most reference laboratories. Depending on the testing format, the costs will vary. In general, NAAT testing will be more costly than viral culture (Table 1).
Rapid antigen assays that show poor sensitivity and specificity compared to virus isolation in cell cultures should be either discontinued completely or replaced with assays with proven better performance. In addition, data (generated preferably in-house or, alternatively, from the published literature) on the sensitivity and specificity of the assays used and, when indicated, recommendations for additional testing by more sensitive tests (such as virus isolation in cell culture) should be included along with the rapid test results.
Samples from certain groups of patients or targeting particular viruses warrant strategies different from those suggested above. Suggestions for use of cell culture and other respiratory virus detection methods in these special cases follow.
Exceptions in Respiratory Virus Testing
Samples from persons with immunosuppression.
In persons with immunosuppression (e.g., patients with HIV disease or neutropenia due to chemotherapy, bone marrow or solid organ transplant recipients, or patients with comorbidities or underlying chronic pulmonary disease), there is a heightened potential for coinfection with more than one respiratory pathogen or with viruses such as CMV, HSV, enteroviruses, and rhinovirus that are not routinely detected in respiratory IF panels or in cell culture systems such as R-Mix. Although the frequency of mixed infections is difficult to estimate, it is suggested that >10% of respiratory samples from immunocompromised patients contain more than one virus (158). Identification of all agents is essential to ensure appropriate antiviral therapy in these groups of highly susceptible patients. Therefore, samples from these groups of patients, independent of rapid antigen testing results, should be tested in full respiratory cultures supplemented with tube cultures or shell vials for CMV. NAAT testing should be considered to increase the sensitivity of virus detection and to detect viruses normally not detected by routine culture.
Samples from persons considered clinically as having possible cases of avian influenza or SARS or exposure to other emerging pathogens or viral agents of bioterrorism.
It is essential that the ordering physician notify the laboratory immediately when avian influenza, SARS, or an unusual agent is suspected. This information is key in ensuring that these samples are handled properly. Laboratories should access the appropriate CDC website pages to view current recommendations. “Severe Acute Respiratory Syndrome (SARS)” (27) and “Bioterrorism Agents/Diseases” (26) are two helpful sites. In general, testing for such agents in biosafety level 2 (BSL-2) facilities such as hospital diagnostic laboratories should be limited to rapid antigen testing or molecular methods performed in a biological safety cabinet. Samples should not be inoculated into cell cultures by local laboratories but should be referred immediately to local public health officials and/or the CDC. The R-Mix Too line of cocultured cells (Diagnostic Hybrids, Inc.) was developed specifically to prevent accidental isolation of the SARS virus (see “VIRUS ISOLATION IN COCULTURED CELLS” above). The use of this cell line would be very helpful in the event of accidental inoculation of a SARS-CoV-positive sample into a cell culture in a BSL-2 laboratory. However, intentional culturing of possible SARS-CoV samples should not be done at BSL-2 laboratories even if R-Mix Too is used.
The capacity of detection by rapid antigen assays and conventional and other cell lines to support the growth of avian influenza virus (H5N1) has not been fully tested because of restrictions on the availability of the virus. All testing on possible avian influenza samples should be referred to the appropriate agency.
Samples from pediatric patients.
If possible, samples from pediatric patients, particularly those younger than 5 years, should be tested for hMPV and the pediatric coronaviruses. NAAT testing may be the best approach due to the limited choice of susceptible cell lines, the lack of FDA-cleared MAbs, and the delayed time to detection by culture-based methods. As more data are generated about the epidemiology of these pathogens, routine testing recommendations may be expanded to include patients in other age groups.
Samples from seriously ill pediatric patients.
Samples from seriously ill pediatric patients who are hospitalized after a positive RSV antigen screen should receive a full viral culture to rule out coinfection, especially in pediatric hospitals where isolation rooms are not available and patients' hospital room assignments are based on initial virus screening. The detection of additional viruses, such as hMPV or adenovirus, may affect the patient's therapy as well as hospital infection control measures.
When adenovirus is the viral suspect.
When adenovirus is the viral suspect in any sample, virus isolation in cell cultures may be necessary. EIA, OIA, and lateral-flow devices are not available for detecting adenovirus antigen. Both DFA and IFA methods for adenovirus antigen detection are available, and a positive result with either is excellent evidence of infection. However, both DFA and IFA have shown poor sensitivity in adenovirus antigen detection (84, 90). Therefore, samples strongly suspected of adenovirus infection should be inoculated into susceptible cell cultures such as R-Mix cells, and/or a full viral culture using traditional tube cultures should be performed. NAAT testing for adenovirus may be available at some large laboratories.
Viral Testing of Vesicular Lesions
If possible, DFA testing for HSV-1, HSV-2, and/or VZV should be performed initially on direct smears made from vesicular lesions. If the DFA is positive, virus isolation in cell culture is usually not useful. However, isolation of virus in culture should be done if antiviral susceptibility testing is required for patients with recurrent HSV that appears to be refractory to treatment. Although the DFA for VZV has been reported to be more sensitive than culture (33, 61), if DFA is not performed or is negative, cell culture-based methods such as traditional tube culture, shell vial, H & V shell vial for HSV and VZV, or ELVIS for HSV-1 and HSV-2 should be used. If NAAT testing is available, it should be considered because of its generally superior sensitivity. However, laboratories that select NAATs for the detection of HSV in lesions must be extremely careful to avoid cross contamination of samples that may occur due to the high titers of virus generally present in these types of samples.
Exceptions in Viral Testing of Vesicular Lesions
Specimens from vesicles suspicious for variola virus.
Specimens from vesicles suspicious for variola virus should not be inoculated into cell cultures in the laboratory and must be referred immediately to the public health laboratories and CDC. Variola virus proliferates in several cell lines that are routinely available, such as Vero, HeLa, and others.
Specimens from vesicles suspicious for monkey pox or other poxvirus infections.
The reagents and expertise needed to identify monkey pox virus or other poxviruses are not available at most hospital laboratories. Virology laboratories should develop their own protocols for handling such samples. Testing of such materials in-house to rule out VZV may present a risk if, indeed, the suspected poxvirus is present. Samples should be referred immediately to the public health laboratories or CDC.
Viral Testing of CSF
In clinical virology laboratories the detection of viral CNS pathogens by culture has demonstrated very poor sensitivity in comparison to NAATs, which are now accepted in most cases as the gold standard (59). The enteroviruses, the viruses in the Herpesviridae family (CMV, HSV-1, HSV-2, VZV, EBV, and HHV-6), JC virus, and the arboviruses are most frequently associated with CNS infection. These are discussed below.
Enteroviruses in CSF.
Isolation of CSF enterovirus in conventional cell culture is 75% less sensitive than that in NAATs, and NAATs are currently accepted as the gold standard for diagnosis of enterovirus infection in CSF (18, 59, 64, 112, 128, 135, 138, 157). Methods using 5′ UTR pan-enteroviral primers detect a broad spectrum of enteroviruses, including the nonculturable coxsackievirus strains. These methods do not detect the parechoviruses, which require different primers and probes (60, 87). If NAAT testing is available in-house or from a nearby reference laboratory that can ensure a rapid turnaround time, NAAT testing alone is sufficient. However, if NAAT results cannot be obtained rapidly, inoculation of cell cultures in-house (in addition to referring the sample for NAAT), especially the rapid cultures and Super E-Mix, which yield results within 24 to 48 h, may yield a positive result before the NAAT results are available. Of the CSF pathogens, enteroviruses have the highest recovery rate in cell culture, depending on the cell line used. Super E-Mix has shown very good sensitivity in isolation of many strains of enteroviruses from various specimen sources (73).
Herpesviruses in CSF.
HSV-1, HSV-2, and VZV are seldom isolated in culture from CSF (59). In the last 10 years, the North Shore University Hospital Virology Laboratory, Manhasset, NY, has isolated only six HSVs from cultures of CSF; all positive samples were from newborns with overwhelming disseminated HSV-2 disease (C. C. Ginocchio, unpublished data). In contrast, since the advent of herpesvirus PCR testing in 2000, the same laboratory has detected a Herpesviridae family virus (HSV-1, HSV-2, VZV, EBV, HHV-6, or CMV) in more than 300 CSF samples (10% of the total tested) and also has identified several cases with mixed infections and/or reactivations of latent viruses (HSV, VZV, and/or CMV) (C. C. Ginocchio, unpublished data). Detection of HHV-6 in the CSF of children with a febrile seizure is important for identifying those with a potential for the recurrence of seizure episodes due to ongoing HHV-6 infection. Immunocompromised patients can manifest severe postprimary infections, including encephalitis, due to HHV-6 reactivation. Therefore, NAATs are the standard of care for detection of the herpesviruses in CSF specimens.
JC virus in CSF.
Disease due to JC virus usually occurs in immunocompromised individuals in the form of progressive multifocal leukoencephalopathy, which is rare but fatal and involves oligodendrocytes in the brain. Traditionally, diagnosis of progressive multifocal leukoencephalopathy has been by histopathology of brain tissue or by in situ hybridization of paraffin sections of the brain with a JC virus probe. Although the virus is reported to grow in primary human fetal glial cells and in primary urothelial cell cultures, these cell lines are not routinely maintained in most diagnostic laboratories and culturing is very inefficient, requiring several weeks before the virus is detectable (144). Cell culture is not recommended for JC virus isolation. NAAT testing for JC virus is now recommended (101).
Arboviruses in CSF.
Due to the transient nature of the viremic stage of arboviral infections and the often low levels of virus in the CSF and serum, virus isolation using cell culture has generally been unsuccessful and not practical for most clinical laboratories for detection of the arboviruses. Molecular methods are more sensitive than cell culture, and a positive result with a NAAT is diagnostic. However, a negative result does not preclude the possibility of an arboviral infection. For example, studies have shown that up to 55% of CSF samples and only approximately 10% of serum samples were positive by NAAT in patients with serologically confirmed cases of West Nile virus infection (81). Therefore, serology remains the gold standard for diagnosis and/or confirmation of infections caused by arboviruses.
Viral Testing of Other Types of Samples
Fecal samples for detection of enteric viruses.
Detection of enteroviruses, CMV, and HSV can be performed using traditional and/or rapid cell culture methods. Rotavirus and adenovirus type 40 and 41 antigens can be detected using FDA-cleared EIA-based assays. Calicivirus, astrovirus, and norovirus detection is best achieved by NAATs or electron microscopy. Outbreaks should be referred to the local public health officials.
Peripheral blood samples.
Only a few types of virus are isolated from peripheral blood. The most common blood isolate is CMV. Rapid shell vial cultures of fibroblasts or H & V Mix yield sensitive results within 24 to 48 h or sooner. Although detection in shell vial culture of CMV in blood samples is less sensitive than CMV isolation in traditional tube cell cultures, the lengthy incubation required (up to 30 days) to produce CPE in tube cell cultures makes shell vial cultures a more attractive option. However, the tube cell cultures should be included, especially for samples that have very low levels of virus or if an isolate is needed for additional studies. Quantitative measurement of CMV in blood is achieved either by performing a CMV pp65 antigenemia assay on peripheral blood granulocytes or by NAAT methods. Quantitative CMV testing of blood samples collected sequentially allows for monitoring the progress of patients with CMV disease.
Additionally, when indicated for transplant patients, quantitation of EBV (which is associated with posttransplantation lymphoproliferatiave disorder), HHV-6, and/or BK virus (which is associated with problems in renal transplant patients) should be performed by NAATs. HSV and adenovirus may also be isolated in cell cultures of blood, but this is rare. Full viral cultures, which include both rapid shell vial testing and prolonged (30-day) incubation of traditional tube cell cultures, are recommended if the viral suspect in a blood sample is a virus other than CMV. NAATs can be used to detect enteroviruses and HSV in blood from infants with neonatal sepsis.
Urine samples.
Shell vial and traditional tube cultures can be used for the detection of CMV and adenovirus in urine. When indicated in renal transplant patients, quantitation of BK virus should be performed using NAATs.
General Suggestions for Viral Diagnostic Laboratories
(i) Use cell cultures to monitor the sensitivity and specificity of NAATs and of rapid antigen assays each year, and, if possible, provide clinicians with the performance data. Encourage further testing by cell culture for specimens with negative rapid antigen test results obtained during high-prevalence seasons from patients with clinical signs and symptoms of infection and for specimens with positive rapid antigen results obtained during periods of low viral prevalence. For laboratories that do not offer virus isolation in cell culture in-house, monitoring may include sending a certain portion of specimens to a reference laboratory for culture confirmation, comparison of the rapid test result with clinical assessment, and review of peer-reviewed journal articles of studies using the assays in question.
(ii) Improve time to virus detection through the use of rapid cell culture methods such as shell vial cultures. Use traditional tube cell cultures to evaluate the sensitivity and specificity of these rapid culture systems.
(iii) Use traditional tube cell cultures for patients with compromised immune systems in order to allow for detection of a wide variety of viruses (rather than testing for only particular viruses), and use traditional tube cultures or combinations of rapid shell vial cultures to cover a variety of viral pathogens in all patients when coinfection is suspected. Use cell culture systems to differentiate viable from nonviable viral particles and when a viral isolate is needed for further testing, such as antiviral susceptibility testing or strain typing.
(iv) Use cell culture to obtain early- and late-in-the-season influenza virus isolates. Submit these to local health departments for strain typing. This is important for ensuring appropriate vaccine strains for the following year.
(v) Use NAAT rather than cell culturing for viruses that (a) do not grow in cell culture (e.g., HCV), (b) should not be isolated in the routine viral diagnostic laboratory (e.g., SARS-CoV), (c) grow too slowly (e.g., hMPV), (d) have titers that are too low (e.g., HSV in CSF), or (e) need to be quantitated (e.g., HIV and HCV).
CONCLUSION
This decade has seen numerous innovations in cell culture formats and technologies. Through the use of shell vials and microwell plates, applications of cryopreservation, and use of centrifugation-enhanced culture inoculation and pre-CPE detection, virologists are no longer at the mercy of biological supply house production and shipping schedules for access to viable cell cultures, virus isolation is no longer confined to the detection of viral CPE in traditional cell culture tubes, and the average time for virus detection in cell culture has been reduced from 5 to 10 days to only 24 to 48 h for many viruses. The technical experience required for virus detection has been brought to a level that is within reach of most technologists. The use of cocultured virus-susceptible cell preparations reduces the number of individual cell lines needed in the virology laboratory, while facilitating isolation of the same wide range of viruses. In addition, transgenic cells that permit infection by only a single type of virus have been developed. In these cells viral presence is signaled by production of an easily detectable enzyme; this approach further simplifies the task of detecting and identifying the infecting virus.
This review of the technology used at present in viral disease diagnosis shows that no single approach, whether molecular detection, antigen identification, or virus isolation, meets the needs of all diagnostic virology laboratories in all clinical situations involving all types of viruses. Virologists are challenged to use the available technology that best fits the particular situation and yields the most useful results. With the improvements in cell culture technology, this approach remains one that is within the realm of possibilities for most laboratories. Virus isolation, in general, continues to provide more sensitive virus detection than rapid antigen assays and remains less costly and generally better suited for detecting a wider range of viruses than the current commercially available molecular assays. Today, virus isolation in cell culture remains a useful approach for viral disease diagnosis. Tomorrow, as more sophisticated, yet simpler-to-use, broad-range molecular platforms become available for clinical diagnostics, virus isolation in cell culture may once again become mainly a research tool.
Acknowledgments
We thank the virologists at Clarian Health Partners in Indianapolis, IN, and at North Shore University Hospital, Manhasset, NY, and the molecular virologists at the North Shore Long Island Jewish Health Systems Laboratories in Lake Success, NY, for their advice concerning technical aspects of this work. We thank Diagnostic Hybrids, Inc., for kindly providing photographic images.
REFERENCES
- 1.Ahluwalia, G., J. Embree, P. McNicol, B. Law, and G. W. Hammond. 1987. Comparison of nasopharyngeal aspirate and nasopharyngeal swab specimens for respiratory syncytial virus diagnosis by cell culture, indirect immunofluorescence assay, and enzyme-linked immunosorbent assay. J. Clin. Microbiol. 25:763-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldous, W. K., K. Gerber, E. W. Taggart, J. Rupp, J. Wintch, and J. A. Daly. 2005. A comparison of Thermo Electron RSV OIA to viral culture and direct fluorescent assay testing for respiratory syncytial virus. J. Clin. Microbiol. 32:224-228. [DOI] [PubMed] [Google Scholar]
- 3.Aldous, W. K., K. Gerber, E. W. Taggart, J. Thomas, D. Tidwell, and J. A. Daly. 2004. A comparison of Binax NOW to viral culture and direct fluorescent assay testing for respiratory syncytial virus. Diagn. Microbiol. Infect. Dis. 49:265-268. [DOI] [PubMed] [Google Scholar]
- 4.Arden, K. E., P. McErlean, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2006. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J. Med. Virol. 78:1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley, R. L., J. Dalessio, and R. E. Sekulovich. 1997. A novel method to assay herpes simplex virus neutralizing antibodies using BHKICP6LacZ-5 (ELVIS) cells. Viral Immunol. 10:213-220. [DOI] [PubMed] [Google Scholar]
- 5a.Bankowski, M., C. Hodges-Savola, S. W. Belzer, B. D. Lembke, and S. M. Anderson. 2004. Abstr. Clin. Virol. Symp., abstr. T33.
- 6.Barenfanger, J., C. Drake, N. Leon, T. Mueller, and T. Troutt. 2000. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J. Clin. Microbiol. 38:2824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barenfanger, J., C. Drake, T. Mueller, T. Troutt, J. O'Brien, and K. Gutman. 2001. R-Mix cells are faster, at least as sensitive and marginally more costly than conventional cell lines for the detection of respiratory viruses. J. Clin. Virol. 22:101-110. [DOI] [PubMed] [Google Scholar]
- 8.Beckham, J. D., A. Cadena, J. Lin, P. A. Piedra, W. P. Glezen, S. B. Greenberg, and R. L. Atmar. 2005. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J. Infect. 50:322-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackmer, F., K. A. Reynolds, C. P. Gerba, and I. L. Pepper. 2000. Use of integrated cell culture-PCR to evaluate the effectiveness of poliovirus inactivation by chlorine. Appl. Environ. Microbiol. 65:3267-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeckh, M., and G. Boivin. 1998. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin. Microbiol. Rev. 11:533-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boivin, G., M. Baz, S. Cote,. R. Gilca, C. Deffrasnes, E. Leblanc, M. G. Bergeron, P. Dery, and G. Serres. 2005. Infections by human coronavirus-NL in hospitalized children. Pediatr. Infect. Dis. J. 24:1045-1048. [DOI] [PubMed] [Google Scholar]
- 12.Boivin, G., I. Hardy, and A. Kress. 2001. Evaluation of a rapid optical immunoassay for influenza viruses (FLU OIA Test) in comparison with cell culture and reverse transcription-PCR. J. Clin. Microbiol. 39:730-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnafous, P. A. Gautheret-Dejean, D. Boutolleau, D. Calola, and H. Agut. 2005. Persistence of DNA in cell cultures may jeopardize the analysis of human herpesvirus 6 dynamics by means of real-time PCR. J. Virol. Methods 125:95-98. [DOI] [PubMed] [Google Scholar]
- 13a.Booth, M. S. Castriciano, C. Robinson, M. MacPherson, A. Campanella, K. Luinstra, M. Smieja, J. Mahony, and A. Petrich. 2006. Abstr. Clin. Virol. Symp., abstr. T-PM23.
- 14.Borek, A. P., S. H. Clemens, V. K. Gaskins, D. Z. Aird, and A. Valsamakis. 2006. Respiratory syncytial virus detection by Remel Xpect, Binax NOW RSV, direct immunofluorescent staining, and tissue culture. J. Clin. Microbiol. 44:1105-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinker, J. P., and G. V. Doern. 1998. Enhancement of varicella-zoster virus detection in A-549 shell vials by use of freeze-thawed specimens, extended incubation, and “a centrifuged, not incubated” direct detection method. Diagn. Microbiol. Infect. Dis. 31:555-558. [DOI] [PubMed] [Google Scholar]
- 16.Brumback, B. G., S. N. Bolejack, M. V. Morris, C. Mohla, and T. E. Schutzbank. 1997. Comparison of culture and the antigenemia assay for detection of cytomegalovirus in blood specimens submitted to a reference laboratory. J. Clin. Microbiol. 35:1819-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brumback, B. G., and C. D. Wade. 1994. Simultaneous culture for adenovirus, cytomegalovirus, and herpes simplex virus in same shell vial by using three-color fluorescence. J. Clin. Microbiol. 32:2289-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck, G. E., M. Wiesemann, and L. Stewart. 2002. Comparison of mixed cell culture containing genetically engineered BGMK and CaCo-2 cells (Super E-Mix) with RT-PCR and conventional cell culture for the diagnosis of enterovirus meningitis. J. Clin. Virol. 24:S13-S18. [DOI] [PubMed] [Google Scholar]
- 19.Buller, R. S., T. C. Bailey, N. A. Ettinger, M. Keener, T. Langlois, J. P. Miller, and G. A. Storch. 1992. Use of a modified shell vial technique to quantitate cytomegalovirus viremia in a population of solid-organ transplant recipients. J. Clin. Microbiol. 30:2620-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bustin, S. A., and R. Mueller. 2005. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin. Sci. (London) 109:365-379. [DOI] [PubMed] [Google Scholar]
- 21.Caliendo, A. M., and N. S. Lurain. 2006. Cytomegalovirus, p. 473-482. In W. B. Coleman and G. J. Tsongolis (ed.), Molecular diagnostics for the clinical laboratorian, 2nd ed. Humana Press, Inc., Totowa, NJ.
- 22.Caliendo, A. M., R. Schuurman, B. Yen-Lieberman, S. A. Specter, J. Andersen, R. Manjiry, C. Crumpacker, N. S. Lurain, A. Erice, CMV Working Group of the Complications of HIV Disease R. A. C., and AIDS Clinical Trials Group. 2001. Comparison of qualitative and quantitative PCR assays for cytomegalovirus DNA in plasma. J. Clin. Microbiol. 39:1334-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cazacu, A. C., S. E. Chung, J. Greer, and G. J. Demmler. 2004. Comparison of the Directigen Flu A + B membrane enzyme immunoassay with viral culture for rapid detection of influenza A and B viruses in respiratory specimens. J. Clin. Microbiol. 42:3707-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cazacu, A. C., G. J. Demmler, M. A. Neuman, B. A. Forbes, S. Chung, J. Greer, A. E. Alvarez, R. Williams, and N. Y. Bartholoma. 2004. Comparison of a new lateral-flow chromatographic membrane immunoassay to viral culture for rapid detection and differentiation of influenza A and B viruses in respiratory specimens. J. Clin. Microbiol. 42:3661-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cazacu, A. C., J. Greer, M. Taherivand, and G. J. Demmler. 2003. Comparison of lateral-flow immunoassay and enzyme immunoassay with viral culture for rapid detection of influenza virus in nasal wash specimens from children. J. Clin. Microbiol. 41:2132-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Bioterrorism agents/diseases. www.bt.cdc.gov/agent/agentlist.asp. Accessed July 2006.
- 27.Centers for Disease Control and Prevention. 2005. Severe acute respiratory syndrome (SARS). http://www.cdc.gov/ncidod/sars/sarslabguide.htm. Accessed July 2006.
- 28.Centers for Disease Control and Prevention. 2005. Good laboratory practices for waived testing sites. Morb. Mortal. Wkly. Rep. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5413a1.htm. Accessed March 2006.
- 29.Chan, E. L., K. Brandt, and G. B. Horsman. 2001. Comparison of Chemicon SimulFluor direct fluorescent-antibody staining with cell culture and shell vial direct immunoperoxidase staining for detection of herpes simplex virus and with cytospin direct immunofluorescence staining for detection of varicella-zoster virus. Clin. Diagn. Lab. Immunol. 18:909-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan, K. H., N. Maldeis, W. Pope, A. Yup, A. Ozinskas, J. Gill, W. H. Seto, K. F. Shortridge, and J. S. M. Peiris. 2002. Evaluation of the Directigen Flu A+B test for rapid diagnosis of influenza virus type A and B infections. J. Clin. Microbiol. 40:1675-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesebro, B., and K. Wehrly. 1988. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J. Virol. 63:3779-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke, L. 1998. Viruses, rickettsiae, chlamydiae, and mycoplasmas, p. 451-559. In H. D. Isenberg (ed.), Essential procedures for clinical microbiology. ASM Press, Washington, DC.
- 33.Coffin, S. E., and R. L. Hodinka. 1995. Utility of direct immunofluorescence and virus culture for detection of varicella-zoster virus in skin lesions. J. Clin. Microbiol. 33:2792-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman, W. B., and G. J. Tsongalis (ed.). 2003. Molecular diagnostics for the clinical laboratorian, 2nd ed. Humana Press, Inc., Totowa, NJ.
- 35.Constantine, N., and R. Zhao. 2005. Molecular-based laboratory testing and monitoring for human immunodeficiency virus infections. Clin. Lab. Sci. 18:263-270. [PubMed] [Google Scholar]
- 36.Crist, G. A., J. M. Langer, G. L. Woods, M. Procter, and D. R. Hillyard. 2004. Evaluation of the ELVIS plate method for the detection and typing of herpes simplex virus in clinical specimens. Diagn. Microbiol. Infect. Dis. 49:173-177. [DOI] [PubMed] [Google Scholar]
- 37.Cruz, A. T., A. C. Cazacu, L. J. McBride, J. M. Greer, and G. J. Demmler. 2006. Performance characteristics of a rapid immunochromatographic assay for detection of influenza virus in children during the 2003 to 2004 influenza season. Ann. Emergency Med. 47:250-254. [DOI] [PubMed] [Google Scholar]
- 38.DeBiasi, R. L., and K. L. Tyler. 2004. Molecular methods for diagnosis of viral encephalitis. Clin. Microbiol. Rev. 17:903-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Jong, M. D., and T. T. Hien. 2006. A review: avian influenza A. J. Clin. Virol. 35:2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domiati-Saad, R., and R. H. Scheuermann. 2006. Nucleic acid testing for viral burden and viral genotyping. Clin. Chem. Acta 363:197-205. [DOI] [PubMed] [Google Scholar]
- 41.Dunn, J. J., C. Gordon, C. Kelley, and K. C. Carroll. 2003. Comparison of the Denka-Seiken INFLU AB-Quick and BD Directigen Flu A + B kits with direct fluorescent-antibody staining and shell vial culture methods for rapid detection of influenza viruses. J. Clin. Microbiol. 41:2180-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn, J. J., R. D. Woolstenhulme, J. Langer, and K. C. Carroll. 2004. Sensitivity of respiratory virus culture when screening with R-Mix Fresh Cells. J. Clin. Microbiol. 42:79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enders, J. F., T. H. Weller, and F. C. Robbins. 1949. Cultivation of the Lansing strain of poliomyelitis virus in cultures of various human embryonic tissues. Science 109:85-87. [DOI] [PubMed] [Google Scholar]
- 44.Erice, A., M. A. Holm, P. C. Gill, S. Henry, C. L. Dirksen, D. L. Dunn, R. P. Hillam, and H. H. Balfour, Jr. 1992. Cytomegalovirus (CMV) antigenemia assay is more sensitive that shell vial cultures for rapid detection of CMV in polymorphonuclear blood leukocytes. J. Clin. Microbiol. 30:2822-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Eskey, K., D. Z. Aird, and A. Valsamakis. 2006. Abstr. Clin. Virol. Symp., abstr. T-PM41.
- 45.Espy, M. J., T. F. Smith, M. W. Harmon, and A. P. Kendal. 1986. Rapid detection of influenza virus by shell vial assay with monoclonal antibodies. J. Clin. Microbiol. 24:677-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espy, M. J., J. R. Uhl, L. M. Sloan, S. P. Buckwalter, M. F. Jones, E. A. Vetter, J. D. C. Yao, N. L. Wengenack, J. E. Rosenblatt, F. R. Cockerill III, and T. F. Smith. 2006. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin. Microbiol. Rev. 19:165-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Espy, M. J., S. K. Schneider, P. A. Wright, S. Kadiyala, M. F. Jones, and T. F. Smith. 2004. Abstr. Clin. Virol. Symp., abstr M51.
- 47.Fader, R. C. 2005. Comparison of the Binax NOW Flu A enzyme immunochromatographic assay and R-Mix shell vial culture for the 2003-2004 influenza season. J. Clin. Microbiol. 43:6133-6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedorko, D. P., N. A. Nelson, J. M. McAuliffe, and K. Subbarao. 2006. Performance of the rapid tests for detection of avian influenza A virus types H5N1 and H9N2. J. Clin. Microbiol. 44:1596-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felber, B. K., and G. N. Pavlakis. 1988. A quantitative bioassay for HIV-1 based on trans-activation. Science 239:184-187. [DOI] [PubMed] [Google Scholar]
- 50.Fife, K. H., and L. Corey. 1989. Herpes simplex virus, p. 941-952. In K. K. Holmes, P. A Mardh, P. F. Sparling, and P. J. Weisner (ed.), Sexually transmitted disease, 2nd ed. McGraw Hill, New York, NY.
- 51.Fong, C. K., M. K. Lee, and B. P. Grith. 2000. Evaluation of R-Mix FreshCells in shell vials for detection of respiratory viruses. J. Clin. Microbiol. 38:4660-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forman, M. S., and A. Valsamakis. 2003. Specimen collection, transport, and processing: virology, p. 1227-1241. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 53.Forns, X., and J. Costa. 2006. HCV virological assessment. J. Hepatol. 44(Suppl.):S35-S39. [DOI] [PubMed] [Google Scholar]
- 54.Foulonge, V., G. Guyon, M. Rodiere, and M. Segondy. 2006. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr. Infect. Dis. J. 25:354-359. [DOI] [PubMed] [Google Scholar]
- 55.Freshney, R. I. 2000. Culture of animal cells, a manual of basic technique, 4th ed. Wiley-Liss, New York, NY.
- 56.Germann, D., M. Gorgievski, A. Strohle, and L. Matter. 1998. Detection of mumps virus in clinical specimens by rapid centrifugation culture and conventional tube cell culture. J. Virol. Methods 73:59-64. [DOI] [PubMed] [Google Scholar]
- 57.Gillim-Ross, L., J. Taylor, D. R. Scholl, J. Ridenour, P. S. Masters, and D. E. Wentworth. 2004. Discovery of novel human and animal cells infected by the severe acute respiratory syndrome coronavirus by replication-specific multiplex reverse transcription-PCR. J. Clin. Microbiol. 42:3196-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ginocchio, C. C. 2000. Laboratory diagnosis of human cytomegalovirus (HCMV) central nervous system disease in AIDS patients. Int. J. Antimicrob. Agents 16:447-453. [DOI] [PubMed] [Google Scholar]
- 59.Ginocchio, C. C. 2006. Viral infections of the central nervous system, p. 437-446. In D. G. B. Leonard (ed.), Molecular pathology in clinical practice. Springer-Verlag, Inc., New York, NY.
- 60.Ginocchio, C. C., F. Zhang, A. Malholtra, R. Manji, P. Sillekens, H. Foolen, M. Overdyk, and M. Peeters. 2005. Development, technical performance and clinical evaluation of a NucliSens basic kit application for the detection of enterovirus RNA in cerebrospinal fluid. J. Clin. Microbiol. 43:2616-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gleaves, C. A., C. F. Lee, C. I. Bustamante, and J. D. Meyers. 1988. Use of murine monoclonal antibodies for laboratory diagnosis of varicella-zoster virus infection. J. Clin. Microbiol. 26:1623-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gleaves, C. A., T. F. Smith, E. A. Shuster, and G. R. Pearson. 1984. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J. Clin. Microbiol. 19:917-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gleaves, C. A., D. J. Wilson, A. D. Wold, and T. F. Smith. 1985. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J. Clin. Microbiol. 21:20-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorgievski-Hrisoho, M., J. D. Schumacher, N. Vilimonovic, D. Germann, and L. Matter. 1998. Detection by PCR of enteroviruses in cerebrospinal fluid during a summer outbreak of aseptic meningitis in Switzerland. J. Clin. Microbiol. 36:2408-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grazia Revello, M., M. Zavattoni, E. Percivalle, P. Grossi, and G. Gerna. 1989. Correlation between immunofluorescent detection of human cytomegalovirus immediate early antigens in polymorphonuclear leukocytes and viremia. J. Infect. Dis. 160:159-160. [DOI] [PubMed] [Google Scholar]
- 66.Habib-Bein, N. F., W. H. Beckwith III, D. Mayo, and M. L. Landry. 2003. Comparison of Smart Cycler real-time reverse transcription-PCR assay in a public health laboratory with direct immunofluorescence and cell culture assays in a medical center for detection of influenza A virus. J. Clin. Microbiol. 41:3597-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henrickson, K. J. 2004. Advances in the laboratory diagnosis of viral respiratory disease. Pediatr. Infect. Dis. J. 23(Suppl. 1):S6-S10. [DOI] [PubMed] [Google Scholar]
- 67a.Henry, R. K. A. Fauntleroy, and D. H. Larone. 2006. Abstr. Clin. Virol. Symp., abstr. T-PM21.
- 68.Hijazi, Z., A. Pacsa, S. Eisa, A. el Shazli, and R. A abd el-Salam. 1996. Laboratory diagnosis of acute lower respiratory tract viral infections in children. J. Trop. Pediatr. 42:276-280. [DOI] [PubMed] [Google Scholar]
- 68a.Hill, R. J. D. Wisotzkey, D. Bankert, C. Vaughn, S. Holtzapple, M. Smith, S. Nelson, R. Peters, and A. C. Crist. 2004. Abstr. Clin. Virol. Symp., abstr. T32.
- 69.Hindiyeh, M., C. Goulding, H. Morgan, B. Kenyon, J. Langer, L. Fox, G. Dean, D. Woolstenhulme, A. Turnbow, E. Billetdeaux, S. Shakib, C. Gordon, A. Powers, G. Vardey, M. Johnson, L. Skodack-Jones, and K. Carroll. 2000. Evaluation of BioStar FLU OIA assay for rapid detection of influenza A and B viruses in respiratory specimens. J. Clin. Virol. 17:119-126. [DOI] [PubMed] [Google Scholar]
- 70.Hosoya, M., K. Honzumi, M. Sato, M. Katayose, K. Kato, and H. Suzuki. 1998. Application of PCR for various neurotrophic viruses on the diagnosis of viral meningitis. J. Clin. Virol. 11:117-124. [DOI] [PubMed] [Google Scholar]
- 71.Hsiung, G. D. 1984. Diagnostic virology: from animals to automation. Yale J. Biol. Med. 57:727-733. [PMC free article] [PubMed] [Google Scholar]
- 72.Huang, Y. T., S. Hite, V. Duane, and H. Yan. 2002. CV-1 and MRC-5 mixed cells for simultaneous detection of herpes simplex virus and varicella zoster virus in skin lesions. J. Clin. Virol. 24:37-43. [DOI] [PubMed] [Google Scholar]
- 73.Huang, Y. T., P. Yam, H. Yan, and Y. Sun. 2002. Engineered BGMK cells for sensitive and rapid detection of enteroviruses. J. Clin. Microbiol. 40:366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang, Y. T., H. Yan, Y. Sun, J. A. Jollick, Jr., and H. Baird. 2002. Cryopreserved cell monolayers for rapid detection of herpes simplex virus and influenza virus. J. Clin. Microbiol. 40:4301-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hughes, J. D. 1993. Physical and chemical methods for enhancing rapid detection of viruses and other agents. Clin. Microbiol. Rev. 6:150-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hulson, T. D., J. W. Mold, D. Scheid, M. Aaron, C. B. Aspy, N. L. Ballard, N. Boren, M. E. Gregory, and T. C. Truong. 2001. Diagnosing influenza: the value of clinical clues and laboratory tests. J. Family Practice 50:1051-1056. [PubMed] [Google Scholar]
- 76a.Jollick, J., Jr., Y. Li, J. Pappas, D. Scholl, J. Dyall, P. D. Olivo, and A. Pekosz. 2005. Abstr. Clin. Virol. Symp., abstr. M2.
- 76b.Karchava, M., M. Fuschino, J. Kinne, K. Rush-Wilson, and K. St. George. 2006. Abstr. Clin. Virol. Symp., abstr. T-AM42.
- 77.Klespies, S. L., D. E. Cebula, C. L. Kelley, D. Galehouse, and C. C. Maurer. 1996. Detection of enteroviruses from clinical specimens by spin amplification shell vial culture and monoclonal antibody assay. J. Clin. Microbiol. 34:1465-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kok, T. W., T. Pryor, and L. Payne. 1998. Comparison of rhabdomyosarcoma, buffalo green monkey kidney epithelial, A549 (human lung epithelial) cells and human embryonic lung fibroblasts for isolation of enteroviruses from clinical samples. J. Clin. Virol. 11:61-65. [DOI] [PubMed] [Google Scholar]
- 79.Kowalski, R. P., L. M. Karenchak, C. Shad, and J. S. Gordon. 2002. A new 24-hour culture test for detecting herpes simplex virus from ocular samples. Arch. Ophthalmol. 120:960-962. [DOI] [PubMed] [Google Scholar]
- 80.Kumar, R. 2005. Aseptic meningitis: diagnosis and management. Indian J. Pediatr. 72:57-63. [DOI] [PubMed] [Google Scholar]
- 81.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landry, M. L., S. Cohen, and D. Ferguson. 2000. Impact of sample type on rapid detection of influenza virus A by cytospin-enhanced immunofluorescence and membrane enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:429-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Landry, M. L., S. Cohen, and D. Ferguson. 2004. Comparison of Binax NOW and Directigen for rapid detection of influenza A and B. J. Clin. Virol. 31:113-115. [DOI] [PubMed] [Google Scholar]
- 84.Landry, M. L., and D. Ferguson. 2000. SimulFluor respiratory screen for rapid detection of multiple respiratory viruses in clinical specimens by immunofluorescence staining. J. Clin. Microbiol. 38:708-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Landry, M. L., D. Ferguson, S. Cohen, T. C. T. Peret, and D. D. Erdman. 2005. Detection of human metapneumovirus in clinical samples by immunofluorescence staining of shell vial centrifugation cultures prepared from three different cell lines. J. Clin. Microbiol. 43:1950-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Landry, M. L., D. Ferguson, and J. Wlochowski. 1997. Detection of herpes simplex virus in clinical specimens by cytospin-enhanced direct immunofluorescence. J. Clin. Microbiol. 35:302-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Landry, M. L., R. Garner, and D. Ferguson. 2003. Rapid enterovirus RNA detection in clinical specimens by using nucleic acid sequence-based amplification. J. Clin. Microbiol. 41:346-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Landry, M. L., and G. D. Hsiung. 2000. Primary isolation of viruses, p. 27-42. In S. Specter, R. L. Hodinka, and S. A. Young (ed.). Clinical virology manual, 3rd ed. ASM Press, Washington, DC.
- 89.LaRocco, M. T. 2000. Evaluation of an enzyme-linked viral inducible system for the rapid detection of herpes simplex virus. Eur. J. Clin. Microbiol. Infect. Dis. 19:233-235. [DOI] [PubMed] [Google Scholar]
- 90.Leland, D. S. 1996. Clinical virology. W. B. Saunders, Philadelphia, PA.
- 91.Leland, D. S., R. L. Hansing, and M. L. V. French. 1989. Clinical experience with cytomegalovirus isolation using both conventional cell cultures and rapid shell vial techniques. J. Clin. Microbiol. 27:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Limaye, A. P., R. Bakthavatsalam, H. W. Kim, C. S. Kuhr, J. B. Halldorson, P. J. Healey, and M. Boeckh. 2004. Late onset cytomegalovirus disease in liver transplant recipients despite antiviral prophylaxis. Transplantation 78:1390-1396. [DOI] [PubMed] [Google Scholar]
- 93.Limaye, A. P., L. Corey, D. M. Koelle, C. L. Davis, and M. Boeckh. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 356:645-649. [DOI] [PubMed] [Google Scholar]
- 94.Lloyd, W., M. Theiler, and N. I. Ricci. 1936. Modification of the virulence of yellow fever virus by cultivation in tissues in vitro. Trans. R. Soc. Trop. Med. Hyg. 19:481-529. [Google Scholar]
- 94a.Lotlikar, M., L. Falk, M. Bornfreund, and C. C. Ginnochio. 2003. Abstr. Clin. Virol. Symp., abstr. S19.
- 94b.Lotlikar, M., L. Falk, M. Bornfreund, and C. C. Ginnochio. 2004. Abstr. Clin. Virol. Symp., abstr. T7.
- 95.Lublin, D. M., and J. P. Atkinson. 1989. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu. Rev. Immunol. 7:35-58. [DOI] [PubMed] [Google Scholar]
- 96.Lutz, A., J. Dyall, P. D. Olivo, and A. Pekosz. 2005. Virus-inducible reporter genes as a tool for detecting and quantifying influenza A virus replication. J. Virol. Methods 126:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lyall, E. G., M. M. Ogilvie, N. M. Smith, and S. Burns. 1994. Acyclovir resistant varicella zoster and HIV infection. Arch. Dis. Child. 70:133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mackay, I. M., K. E. Arden, and A. Nitsche. 2002. Real-time PCR in virology. Nucleic Acids Res. 30:1292-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mackie, P. L., E. M. McCormick, and C. Williams. 2004. Evaluation of Binax NOW RSV as an acute point-of-care screening test in a paediatric accident and emergency unit. Communic. Dis. Public Health 7:328-330. [PubMed] [Google Scholar]
- 100.Madeley, C. R. 2004. Molecular and diagnostic clinical virology in real time. Clin. Microbiol. Infect. 10:5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100a.Mahony, J., S. Chong, F. Merante, K. Luinstra, T. Sinha, A. Petrich, M. Smieja, C. Lisle, S. Yaghoubian, and R. Janeczko. 2006. Abstr. Clin. Virol. Symp., abstr. T-AM56.
- 101.Major, E. O., M. Gravell, and J. Hou. 2003. Human polyomaviruses, p. 1524-1533. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.). Manual of clinical microbiology, 8th ed., vol. 2. ASM Press, Washington, DC. [Google Scholar]
- 102.Mavromoustakis, C. T., D. T. Witiak, and J. H. Hughes. 1988. Effect of high-speed rolling on herpes simplex virus detection and replication. J. Clin. Microbiol. 26:2328-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazzulli, T., L. Drew, B. Yen-Lieberman, D. Jekic-McMullen, D. J. Kohn, C. Isada, G. Moussa, R. Chua, and S. Walmsley. 1999. Multicenter comparison of the Digene Hybrid Capture CMV DNA assay (verson 2.0), the pp65 antigenemia assay, and cell culture for detection of cytomegalovirus viremia. J. Clin. Microbiol. 37:958-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mazzulli, T., R. H. Rubin, M. J. Ferraro, R. T. D'Aqila, S. A. Doveikis, B. R. Smith, T. H. The, and M. S. Hirsch. 1993. Cytomegalovirus antigenemia: clinical correlations in transplant recipients and in persons with AIDS. J. Clin. Microbiol. 31:2824-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Minnich, L. L., and G. C. Ray. 1987. Early testing of cell cultures for detection of hemadsorbing viruses. J. Clin. Microbiol. 25:421-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mommeja-Marin, H., E. Mondou, M. R. Blum, and F. Rousseau. 2003. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection. Analysis and review of the literature. Hepatology 37:1309-1319. [DOI] [PubMed] [Google Scholar]
- 107.Murdock, D. R. 2005. Impact of rapid microbiological testing on the management of lower respiratory tract infection. Clin. Infect. Dis. 41:1445-1447. [DOI] [PubMed] [Google Scholar]
- 108.Navarro-Mari, J. M., S. Sanbonmatsu-Gamez, M. Perez-Ruiz, and M. De La Rosa-Fraile. 1999. Rapid detection of respiratory viruses by shell vial assay using simultaneous culture of Hep-2, LLC-MK2, and MDCK cells in a single vial. J. Clin. Microbiol. 37:2346-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Newton, D. W., C. F. Mellen, B. D. Baxter, R. L. Atmar, and M. A. Menegus. 2002. Practical and sensitive screening strategy for detection of influenza virus. J. Clin. Microbiol. 40:4353-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niesters, H. G. 2001. Quantitation of viral load using real-time amplification techniques. Methods (Duluth) 25:419-429. [DOI] [PubMed] [Google Scholar]
- 111.Niesters, H. G. 2002. Clinical virology in real time. J. Clin. Virol. 25(Suppl. 3):S3-S12. [DOI] [PubMed] [Google Scholar]
- 112.Nix, W. A., M. S. Oberste, and M. A. Pallansch. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 44:2698-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Noyola, D. E., B. Clark, F. T. O'Donnell, R. L. Atmar, J. Greer, and G. J. Demmler. 2000. Comparison of a new neuraminidase detection assay with an enzyme immunoassay, immunofluorescence, and culture for rapid detection of influenza A and B viruses in nasal wash specimens. J. Clin. Microbiol. 38:1161-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogilvie, M. 2001. Molecular techniques should not now replace cell culture in diagnostic virology laboratories. Rev. Med. Virol. 1:351-354. [DOI] [PubMed] [Google Scholar]
- 115.Ohm-Smith, M. J., P. S. Nassos, and B. L. Haller. 2004. Evaluation of the Binax NOW, BD Directigen, and BD Directigen EZ assays for detection of respiratory syncytial virus. J. Clin. Microbiol. 42:2996-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olivo, P. D. 1996. Transgenic cell lines for detection of animal viruses. Clin. Microbiol. Rev. 9:321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olsen, M. A., K. M. Shuck, A. R. Sambol, S. M. Flor, J. O'Brien, and B. J. Cabrera. 1993. Isolation of seven respiratory viruses in shell vials: a practical and highly sensitive method. J. Clin. Microbiol. 31:422-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oosterheert, J. J., A. M. vanLoon, R. Schuurman, A. I. M. Hoepelman, E. Hak, S. Thijsen, G. Nossent, M. M. E. Schneider, W. M. N. Hustinx, and M. J. M. Bunten. 2005. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin. Infect. Dis. 41:1438-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patel, N., L. Kauffmann, G. Baniewicz, M. Forman, M. Evans, and D. Scholl. 1999. Confirmation of low-titer, herpes simplex virus-positive specimen results by the enzyme-linked virus-inducible system (ELVIS) using PCR and repeat testing. J. Clin. Microbiol. 37:3986-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paya, C. V., A. D. Wald, and T. F. Smith. 1987. Detection of cytomegalovirus infections in specimens other than urine by the shell vial assay and conventional tube cell cultures. J. Clin. Microbiol. 25:755-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peterson, E. M., B. L. Hughes, S. L. Aarnaes, and L. M. de la Maza. 1988. Comparison of primary rabbit kidney and MRC-5 cells and two stain procedures for herpes simplex virus detection by a shell vial centrifugation method. J. Clin. Microbiol. 26:222-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121a.Petrich, A., S. Castriciano, K. Luinstra, M. Booth, C. Robinson, M. MacPherson, A. Campanella, S. Chong, M. Smieja, and J. Mahoney. 2006. Abstr. Clin. Virol. Symp., abstr. T-PM22.
- 122.Phipps, P., H., B. G. McCulloch, H. R. Miller, and E. Rossier. 1989. Rapid detection of influenza virus infections in human fetal lung diploid cell cultures. J. Infect. 18:269-278. [DOI] [PubMed] [Google Scholar]
- 123.Pipkin, P. A., D. J. Wood, V. R. Racaniello, and P. D. Minor. 1993. Characterization of L cells expressing the human poliovirus receptor for the specific detection of polioviruses in vitro. J. Virol. Methods 41:333-340. [DOI] [PubMed] [Google Scholar]
- 124.Poehling, K. A., M. R. Griffin, R. S. Dittus, Y.-W. Tang, K. Holland, H. Li, and K. M. Edwards. 2002. Bedside diagnosis of influenza virus infections in hospitalized children. Pediatrics 110:83-88. [DOI] [PubMed] [Google Scholar]
- 125.Proffitt, M. R., and S. A. Schindler. 1995. Rapid detection of HSV with an enzyme-linked virus inducible system (ELVISTM) employing a genetically modified cell line. Clin. Diagn. Virol. 4:175-182. [DOI] [PubMed] [Google Scholar]
- 126.Rabalais, G. P., G. G. Stout, K. L. Ladd, and K. M. Cost. 1992. Rapid diagnosis of respiratory viral infections by using a shell vial assay and monoclonal antibody pool. J. Clin. Microbiol. 30:1505-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ray, C. G., and L. L. Minnich. 1987. Efficiency of immunofluorescence for rapid detection of common respiratory viruses. J. Clin. Microbiol. 25:355-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Read, S. J., K. M. J. Jeffrey, and C. R. M. Bangham. 1997. Aseptic meningitis and encephalitis: the role of PCR in the clinical laboratory. J. Clin. Microbiol. 35:691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reina, J. 1998. An increase in the number of polymorphonuclear leukocytes inoculated on shell-vial culture increases the sensitivity of this assay in the detection of cytomegalovirus in the blood of immunocompromised patients. Diagn. Microbiol. Infect. Dis. 31:425-428. [DOI] [PubMed] [Google Scholar]
- 130.Reina, J., V. Fernandez-Baca, I. Blanco, and M. Munar. 1997. Comparison of Madin-Darby canine kidney cells (MDCK) with a green monkey continuous cell line (Vero) and human lung embryonated cells (MRC-5) in the isolation of influenza A virus from nasopharyngeal aspirates by shell vial culture. J. Clin. Microbiol. 35:1900-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ribes, J. A., J. P. Seabolt, and S. B. Overman. 2002. Performance characteristics of VIDAS and Directigen respiratory syncytial virus (RSV) antigen detection assays and culture for the identification of RSV in respiratory specimens. J. Clin. Microbiol. 40:1818-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rigonan, A. S., L. Mann, and T. Chonmaitree. 1998. Use of monoclonal antibodies to identify serotypes of enterovirus isolates. J. Clin. Microbiol. 36:1877-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rivers, T. M., and S. M. Ward. 1935. Jennerian prophylaxis by means of intradermal injections of culture vaccine virus. J. Exp. Med. 62:549-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robbins, F. C., J. F. Enders, and T. H. Weller. 1950. Cytopathogenic effect of poliomyelitis viruses in vitro on human embryonic tissues. Proc. Soc. Exp. Biol. Med. 75:370-374. [DOI] [PubMed] [Google Scholar]
- 134a.Robinson, C., N. R. Jones, and J. Hansen. 2005. Abstr. Clin. Virol. Symp., abstr. M22.
- 135.Robinson, C. C., M. Willis, A. Meagher, K. E. Gieseker, H. Rotbart, and M. P. Glode. 2002. Impact of rapid polymerase chain reaction results on management of pediatric patients with enteroviral meningitis. Pediatr. Infec. Dis. J. 21:283-286. [DOI] [PubMed] [Google Scholar]
- 136.Roche, R. R., M. Alvarez, M. G. Guzman, M. Luis, and G. Kouri. 2000. Comparison of rapid centrifugation assay with conventional tissue culture method for isolation of dengue 2 virus in C6/36-HT cells. J. Clin. Microbiol. 38:3508-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rodriguez, W. J., R. H. Schwartz, and M. M. Thorne. 2002. Evaluation of diagnostic tests for influenza in a pediatric practice. Pediatr. Infect. Dis. J. 21:193-196. [DOI] [PubMed] [Google Scholar]
- 138.Rotbart, H. A., and J. R. Robero. 1995. Laboratory diagnosis of enteroviral infections, p. 401-418. In H. A. Rotbart (ed.), Human enterovirus infections. ASM Press, Washington, DC.
- 139.Ruest, A., S. Michaud, S. Deslandes, and E. H. Frost. 2003. Comparison of the Directigen Flu A + B Test, the QuickVue Influenza Test, and clinical case definition to viral culture and reverse transcription-PCR for rapid diagnosis of influenza virus infection. J. Clin. Microbiol. 41:3487-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salmon, V. C., B. R. Kenyon, and J. C. Overall, Jr. 1990. Cross contamination of viral specimens related to shell vial caps. J. Clin. Microbiol. 28:2820-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Savatier, N., D. Racancourt, C. Bonnerot, and J. F. Nicholas. 1989. A novel system for screening antiretroviral agents. J. Virol. Methods 26:229-235. [DOI] [PubMed] [Google Scholar]
- 142.Schmidt, N. J. 1969. Tissue culture technics for diagnostic virology, p. 81-178, In E. H. Lennette and N. J. Schmidt (ed.), Diagnostic procedures for viral and rickettsial infections, 4th ed. American Public Health Association, Inc., New York, NY.
- 143.Schultze, D., Y. Thomas, and W. Wunderli. 2001. Evaluation of an optical immunoassay for the rapid detection of influenza A and B viral antigens. Eur. J. Clin. Microbiol. Infect. Dis. 20:280-283. [DOI] [PubMed] [Google Scholar]
- 143a.Setterquist, S., and G. C. Gray. 2003. Abstr. Clin. Virol. Symp., abstr. S27.
- 144.Shah, K. 2000. Papovaviruses, p. 374-383. In S. Specter, R. L. Hodinka, and S. A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, DC.
- 145.She, R. C., G. Crist, E. Billetdeaus, J. Langer, and A. Petti. 2006, posting date. Comparison of multiple shell vial cell lines for isolation of enteroviruses: a national perspective. J. Clin. Virol. 37:151-155. [DOI] [PubMed] [Google Scholar]
- 146.Smith, T. F. 2000. Specimen requirements: selection, collection, transport, and processing, p. 11-26. In S. Specter, R. L. Hodinka, and S. A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, DC.
- 147.Stabell, E. C., and P. D. Olivo. 1992. Isolation of a cell line for rapid and sensitive histochemical assay for the detection of herpes simplex virus. J. Virol. Methods 38:195-204. [DOI] [PubMed] [Google Scholar]
- 148.Stabell, E. C., R. R. O'Rourke, G. A. Storch, and P. D. Olivo. 1993. Evaluation of a genetically engineered cell line and a histochemical β-galactosidase assay to detect herpes simplex virus in clinical specimens. J. Clin. Microbiol. 31:2796-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.St. George, K., M. J. Boyd, S. M. Lipson, D. Ferguson, G. F. Cartmell, L. H. Falk, C. R. Rinaldo, and M. L. Landry. 2000. A multisite trial comparing two cytomegalovirus (CMV) pp65 antigenemia test kits, Biotest CMV Brite and Bartels/Argene CMV Antigenemia. J. Clin. Microbiol. 38:1430-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.St. George, K., N. M. Patel, R. A. Hartwig, D. R. School, J. A. Jollick, Jr., L. M. Kauffmann, M. R. Evans, and C. R. Rinaldo, Jr. 2002. Rapid and sensitive detection of respiratory virus infections for directed antiviral treatment using R-Mix cultures. J. Clin. Virol. 24:107-115. [DOI] [PubMed] [Google Scholar]
- 151.St. George, K., D. T. Rowe, and C. R. Rinaldo. 2000. Cytomegalovirus, varicella-zoster virus, and Epstein-Barr virus, p. 410-449. In S. Specter, R. L. Hodinka, and S. A. Young (ed.), Clinical virology manual, 3rd ed. ASM Press, Washington, DC.
- 152.Stinehardt, E., C. Israeli, and R. Lambert. 1913. Studies on the cultivation of the virus of vaccinia. J. Infect. Dis. 13:204-300. [Google Scholar]
- 153.Storch, G. A. 2003. Rapid diagnostic tests for influenza. Curr. Opin. Pediatr. 15:77-84. [DOI] [PubMed] [Google Scholar]
- 154.Turchek, B. M., and Y. T. Huang. 1999. Evaluation of ELVIS™ HSV ID/Typing System for the detection and typing of herpes simplex virus from clinical specimens. J. Clin. Virol. 12:65-69. [DOI] [PubMed] [Google Scholar]
- 155.Van Doornum, G. J., and J. C. de Jong. 1998. Rapid shell vial culture technique for detection of enteroviruses and adenoviruses in fecal specimens: comparison with conventional virus isolation method. J. Clin. Microbiol. 36:2865-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vernet, G. 2004. Diagnosis of zoonotic viral encephalitis. Arch. Virol. Suppl. 18:231-244. [DOI] [PubMed] [Google Scholar]
- 156a.Vestal, D., M. Barger, and B. A. Body. 2003. Abstr. Clin. Virol. Symp., abstr. T39.
- 157.Vuorinen, T., R. Vainionpaa, and T. Hypia. 2003. Five years' experience of reverse transcriptase polymerase chain reaction in daily diagnosis of enterovirus and rhinovirus infections. Clin. Infect. Dis. 37:452-455. [DOI] [PubMed] [Google Scholar]
- 158.Waner, J. L. 1994. Mixed viral infections: detection and management. Clin. Microbiol. Rev. 7:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weinberg, A., L. Brewster, J. Clark, E. Simoes, and the ARIVAC Consortium. 2004. Evaluation of R-Mix shell vials for the diagnosis of viral respiratory tract infections. J. Clin. Virol. 30:100-105. [DOI] [PubMed] [Google Scholar]
- 160.Weinberg, A., and M. L. Walker. 2005. Evaluation of three immunoassay kits for rapid detection of influenza virus A and B. Clin. Diagn. Lab. Immunol. 12:367-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.West, P. G., B. Aldrich, R. Hartwig, and G. J. Haller. 1988. Increased detection rate for varicella-zoster virus with combination of two techniques. J. Clin. Microbiol. 26:2680-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161a.Wilkey, Z., J. Langer, and C. A. Petti. 2006. Abstr. Clin. Virol. Symp., abstr. T-PM42.
- 162.World Health Organization. 2005. WHO recommendations on the use of rapid testing for influenza diagnosis. World Health Organization, Geneva, Switzerland. www.who.int/csr/disease/avian_influenza/guidelines/rapidtestinfluenza_web.pdf.
- 163.Yang, W., S. Hite, and Y. T. Huang. 2005. Enhancement of cytomegalovirus detection in mink lung cells using CMV TurboTreat™. J. Clin. Virol. 34:125-128. [DOI] [PubMed] [Google Scholar]
- 164.Yolken, R. H., F. Coutlee, and R. P. Viscidi. 1989. New prospects for the diagnosis of viral infections. Yale J. Biol. Med. 62:131-139. [PMC free article] [PubMed] [Google Scholar]
- 165.Yuen, K. Y., P. K. S. Chang, M. Peiris, D. N. C. Tsang, T. L. Que, K. F. Shortridge, P. T. Cheung, W. K. To, E. T. F. Ho, R. Sung, A. F. B. Cheng and members of the H5N1 Study Group. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351:467-471. [DOI] [PubMed] [Google Scholar]
- 166.Zavattoni, M., E. Percivalle, E. Cattaneo, M. G. Revello, M. Torsellini, and G. Gerna. 2003. Optimized detection of respiratory viruses in nasopharyngeal secretions. New Microbiol. 26:133-140. [PubMed] [Google Scholar]
- 167.Zhang, F., S. Tetali, X. P. Wang, M. H. Kaplan, F. V. Cromme, and C. C. Ginocchio. 2000. Detection of human cytomegalovirus pp67 late gene transcripts in cerebrospinal fluid of human immunodeficiency virus type 1-infected patients by nucleic acid sequence-based amplification. J. Clin. Microbiol. 38:1920-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ziegler, T., M. Waris, M. Rautiainen, and P. Arstila. 1988. Herpes simplex virus detection by macroscopic reading after overnight incubation and immunoperoxidase staining. J. Clin. Microbiol. 26:2013-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]