Abstract
The “amitochondriate” protozoan parasites of humans Entamoeba histolytica, Giardia intestinalis, and Trichomonas vaginalis share many biochemical features, e.g., energy and amino acid metabolism, a spectrum of drugs for their treatment, and the occurrence of drug resistance. These parasites possess metabolic pathways that are divergent from those of their mammalian hosts and are often considered to be good targets for drug development. Sulfur-containing-amino-acid metabolism represents one such divergent metabolic pathway, namely, the cysteine biosynthetic pathway and methionine γ-lyase-mediated catabolism of sulfur-containing amino acids, which are present in T. vaginalis and E. histolytica but absent in G. intestinalis. These pathways are potentially exploitable for development of drugs against amoebiasis and trichomoniasis. For instance, l-trifluoromethionine, which is catalyzed by methionine γ-lyase and produces a toxic product, is effective against T. vaginalis and E. histolytica parasites in vitro and in vivo and may represent a good lead compound. In this review, we summarize the biology of these microaerophilic parasites, their clinical manifestation and epidemiology of disease, chemotherapeutics, the modes of action of representative drugs, and problems related to these drugs, including drug resistance. We further discuss our approach to exploit unique sulfur-containing-amino-acid metabolism, focusing on development of drugs against E. histolytica.
INTRODUCTION
Three protozoan parasites of humans, Entamoeba histolytica, Giardia intestinalis, and Trichomonas vaginalis, share various biological and biochemical characteristics, including anaerobic carbohydrate metabolism and the lack of typical mitochondria (“amitochondriate”). The ATP generation in these parasites occurs exclusively through substrate-level phosphorylation, despite differences in their life cycles and pathogenic properties (216, 217). As obligatory parasites, these organisms have a reduced ability for the de novo synthesis of essential building blocks of DNA and proteins, including nucleic acid precursors (7, 10, 322) and amino acids (7, 233, 247). As a consequence, certain metabolic pathways either are missing in these organisms or are divergent from those of mitochondriate organisms. Sulfur-containing-amino-acid metabolism represents one such divergent metabolic pathway in these three “amitochondriate” protists. Sulfur-containing amino acids are essential for a variety of biological activities, including protein synthesis, methylation, polyamine synthesis, coenzyme A production, cysteamine production, taurine production, iron-sulfur cluster (ISC) biosynthesis, and antioxidative stress defense (233). Besides the general importance of sulfur-containing amino acids, it was previously shown that a high concentration of extracellular cysteine is required for the growth, attachment, and survival of E. histolytica, T. vaginalis, and G. intestinalis under oxidative stress (2, 36, 106-110, 298).
Recent molecular and biochemical characterization of the sulfur-containing-amino-acid metabolism in these organisms revealed that metabolic pathways for sulfur-containing amino acids in E. histolytica, G. intestinalis, and T. vaginalis are distinct from those of their mammalian hosts in several ways. First, they lack part of both the forward and reverse transsulfuration pathways and thus are unable to complete transsulfuration sequences in either direction between methionine and cysteine. Second, they lack the enzymes responsible for cysteine and homocysteine degradation in mammals. Instead, E. histolytica and T. vaginalis possess a unique enzyme for the degradation of methionine, homocysteine, and cysteine called methionine γ-lyase. Third, E. histolytica and T. vaginalis are capable of sulfur-assimilatory de novo cysteine biosynthesis. Since aspects of sulfur-containing-amino-acid metabolism differ significantly between parasites and their mammalian hosts, molecular dissection and characterization of the unique properties of the sulfur-containing-amino-acid metabolism of these “amitochondriate” parasites should lead to the exploitation of new chemotherapeutic agents against infections caused by these pathogens. This review discusses the current therapeutic agents against infections by these “amitochondriate” protozoan parasites, their targets and mode of action, and the molecular mechanism of drug resistance. We also summarize various aspects of the unique sulfur-containing-amino-acid metabolism in these protozoa and discuss how these metabolic pathways could be exploited as novel targets for development of drugs against these infections.
EPIDEMIOLOGY, BIOLOGY, AND DISEASE
Entamoeba histolytica
The World Health Organization (WHO) estimated that 280 million people are infected each year and 2.5 million deaths occur annually from diarrheal diseases (314, 333). Entamoeba histolytica is an enteric unicellular protozoan parasite belonging to the Entamoebidae family. It causes amoebic colitis and extraintestinal abscesses in approximately 50 million inhabitants of areas of endemicity, resulting in an estimated 40,000 to 110,000 deaths annually and making this disease the second leading cause of death from parasitic diseases (321; WHO/PAHO/UNESCO report, presented at the WHO/PAHO/UNESCO meeting, Mexico City, Mexico, 28 to 29 January 1997). Other than imported cases, E. histolytica infection is rarely found in most industrialized countries, although infection with the closely related but commensal (noninvasive) species Entamoeba dispar is frequently found in these countries. E. dispar does not usually invade tissues and at most produces only superficial erosion of the colonic mucosa. In developed countries, travelers and immigrants are at risk of amoebiasis infections (293). In some developed countries, amoebiasis is domestically transmitted only in the restricted populations of the mentally handicapped and male homosexuals (232, 236).
E. histolytica has a simple life cycle consisting of two stages, an infective cyst stage and a proliferating trophozoite form. Human and certain nonhuman primates are its only natural hosts. Infection of the host occurs upon ingestion of water or food contaminated with cysts. E. histolytica cysts are round, usually 10 to 15 μm in diameter, and surrounded by a refractive wall containing chitin. After ingestion, the cyst excysts in the small intestine and forms the amoeboid trophozoite, which then colonizes the large intestine of the host. Colonization by E. histolytica trophozoites often results in an asymptomatic intestinal infection similar to that resulting from E. dispar (67). Unlike the inert cysts, E. histolytica trophozoites are highly motile, with polymorphic shapes and sizes varying from 10 to 50 μm in diameter. Trophozoites reproduce by binary fission, ingest bacteria and food particles, and adhere to and destroy epithelial cells in the bowel. The destruction of the epithelial tissue causes disease and symptoms. After penetration into the blood vessels, trophozoites are occasionally transported to extraintestinal organs, including the liver, lung, brain, and skin, and produce abscesses, often with lethal outcomes. Trophozoites transform into dormant and infectious cysts which are excreted into the environment. Although signals leading to encystation and excystation of E. histolytica are still poorly understood, osmolality changes, nutrient depletion (278), and adherence via galactose-binding lectin (79) are likely involved in encystation. In vitro encystation of the related reptilian species Entamoeba invadens was established by manipulating osmotic and nutrient conditions of axenic cultures (20, 278, 317). However, encystation has not been achieved with an axenic E. histolytica line.
Many individuals infected with E. histolytica have no symptoms and clear their infection without disease. However, up to 10% of asymptomatic infected individuals develop disease within a year of being infected (293). Clinical symptoms of amoebic colitis include bloody diarrhea and abdominal pain and tenderness. Multiple mucoid stools are common and are almost invariably heme positive. Fever is unusual except in cases with concurrent amoebic liver abscess. Fulminant amoebic colitis characterized by profuse bloody diarrhea, fever, pronounced leucocytosis, and severe abdominal pain is occasionally seen in individuals at risk, including pregnant women, immunocompromised individuals, patients receiving corticosteroids, and individuals with diabetes and alcoholism. Liver abscess is the most common extraintestinal manifestation of amoebic infection, most likely caused by hematogenous spread of amoebic trophozoites from the colon. Symptoms associated with amoebic liver abscess are fever, right upper quadrant pain, and hepatic tenderness and sometimes include cough, anorexia, and weight loss. Pleuropulmonary amoebiasis may also develop when an amoebic liver abscess is ruptured through the diaphragm. Patients with pleuropulmonary amoebiasis have chest pain, pleural effusions, atelectasis, and respiratory distress. Rupture into the peritoneum occurs occasionally, leading to peritonitis and shock in some individuals with amoebic liver abscess. Patients with amoebic liver abscess infrequently develop rupture into the pericardium, leading to pericarditis, dyspnoea, tachycardia, and cardiac tamponade. Amoebic brain abscess, although very rare, may also occur with concomitant amoebic liver abscess. Clinical symptoms include headache, vomiting, and seizures. The onset and progression of amoebic brain abscess is very rapid, and outcomes are often lethal. Comprehensive reviews of the pathophysiology and clinical signs and symptoms are found elsewhere (122, 293).
E. histolytica is considered to be anaerobic or microaerophilic because it is sensitive to oxygen yet is able to reduce it to water (296). E. histolytica can grow only under reduced oxygen tension in vitro (325). Toxic reactive oxygen derivatives, including superoxide, are produced by the electron transfer that occurs during energy metabolism. E. histolytica possesses iron-containing superoxide dismutase (SOD) (39) for the detoxification of reactive oxygen intermediates, but it lacks catalase, peroxidase, and enzymes for glutathione synthesis and metabolism (92, 205, 325), which are almost ubiquitously present in aerobes and are responsible for the removal of hydrogen peroxide. However, E. histolytica possesses alternative mechanisms for detoxification of the reactive oxygen species. The alternative mechanism depends on reducing agents (thiols), especially cysteine. In glutathione-depleted medium, cysteine was the main thiol compound in E. histolytica and was present largely in the thiol rather than the disulfide form (92). As mentioned above, extracellular cysteine is required to maintain a reducing environment and is essential for growth, attachment, and survival of E. histolytica trophozoites. It is conceivable that cysteine and other, as-yet-uncharacterized thiols are involved in the regulation of antioxidative defense mechanisms that likely consist of SOD (39, 297), peroxiredoxin (a thiol-specific antioxidant) (323), p34 NADPH oxidoreductase (38), and rubrerythrin (185, 205). However, several key components of the pathway have not been identified, e.g., a reducing system for peroxiredoxin and rubrerythrin.
Giardia intestinalis
G. intestinalis, also known as Giardia lamblia or Giardia duodenalis, is a flagellated protist that belongs to the order Diplomonadida and is the most commonly detected protozoan parasite in the intestinal tract (199). G. intestinalis infects >40 animal species, and the disease is considered zoonotic (199). WHO estimates that 280 million people are infected each year with G. intestinalis (314, 333). The prevalence is very high in children in developing countries but decreases to 2 to 7% in developed nations (95, 199). Water- and food-borne transmission is the main route of transmission of giardiasis in developing countries (241). While food-borne outbreaks of Giardia infections are common in developing countries, waterborne outbreaks have occurred and could reoccur in developed countries (25, 212). Waterborne infections can be prevented by proper filtration of surface water supplies. It has also been reported that in certain areas of world, water contaminated with G. lamblia cysts commonly causes travel-related giardiasis in tourists (33). In addition, direct person-to-person transmission via a fecal-oral route during sexual intercourse, in the mentally handicapped population, and in day care settings has been demonstrated (7, 246).
The life cycle of G. intestinalis consists of two stages, an infective cyst and a proliferating trophozoite form. The cyst is the infective form of the parasite and can survive in water for several months (320). The ingested cyst excysts in the duodenum and then becomes a motile trophozoite after passing through the acidic environment of the stomach. The trophozoite reproduces by binary fission and attaches to the duodenal or jejunal mucosa (7, 8, 88), where it causes symptoms. Environmental stimuli, including cholic acid and low cholesterol, induce trophozoites to encyst (111, 189). However, the biochemical and physiological significance of cholesterol deprivation and the role of bile salts and fatty acids in the encystation process remain controversial. For the related issues of encystation and excystation of G. intestinalis and E. histolytica, recent reviews should be consulted (7, 78).
A range of symptoms, including nausea, vomiting, stomach cramps, and diarrhea, occur after an incubation period of 1 to 2 weeks. The acute phase of infection usually lasts 3 to 4 days, but symptoms sometimes persist for a longer period (8). In children, a failure-to-thrive syndrome caused by infection with high parasite burdens and malnutrition occasionally results in severe loss of body weight (up to 20%) (95). Thus, in the developing world, giardiasis is considered to be an important cause of morbidity (43). In the small intestine, infestation with the organism reduces the number of microvilli and the surface area at the brush border, causing atrophy of the villi and enterocyte immaturity. It also decreases disaccharidase and other luminal enzymes and causes malabsorption of electrolytes (43). A number of other symptoms are associated with giardiasis (43), but it is unlikely that these symptoms are causally connected to the infection. Consult recent reviews for updates on the pathogenesis, pathology, and cell biology of giardiasis (280).
The pathogenesis of giardiasis is partially attributable to the enzymes produced by the parasite, including serine and cysteine proteases which disrupt the epithelial barrier in the intestine, causing inflammation and an immune response in the host. Giardia trophozoites induce apoptosis of enterocytes, which is associated with disruption of the cytoskeleton and tight junctions. It has also been reported that disruption of cellular F-actin and ZO-1 in tight junctions and the concurrent increase in enterocyte permeability are modulated by caspase-3 and myosin light chain kinase. Giardia proteases are proposed to activate protease-activated receptor 1, a unique class of G protein-coupled signaling receptors which modulate apoptosis and increase enterocyte permeability (280).
Trichomonas vaginalis
T. vaginalis is a flagellated protozoan parasite belonging to the Trichomonadidae family. Worldwide, it is responsible for 180 million trichomoniasis infections annually (261). Trichomoniasis is a sexually transmitted disease (STD) of the genital tract, and the reported prevalence is extremely high in some developing countries. During an antenatal class in a rural region of South Africa, 65% of pregnant women were reportedly severely infected with T. vaginalis (135, 279). It was also reported that 67% of women who visited an STD clinic in Ulaanbaatar, Mongolia, were infected with T. vaginalis (281, 282). T. vaginalis was isolated from 14% to 60% of the male partners of infected women and from 67% to 100% of the female partners of infected men. Trichomoniasis is often associated with human immunodeficiency virus (HIV)/AIDS transmission and is known to be worsened by HIV infection (91, 211, 282, 292). In addition to venereal infection, rare cases of perinatal transmission have also been reported (121, 198).
The T. vaginalis life cycle consists of a single dividing trophozoite stage; no dormant (e.g., cyst) stage has been identified in this parasite. The trophozoite varies in size and shape, with an average length and width of 10 μm and 7 μm, respectively (134). In axenic culture, the shape of the trophozoite tends to be more uniform, e.g., pear or oval shaped, but the parasite takes on a more amoeboid appearance when attached to vaginal epithelial cells (19, 124). The trophozoite has four free anterior flagella [9(2) + 2 arrangement] and a single recurrent flagellum incorporated into an undulating membrane which is supported by a noncontractile costa (282). The trophozoite divides by binary fission (247).
T. vaginalis infection in women ranges from an asymptomatic carrier state to profound acute inflammatory disease (261). The parasite generally infects the squamous epithelium of the genital tract, but it is occasionally recovered from the urethra, fallopian tubes, and pelvis (117, 247). The incubation period is 4 to 28 days in about 50% of infected individuals (247). The clinical picture in acute infection includes vaginal discharge, odor, and edema or erythema. The discharge is classically described as frothy, but actually it is frothy in only 10% of patients (282). Small punctuate hemorrhagic spots may be found on the vaginal and cervical mucosae in 2 to 3% of patients. These signs and symptoms are cyclic and worsen around the time of menses. Other complications associated with trichomoniasis include adnexitis, pyosalpinx, and endometritis (260); infertility and low birth weight (123); and cervical erosion (204). In males, T. vaginalis infection is often asymptomatic but occasionally causes urethritis and prostatitis (166). Urogenital trichomoniasis in men is categorized into three groups: an asymptomatic carrier state identified through an investigation of the sexual contacts of infected women, acute trichomoniasis characterized by profuse purulent urethritis, and mild symptomatic disease which is clinically indistinguishable from other causes of nongonococcal urethritis. The duration of infection is 10 days or less in most male patients. In symptomatic men, common complaints include scanty clear to mucopurulent discharge, dysuria, and mild pruritus or a burning sensation immediately after sexual intercourse (166). Complications associated with trichomoniasis include nongonococcal urethritis, prostatitis, balanoposthitis, urethral disease, and infertility (131, 167, 197). Pneumonia, bronchitis, and oral infection caused by T. vaginalis have also been documented (132). In rare cases, respiratory infections are acquired perinatally from infected mothers (132, 158). In children, T. vaginalis can infect the urinary tract as well as the vagina. It has been suggested that T. vaginalis infection is associated with sterility, but there is no unequivocal report available. In contrast, there is an established causal relationship between T. vaginalis infection and adverse pregnancy outcomes (9). Similarly, T. vaginalis infection was associated with low birth weight and preterm delivery in 40% of black women (57, 279). In that study, in which 14,000 American women were examined, Trichomonas was also associated with high infant mortality. In cattle, Trichomonas foetus infection causes infertility, which results in a tremendous economic loss (55, 133). It was suggested that T. vaginalis infection, as well as other STDs, increases susceptibility to HIV infection due to local inflammation and microscopic breaches in mucosal barriers (282). It was also suggested that T. vaginalis infection predisposes HIV carriers to symptomatic AIDS (211, 292). However, this issue is still debatable. Conversely, immunosuppression from HIV infection increases susceptibility to STDs (173, 211, 250, 282, 283, 292).
ENERGY METABOLISM OF “AMITOCHONDRIATE” PARASITES
Divergence of Mitochondrion-Related Organelles
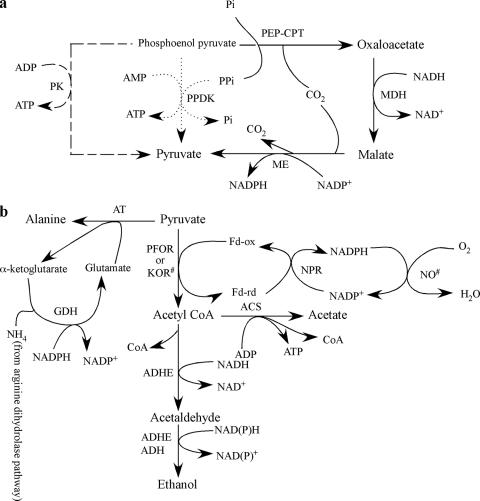
Most higher eukaryotic organisms, including mammals, depend primarily on aerobic metabolism for their energy production. In contrast, certain anaerobic/microaerophilic eukaryotes, including Entamoeba, Giardia, and Trichomonas, lack typical and morphologically discernible mitochondria and the cytochrome-mediated oxidative phosphorylation found in aerobic organisms (217). Accordingly, these three parasites are often misleadingly referred to as “amitochondriate” protists. However, it is now well established that these organisms possess mitochondrion-related organelles, often with reductive or sometimes divergent functions (49, 104, 179, 194, 303, 304). For detailed discussions of the phylogenetic and biochemical aspects of the mitochondrion-related organelles, consult references 85, 86, 87, and 316. For the most recent reviews on the evolution of “amitochondriate” parasites, see references 84 and 156. These “amitochondriate” protists rely on substrate-level phosphorylation during glycolysis for ATP generation and mostly on fermentative metabolism for energy production (Fig. 1a and b). While the vestigial organelles or “mitosomes” (or “crypton” in E. histolytica) in E. histolytica and G. intestinalis retain only residual mitochondrial functions (49, 104, 194, 303, 304), the highly specialized mitochondrion-related organelle or “hydrogenosome” in T. vaginalis has compartmentalized pyruvate oxidation (168, 179, 215-219) (Fig. 2).
FIG. 1.
Biosynthesis of pyruvate and its end products in “amitochondriate” protozoan parasites. (a) Synthesis of pyruvate from phosphoenolpyruvate in G. intestinalis, E. histolytica, and T. vaginalis. CPT, carboxyphosphotransferase; MDH, malate dehydrogenase (oxaloacetate to malate); ME, malic enzyme (decarboxylating, malate to pyruvate); Pi, inorganic phosphate; PPi, inorganic pyrophosphate. Solid arrows indicate a pathway that is optional in all three protists, while a pathway that generally plays a major role in the conversion of PEP to pyruvate is represented by dotted arrows for E. histolytica and G. intestinalis and by dashed arrows for T. vaginalis. (b) Metabolic pathways from pyruvate to its end products under anaerobic, microaerophilic, or aerobic conditions in E. histolytica and G. intestinalis. AT, alanine aminotransferase; GDH, glutamate dehydrogenase; KOR, 2-keto acid oxidoreductase; NO, NADH oxidase; ACS, acetyl-CoA synthase; ADHE, acetaldehyde/alcohol dehydrogenase (NAD dependent); ADH, alcohol dehydrogenase (NADP dependent); NPR, nitroperoxiredoxin or ferredoxin nitroreductase; Fd-ox, oxidized ferredoxin; Fd-rd, reduced ferredoxin. Acetate is the major product under aerobic conditions, while ethanol or alanine is preferentially produced under microaerophilic or strict anaerobic conditions, respectively. Note that ADHE is a fusion protein of acetaldehyde dehydrogenase and alcohol dehydrogenase. #, enzymes demonstrated only in G. intestinalis.
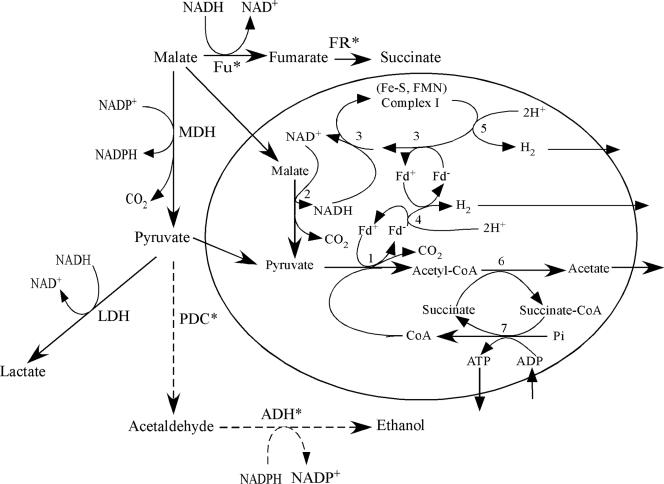
FIG. 2.
Schematic map of pyruvate metabolism in the cytosol and the hydrogenosomes of metronidazole-sensitive and -resistant T. vaginalis and T. foetus. Enzymes marked with asterisks are demonstrated only in T. foetus. Steps depicted by dashed arrows were demonstrated only in metronidazole-resistant T. foetus strains. The major cytosolic end product in metronidazole-susceptible strains of T. vaginalis is lactate, and that in T. foetus is succinate. In metronidazole-resistant T. vaginalis or T. foetus, the major cytosolic end product is lactate or ethanol, respectively. LDH is not present in T. foetus. Acetate is produced only in hydrogenosomes of metronidazole-susceptible T. vaginalis and T. foetus. Abbreviations are as follows: MDH, malate dehydrogenase (decarboxylating, malate to pyruvate); LDH, lactate dehydrogenase; Fu, fumarase; FR, fumarate reductase; PDC, pyruvate decarboxylase; ADH, alcohol dehydrogenase; Fd-ox, oxidized ferredoxin; Fd-rd, reduced ferredoxin; Pi, inorganic phosphate. Steps indicated by numbers are catalyzed by the following enzymes: 1, pyruvate:ferredoxin oxidoreductase; 2, NADH-dependent malate dehydrogenase (decarboxylating); 3, NADH:ferredoxin oxidoreductase activity of 51-kDa and 24-kDa catalytic flavoprotein components of complex I; 4, ferredoxin-dependent Fe-hydrogenase; 5, hypothetical NADH-dependent 65-kDa Fe-hydrogenase; 6, acetate:succinate CoA-transferase; 7, succinate thiokinase.
Besides compartmentalized energy metabolism in the T. vaginalis hydrogenosome, functional differences exist in the mitochondrion-related organelles among these three organisms. It was recently demonstrated that the ISC pathway, involved in iron-sulfur (Fe-S) cluster formation, is localized in the mitosomes of G. intestinalis and the hydrogenosomes of T. vaginalis (294, 304). In contrast, the E. histolytica mitosome lacks the ISC system. Instead, E. histolytica possesses a nitrogen fixation (NIF)-like system localized in the cytosol that is similar to that found in enteric Epsilonprotobacteria (15; V. Ali et al., unpublished data). Fe-S cluster biosynthesis is further described in “Physiological Importance of Cysteine and Fe-S Cluster Biosynthesis” below.
Diversity in the Conversion of Phosphoenolpyruvate to Acetyl Coenzyme A via Pyruvate
In Entamoeba, Giardia, and Trichomonas, glucose is not oxidized to CO2 and H2O as it is in aerobic metabolism but is instead catabolized to acetate, succinate, ethanol, alanine, and CO2. The predominant metabolic end products and catabolic pathways depend on the organism and its environmental and physiological (e.g., drug sensitivity) states (see below). The pathways involved in the biosynthesis of pyruvate from phosphoenolpyruvate (PEP) diverge among these organisms (Fig. 1a). G. intestinalis can utilize three pathways to synthesize pyruvate: through pyruvate kinase (PK), pyruvate phosphate dikinase (PPDK), and a pathway mediated by PEP carboxyphosphotransferase, malate dehydrogenase (oxaloacetate to malate), and malic enzyme (decarboxylating, malate to pyruvate). It was assumed that, in G. intestinalis, the conversion of PEP to pyruvate operated mainly through PPDK (7). Although it was reported earlier that E. histolytica lacks PK activity (255) and that pyruvate is synthesized either by PPDK or through the third pathway mediated by PEP carboxyphosphotransferase, malate dehydrogenase, and malic enzyme, a recent report demonstrated the presence of PK activity and a putative PK gene in E. histolytica (269). No PPDK activity has been detected in T. vaginalis (209), and pyruvate is synthesized predominantly by PK or through the oxaloacetate/malate pathway. Although two PPDK genes were reported recently in T. vaginalis (288), functional characterization of these genes is lacking. The glycolytic pyrophosphate-dependent enzymes PPDK and phosphofructokinase are proposed targets for therapeutic intervention. These pyrophosphate-dependent enzymes are absent in the human host and can be inhibited with pyrophosphate analogues such as bisphosphonates. In order to validate PPDK as a rational drug target, it is essential to understand which pathway plays the major role in pyruvate formation in these protozoan parasites. In particular, mechanisms that control the expression of individual pathways depending upon substrate availability, oxygen tension, and other environmental factors are not well understood.
The conversion of pyruvate to acetyl coenzyme A (acetyl-CoA) is catalyzed by pyruvate:ferredoxin oxidoreductase (PFOR), which utilizes ferredoxin rather than NAD+ as an electron acceptor in E. histolytica (257), G. intestinalis (307, 308), and T. vaginalis (330) in place of the pyruvate dehydrogenase complex found in aerobic bacteria and eukaryotes. Pyruvate dehydrogenase in the mitochondria of aerobic eukaryotes catalyzes an oxidative decarboxylation to form acetyl-CoA and NADH. Energy metabolism in these protists was recently reviewed in more detail (112, 217).
Predominant End Products of Energy Metabolism
The end products of energy metabolism in “amitochondriate” protozoa are influenced by the O2 tension (7). In addition, the major fermentation end products differ remarkably among these three organisms. For instance, in G. intestinalis, which has alanine aminotransferase, alanine is the major product of carbohydrate metabolism under strict anaerobic conditions (Fig. 1b) (243, 244). However, ethanol production is stimulated under conditions of low oxygen (<0.25 μM), while alanine production is suppressed; the major end products are ethanol and CO2 (243). Under conditions of higher oxygen (>46 μM), where alanine production is completely inhibited, the major products from acetyl-CoA are acetate and CO2. In contrast, the major end product in E. histolytica under anaerobic conditions is ethanol. A putative alanine aminotransferase gene is present in the E. histolytica genome, but its functional identity needs to be demonstrated (185). Under microaerophilic conditions, both ethanol and acetate are formed as end products (203, 325).
In Trichomonas, where part of the energy metabolism is compartmentalized in the hydrogenosome, electrons produced by the oxidation of pyruvate catalyzed by PFOR are donated to an Fe-hydrogenase via ferredoxin, which produces hydrogen gas (H2) by transferring electrons to hydrogen ions (Fig. 2). ATP is generated exclusively by substrate-level phosphorylation in the hydrogenosomes (75). In wild-type T. vaginalis, the major end product from pyruvate is lactate produced by lactate dehydrogenase in the cytosol; lactate dehydrogenase is present only in T. vaginalis. The preferred end product also differs between Trichomonas species and between wild-type and drug-resistant strains (168) (see below). In metronidazole-susceptible T. foetus, a Trichomonas species that affects cattle, either succinate or ethanol is the major or minor metabolic end product, respectively, in the cytosol (168), because lactate dehydrogenase is absent in this organism. In the hydrogenosomes, acetate is the only end product of both metronidazole-susceptible T. vaginalis and T. foetus strains. In contrast, major end products in the cytosol of metronidazole-resistant T. vaginalis and T. foetus strains are lactate and ethanol, respectively.
Recently the NADH dehydrogenase module (also called NADH:ubiquinone oxidoreductase) of complex I was identified in T. vaginalis (75, 140, 141). Similar to mitochondrial respiratory complex I, NADH dehydrogenase can reduce a variety of electron carriers, including ubiquinone. Unlike the mitochondrial enzyme, ferredoxin is used as an electron carrier for hydrogen production in a reaction catalyzed by ferredoxin-dependent Fe-hydrogenase. Malate is one of the major hydrogenosomal substrates and is oxidatively decarboxylated to form pyruvate and CO2 by a NAD-dependent malic enzyme. The electrons are transferred from NADH to ferredoxin by an NADH dehydrogenase homologous to the catalytic module of mitochondrial complex I. Thus, the discovery of an NADH dehydrogenase module of complex I solved the long-lasting conundrum of how Trichomonas regenerates NAD+, which is essential for malate oxidation in the hydrogenosome (Fig. 2).
It was previously shown that T. vaginalis possesses two additional 2-keto acid oxidoreductases besides PFOR for energy production (34). These two 2-keto acid oxidoreductases, KOR1 and KOR2, prefer indolepyruvate as a substrate. KOR1 is present in both metronidazole-sensitive and -resistant strains, while KOR2 is present only in metronidazole-resistant strains of T. vaginalis (34, 73, 313). It was reported that 2-keto acid oxidoreductase activity increased in the metronidazole-resistant strain (34). However, neither KOR1 nor KOR2 could donate electrons to ferredoxin in the metronidazole-resistant line, because no detectable ferredoxin was produced in this strain (73) (see below).
Three Major Iron-Sulfur Proteins That Play an Essential Role in Energy Metabolism
Pyruvate:ferredoxin oxidoreductase.
Eukaryotic PFOR is a homodimeric protein with a molecular mass of ∼200 to 300 kDa containing 2[4Fe-4S] clusters and thiamine phosphate. Its structure is similar to that of PFORs from a wide range of eubacteria (138); e.g., PFOR from the eubacterium Clostridium acetobutylicum is a homodimer of 123-kDa subunits (207). PFOR from the thermophilic archaebacterium Pyrococcus furiosus is composed of four fragmented subunits (29, 161), all of which share significant homology to PFORs from eubacteria, fungi, and protists (138). PFORs from E. histolytica, G. intestinalis, and T. vaginalis are homodimers of 240 to 280 kDa containing 2[4Fe-4S] clusters and thiamine phosphate (257, 264, 308, 330). Despite the similar overall structure and the Fe-S clusters of PFORs in these “amitochondriate” protists, the intracellular localization of PFOR is divergent. While PFORs from both archaea and eubacteria are cytosolic, biochemical characterization (179, 330) has established that PFOR is localized to hydrogenosomes and is associated with the hydrogenosome membrane in T. vaginalis (330). In addition, a 120-kDa surface glycoprotein that shares cross-reacting epitopes with PFOR is involved in the adhesion of T. vaginalis trophozoites (213). This may suggest that PFOR or a PFOR-related protein is associated with hydrogenosomes and the plasma membrane, but this premise is still a matter of debate and needs to be independently verified. The PFORs from both G. intestinalis (81, 308) and E. histolytica (263, 274) were also suggested to be associated with the plasma membrane. PFOR was shown to be associated with the plasma membrane and with a cytoplasmic structure in E. histolytica that appeared as a ring form or a compact small body (263). However, the majority of PFOR activity was detected in an 80,000 × g supernatant fraction (257), suggesting that the proposed membrane association of E. histolytica PFOR (263, 274) might be transient and/or weak. At the primary sequence level, T. vaginalis PFOR possesses the putative mitochondrial targeting peptide at the amino terminus (142). In contrast, neither E. histolytica nor G. intestinalis PFOR possesses the mitochondrial targeting sequence. Since pyruvate metabolism is not compartmentalized in either of these organisms like it is in T. vaginalis, the physiological significance of the possible membrane association of PFOR in G. intestinalis and E. histolytica is not well understood.
Ferredoxin.
Ferredoxin is another important class of proteins containing Fe-S clusters. Ferredoxins in the three microaerophilic/anaerobic parasitic protists differ in the nature of the Fe-S clusters they contain and their intracellular localization, i.e., cytosolic or hydrogenosomal. The E. histolytica genome encodes at least three ferredoxins (185), all of which contain either 2[4Fe-4S] clusters or [4Fe-4S] and [3Fe-4S] clusters, which correspond to a molecular mass of ∼6 kDa, as previously characterized (256). E. histolytica apparently lacks [2Fe-2S] ferredoxin (15, 17), but ferredoxins containing these clusters are present in T. vaginalis and G. intestinalis. A larger ferredoxin from Trichomonas with one [2Fe-2S] cluster corresponding to a molecular mass of ∼10 kDa was biochemically characterized and was localized in hydrogenosomes (116). The genome of G. intestinalis encodes at least four ferredoxins: one ferredoxin containing one [2Fe-2S] cluster (ferredoxin I) and three ferredoxins containing either 2[4Fe-4S] clusters or one [4Fe-4S] cluster and one [3Fe-4S] cluster (ferredoxins 1 to 3) (229). Among these ferredoxins, only ferredoxin I was shown to accept electrons from PFOR in vitro (308). The physiological role of [4Fe-4S] ferredoxins in G. intestinalis remains unclear. The small 2[4Fe-4S] ferredoxin of E. histolytica is evolutionarily closest to ferredoxin from anaerobic bacteria (143), while the larger [2Fe-2S] ferredoxin of Trichomonas hydrogenosomes shows a close kinship to ferredoxins of the cytochrome P450-linked mixed-function oxidase systems of bacterial and vertebrate mitochondria (154).
In addition, G. intestinalis possesses two genes, and E. histolytica possesses one gene, encoding a putative ferredoxin nitroreductase which consists of an amino-terminal 2[4Fe-4S] ferredoxin domain and a carboxyl-terminal nitroreductase domain (229). This ferredoxin-nitroreductase may be a physiological acceptor of electrons from ferredoxin or PFOR and may be responsible for the activation of metronidazole. A ferredoxin-nitroreductase with a similar overall structure was also discovered in the whole-genome sequence of Clostridium acetobutylicum (231).
T. vaginalis ferredoxin possesses a [2Fe-2S] cluster and an 8-amino-acid putative mitochondrial targeting sequence at the amino terminus (154), similar to that of G. intestinalis [2Fe-2S] ferredoxin. Recently, the mitosome-targeting signal of G. intestinalis [2Fe-2S] ferredoxin was shown to target ferredoxin to the hydrogenosome of T. vaginalis, suggesting that the mitosome and the hydrogenosome share a common mode of protein targeting similar to the protein import machinery of mitochondria (69). Similarly, the mitochondrial targeting signal from Trypanosoma cruzi was also shown to be interchangeable with that of E. histolytica (303, 316). While import of ferredoxin and IscU, a scaffolding protein for iron-sulfur biosynthesis, relies on the amino-terminal signal, the targeting of other mitosomal proteins (e.g., the catalytic component of iron-sulfur cluster biosynthesis IscS, Cpn60, and mitochondrial Hsp70) into the mitosome does not require this presequence in G. intestinalis (258). The underlying mechanisms of mitosome import of the latter proteins are not clear, but they likely possess cryptic internal signals, as demonstrated in the ADP-ATP carrier protein localized in the hydrogenosome of T. vaginalis (74) and other proteins found in the inner membrane of mitochondria (248). Mitosome import of Cpn60 relies on the presequence in E. histolytica (303), suggesting organism dependence in the targeting mechanisms. It also remains to be determined which ferredoxin(s) among the three types is involved in energy metabolism and redox regulation as well as the activation of metronidazole in E. histolytica and G. intestinalis. It is also unknown why different types of ferredoxins are retained throughout the evolution of these parasites.
Hydrogenase.
Hydrogenases are enzymes responsible for producing hydrogen gas by transferring electrons to two protons. Hydrogenases are oxygen sensitive and are present in a wide range of anaerobic bacteria and hydrogenosomes from certain microaerophilic/anaerobic protozoan parasites, ciliates, fungi, and chytrids (86, 87, 136, 251). Two types of hydrogenases are known; heterodimeric NiFe-hydrogenase and monomeric Fe-hydrogenase. The heteromeric NiFe-hydrogenases consist of a large subunit containing a bimetalic NiFe cluster at the hydrogen-activating site and a small subunit containing up to three Fe-S clusters that transport electrons to the physiological acceptors. NiFe-hydrogenases are generally involved in H2 uptake in archaebacteria and eubacteria (46). In contrast, the mostly monomeric Fe-hydrogenases usually catalyze H2 evolution (98). In some bacteria, e.g., the hyperthermophilic bacterium Thermotoga martima and the anaerobic bacterium Desulfovibrio fructosovorans, Fe-hydrogenase is composed of two or three subunits sharing homology with monomeric Fe-hydrogenases (113, 318). T. vaginalis possesses at least three Fe-hydrogenase genes (42, 137). There are two types of Fe-hydrogenases, referred as “short-form” (50-kDa) and “long-form” (64-kDa) Fe-hydrogenases (86). These Fe-hydrogenases, which differ in size due to the heterogeneity at the amino-terminal end, have been well studied (42, 137). An overlapping region of 450 amino acids of the longer T. vaginalis form of Fe-hydrogenases showed 47% amino acid identity to short-form Fe-hydrogenases (137). While the 50-kDa Fe-hydrogenase contains 2[4Fe-4S] clusters and lacks the mitochondrion/hydrogenosome-targeting sequence, the 64-kDa Fe-hydrogenase possesses an amino-terminal extension containing additional [4Fe-4S] and [2Fe-2S] clusters and the mitochondrion-targeting signal (137), suggesting that long-form Fe-hydrogenase is fully active and accepts electrons from ferredoxin (86).
An Fe-hydrogenase gene was also identified in G. intestinalis and E. histolytica (137, 228), and its enzymatic activity and/or mRNA expression was demonstrated in cultured E. histolytica and G. intestinalis (228). However, a recent report suggests that Giardia produces hydrogen at a 10-fold-lower rate than T. vaginalis does (183). The physiological role of Fe-hydrogenase in E. histolytica and G. intestinalis has not been clearly demonstrated as in T. vaginalis, where hydrogenase plays an indispensable role in the final transfer of electrons to protons (218). Recently, auxiliary Fe-hydrogenase maturases (S-adenosylmethionine-dependent HydG, HydE, and the small GTPase HydF) were identified from the T. vaginalis hydrogenosomes by proteomic analysis and from the genome database (251), which further elucidates the molecular mechanisms of biosynthesis and maturation of Fe-hydrogenase in this parasite.
CURRENT CHEMOTHERAPEUTICS
Current Chemotherapeutics for E. histolytica Infection
Since emetine was first shown to be effective against E. histolytica infection in 1912 (162), the present antiamoebic drugs that are most commonly used are 5-nitroimidazoles, including metronidazole (Flagyl) and tinidazole (Fasigyn). For years, these two drugs have proven to be effective against invasive intestinal and extraintestinal amoebiasis. Although metronidazole is considered the drug of choice (286), nitroimidazoles with longer half-lives such as tinidazole and ornidazole allow for a single dose, better tolerance, and shorter treatment time. Metronidazole (750 mg) and tinidazole (800 mg) three times a day are generally used for 7 and 5 days, respectively. Alternatively, a single dose (2 g) of tinidazole or ornidazole is generally recommended for treatment of amoebiasis or giardiasis. In addition, chloroquine, emetine, and dehydroemetine are used for cases that do not respond to metronidazole or tinidazole alone (26, 286). Since all of these compounds are well absorbed in the intestine, they are generally ineffective for asymptomatic cyst passers. To treat cyst carriers with 5-nitroimidazoles, the course of treatment is generally extended for a minimum of 10 days, which produces some success. Patients occasionally suffer from a relapse of invasive amoebiasis months after a short course of metronidazole therapy (26, 286). A single high-dose therapy with 2 g metronidazole is preferred over therapy with multiple doses or over an extended period, but it is not tolerated in some patients, including pregnant women and alcoholics. Lumen-acting drugs, such as diloxanide furoate (Furamide) (500 mg three times a day for 10 days), iodoquinol (Yodoxin) (650 mg three times a day for 20 days), and paromomycin (Humatin) (25 to 35 mg in three doses for 7 days), have been used to eliminate dormant luminal cysts and prevent relapses. However, asymptomatic cases that fail to respond to this extended therapy are occasionally observed (236, 324). Whether biological differences in the parasite, including differences in drug sensitivity and virulence, are responsible for the treatment failure is unknown. However, high rates of genetic polymorphisms in a limited geographic region are well established (11, 22, 118, 119, 193) and may be partially responsible for the observed therapeutic failure. Alternatively, genetic differences in immune response and drug metabolism may influence the efficacy of antiamoebic drugs (72).
Various nonimidazole drugs, including nitazoxanide, paromomycin, and niridazole, reportedly have the potential to be used for treatment of microaerophilic protozoan parasite infections (62, 73). Nitazoxanide, a nitrothiazoyl-salicylamide derivative, could be used as the first-line agent against amoebiasis and other intestinal parasitic diseases in the future (246). Nitazoxanide has broad-spectrum antiparasitic activity, including activity against the protozoans E. histolytica, G. intestinalis, T. vaginalis, Cryptosporidium parvum, and Isospora belli and the helminths Ascaris lumbricoides, Ancylostoma duodenale, Trichuris trichiura, Taenia saginata, Hymenolepsis nana, and Fasciola hepatica (267). The mode of action for nitazoxanide is unproven, but it is predicted to inhibit PFOR. Clinical efficacy was also demonstrated in a randomized, double-blind, placebo-controlled study (267).
The disadvantages of the current antiamoebic drugs, besides their relative ineffectiveness against luminal cysts, include various side effects. Adverse effects of metronidazole include anorexia, nausea, vomiting, diarrhea, abdominal discomfort, disulfiram-like alcohol intolerance, and hypersensitivity (62, 122). Neurological side effects include dizziness, vertigo, paresthesias, and, rarely, encephalopathy or convulsions which warrant discontinuation of the drug (246). Neutrocytopenia is also associated with metronidazole. Metronidazole is also known to be mutagenic in bacteria and carcinogenic in rodents, making teratogenicity a concern (28, 44, 47, 51, 265). Metronidazole is also known to cross the placental barrier. The Food and Drug Administration (FDA) classified metronidazole as a class B risk factor for pregnancy. Accordingly, use of metronidazole for amoebiasis in pregnant women is not currently recommended (58, 59), although a causal connection between metronidazole exposure during pregnancy and birth defects has not been established (45, 63, 94). Emetine and dehydroemetine hydrochloride have serious side effects, including nausea, vomiting, cardiotoxicity, local pain, and tenderness (162). These drugs are poorly excreted into the gut and urine and accumulate at high concentrations in the liver, heart, and other tissues. Side effects of nitazoxanide include diarrhea, nausea, vomiting, abdominal pain, and flatulence (246).
Current Chemotherapeutics for G. intestinalis Infection
The most commonly used drugs against giardiasis, i.e., metronidazole (250 mg three times a day for 5 to 10 days), tinidazole (100 mg three times a day for 7 days), furazolidone (100 mg four times a day for 7 to 10 days), and quinacrine (100 mg three times a day for 5 to 7 days), show up to 90% efficacy (furazolidone, <80%), which is followed by albendazole (400 mg four times a day for 5 days) and paromomycin (500 mg three times a day for 5 to 10 days) (1, 99, 125, 129). Metronidazole is considered the drug of choice, with tinidazole being an alternative (31, 99). However, metronidazole does not have an FDA indication for this use in the United States, whereas tinidazole is not available in the United States (246). A single-high-dose (2-g) regimen and/or multiple low doses (250 mg three times a day) for 5- to 7-day regimens of metronidazole were previously recommended (31). Tinidazole or ornidazole is generally recommended as a single-high-dose (2-g) regimen for treatment of giardiasis (99). Nitazoxanide received an FDA indication for the treatment of giardiasis and is available as a liquid formulation. Although reports are limited, nitazoxanide appears to be as effective as metronidazole and tinidazole (1, 6). Considering its excellent oral availability and that the major metabolites in serum (tizoxanide and glucuronide tizoxanide) exceed the 90% inhibitory concentration (IC90) against in vitro cultures of E. histolytica, G. intestinalis, and T. vaginalis, nitazoxanide could be used for cases that are refractory to metronidazole treatment in the future. Alternative drugs during pregnancy include paromomycin. Other drugs, including furazolidone, quinacrine, and albendazole, should be reserved for the treatment of cases refractory to the first-line drugs. Furazolidone was often recommended for children because of its availability as a suspension (31, 149). Quinacrine and furazolidone, similar to metronidazole, are not tolerated with alcohol due to a disulfiram-like reaction (99, 149). Five of six cases refractory to metronidazole treatment were successfully treated with a combination regimen of quinacrine and metronidazole (227).
Albendazole, currently used as an anthelminthic, was first successfully used against giardiasis (340, 341) with other benzimidazoles (fenbendazole and mebendazole), with variable success. The first large-scale human study of albendazole, conducted in Bangladesh, showed a lower average efficacy than for metronidazole (120), suggesting that single high doses of metronidazole or tinidazole are advantageous with regard to patient compliance. A recent study demonstrated that combinations of albendazole and phenyl-carbamate derivatives were more effective against albendazole-resistant G. intestinalis strains (150). Nitazoxanide was also effective for treatment of giardiasis cases resistant to metronidazole and albendazole therapy (1). The side effects of benzimidazoles are similar to those of metronidazole (anorexia, vomiting, and metal taste) but of lower intensity (155).
Current Chemotherapeutics for T. vaginalis Infection
Drugs commonly used against T. vaginalis infection are generally similar to those described above for E. histolytica or G. intestinalis. Metronidazole is administered orally in one of the following regimens: 250 mg three times a day for 7 days, 500 mg twice a day for 7 days, or a single 2-g dose. The last regimen is usually favored because of better compliance and because a lower total dose is required for successful treatment. Metronidazole can also be administered intravenously at a dose of 500 mg to 2 g over 20 min (62). Reported cure rates for oral and intravenous regimens were similar (85 to 95%) and increased in cases where the sexual partners were treated simultaneously (186). There has been a reluctance to utilize nitroimidazoles for trichomoniasis in pregnant women, particularly in the United States, due to some concerns over severe side effects and the teratogenecity of metronidazole, as described above (58, 59, 62, 279, 289, 290). It is now considered that the risk to the fetus from maternal trichomoniasis is far greater than any risk related to metronidazole exposure from the mother.
Although metronidazole is still the drug of choice (153), drug-resistant cases of trichomoniasis are considerably more common than in infections with G. intestinalis or E. histolytica. The second drug of choice for treatment of trichomoniasis is tinidazole. A recent study using >100 clinical isolates demonstrated that they showed slightly higher sensitivity to tinidazole (minimum lethal concentration [MLC], 1.0 ± 1.3 mM) than to metronidazole (MLC, 2.6 ± 1.9 mM) under aerobic conditions. However, there was no significant difference in MLCs between these nitroimidazoles under anaerobic conditions (61). The efficacies of a number of nitroimidazole derivatives other than metronidazole and tinidazole for the treatment of T. vaginalis infection have been investigated. Although the modes of action of these derivatives are similar, the pharmacokinetics, tissue distributions, trichomonicidal activities, and toxicities are variable for these compounds (62). Ornidazole and secnidazole are similar to tinidazole in that they have longer half-lives but lower rates of efficacy to cure infection than metronidazole. In contrast, nimorazole, a nitrothiazole derivative, is converted into two major metabolites that possess significantly higher trichomonicidal activity than metronidazole (177). Nimorazole was active against both metronidazole-sensitive and -resistant T. vaginalis strains (334, 335).
Intravaginal application of paromomycin was successfully used to treat recurrent trichomoniasis. However, severe side effects, including pain and mucosal ulceration, made it an unlikely candidate for clinical therapy (249). Nitazoxanide was as active against T. vaginalis as metronidazole (6, 48), similar to the case for infections caused by E. histolytica and G. intestinalis as described above and for C. parvum and Blastocystis (6, 100). Hamycin, an aromatic polyene related to amphotericin B, was shown to induce cell death in T. vaginalis and other eukaryotes by binding to ergosterols in the plasma membrane and forming pores. Although lower concentrations of hamycin effectively killed both metronizole-sensitive and -resistant T. vaginalis strains, toxicity both in vitro and in vivo is likely to make hamycin inapplicable for clinical use (190). Sodium nitrite, sodium nitroprusside, and Roussinn's black salt, traditionally used to prevent food contamination, exhibited trichomonicidal activity against both sensitive and resistant T. vaginalis strains (268). Sulfimidazole, which has two functional groups, sulfonamide and 5-nitroimidazole, exhibited activity comparable to that of metronidazole against metronidazole-sensitive strains (MLC, 10 μg/ml) and was more effective against the metronidazole-resistant strains (MLC, 40 to 60 μg/ml) (73, 196).
DRUG TARGETS AND MECHANISMS OF RESISTANCE
Mechanism of Action of 5-Nitroimidazole and Benzimidazoles
Metronidazole (α-hydroxyethyl-2-methyl-5-nitroimidazole), a synthetic 5-nitroimidazole (56), enters the cell and its organelles via passive diffusion. Metronidazole is relatively inert until its 5-nitro group is reduced within the cell or organelle by an appropriate electron donor such as ferredoxin. Importantly, metronidazole activation occurs only under strong reducing conditions. As oxygen is an efficient electron acceptor, increased levels of oxygen result in the impaired reduction and activation of metronidazole which could theoretically result in metronidazole resistance. Thus, sensitivity to metronidazole is influenced by oxygen tension. Metronidazole is usually activated through the acceptance of electrons from ferredoxin or flavodoxin that is reduced by PFOR (178, 218-220, 264, 308). Among microorganisms, there is a strong correlation between the presence of PFOR activity and metronidazole sensitivity (138, 218, 219, 266, 275, 308). In humans, where PFOR is absent, pyruvate dehydrogenase catalyzes the oxidative decarboxylation to form acetyl-CoA and NADH in mitochondria (see above). It was directly demonstrated by using purified PFOR and ferredoxin from G. intestinalis trophozoites that these Fe-S cluster-containing proteins activate metronidazole in vitro (306-308, 314). The reduction of metronidazole results in the nitro-radical form, which binds transiently to DNA to disrupt or break the nucleotide strands, leading to cell death (65, 76, 77, 182). Studies with Escherichia coli showed that the damage caused by the direct binding of activated metronidazole to DNA is repaired by the excision repair pathway, which was not activated in T. vaginalis trophozoites exposed to metronidazole (172). DNA damage may be the basis for the carcinogenicity of metronidazole in animals, although carcinogenicity of metronidazole in humans has not been demonstrated.
The cellular compartments where metronidazole is activated differ among the “amitochondriate” protists. It was proposed that metronidazole is activated in E. histolytica by ferredoxin in the cytosol (256). As described above, ferredoxin nitroreductase, a fusion protein of 2[4Fe-4S] ferredoxin and nitroreductase, may be a target of metronidazole in E. histolytica and G. intestinalis (229). G. intestinalis also has, in addition to ferredoxin-nitroreductases, an oxygen-insensitive nitroreductase that lacks the ferredoxin domain and which is also present in archaea and bacteria (229). This nitroreductase reduces and activates the nitro groups of metronidazole and furazolidone in Helicobacter pylori and E. coli, respectively. Bacterial mutants lacking this nitroreductase activity were resistant to a corresponding drug (115, 163, 327). Although a physiological electron acceptor from reduced ferredoxin in E. histolytica has not been unequivocally demonstrated, oxygen competes with metronidazole for electrons from the physiological electron donor. Therefore, it is conceivable that the metronidazole sensitivity of E. histolytica is influenced by oxygen tension, as demonstrated for Trichomonas (see below). In G. intestinalis, the terminal oxidase (NADH oxidase), which converts oxygen directly to water to scavenge for oxygen and protect the anaerobic PFOR and ferredoxins (37), seems to be a physiological acceptor of electrons from ferredoxin in the cytosol. In contrast, in T. vaginalis, metronidazole is metabolized and activated in the hydrogenosomes. Metronidazole competes with the terminal enzyme hydrogenase for electrons from ferredoxin (218).
The underlying mode of action of benzimidazoles, including albendazole, fenbendazole, and mebendazole, has been extensively studied in the parasitic nematode Haemonchus contortus (171, 187, 188, 226). Benzimidine binds to the β-tubulin monomer prior to dimerization with α-tubulin to block microtubule formation (171). More specifically, benzimidazoles bind to the high-affinity binding site on the β-tubulin monomer (187). The effect of benzimidazoles on in vitro assembly of microtubules was investigated in benzimidazole binding assays using recombinant α- or β-tubulin and dimeric tubulin (αβ-tubulin) from G. intestinalis, Encephalitozoon intestinalis, and C. parvum (192).
Drug Resistance in E. histolytica
A number of factors are associated with resistance against metronidazole and related 5-nitroimidazoles in “amitochondriate” protists, including a decreased uptake of metronidazole, an altered pyruvate-oxidizing metabolic pathway (168), and high oxygen tension (82). There are no reports of high levels of resistance to metronidazole in clinical isolates of E. histolytica, but resistant cases of Trichomonas and Giardia infections are frequently observed (see below). However, inadequate short-term exposure to metronidazole and exposure to sublethal levels of metronidazole could induce increased drug resistance (312, 314, 323). Indeed, under experimental conditions, a stepwise increase in drug concentration induced metronidazole resistance in two axenic lines of E. histolytica (274). Two independent laboratory strains, HM-I:IMSS and HTH-56:MUTM, developed resistance against metronidazole and grew in the presence of 10 μM metronidazole, which is normally lethal to parasites in vitro (101, 274, 323). A metronidazole-resistant line that grew in the presence of 40 μM metronidazole was developed independently (323). In the former study, the expression of SOD increased three- to fivefold in the metronidazole-resistant line (274). Unlike in Giardia and Trichomonas (see below), PFOR activity did not decrease significantly in this metronidazole-resistant line (274). In the latter study, SOD mRNA and enzyme activity (323) were increased fivefold in the resistant strain, while PFOR mRNA decreased only marginally, supporting the previous finding. Peroxiredoxin (thiol-specific antioxidant) mRNA and enzyme activity also increased by three- to fourfold, while NADPH:flavin reductase mRNA and its activity decreased by 40%. In addition, ferredoxin 1, but not ferredoxin 2, was selectively decreased in this resistant strain (323), which may reflect the specificity of metronidazole. However, further biochemical differentiation of the two ferredoxins has not been done. E. histolytica possesses an unusual pathway for detoxification of superoxide radicals and hydrogen peroxide, using SOD, rubrerythrin (185), NADPH:flavin oxidoreductase (38), and peroxiredoxin (323) to protect oxygen-sensitive PFOR and ferredoxin. Thus, it is conceivable that E. histolytica may possess a mechanism of metronidazole resistance different from those of the other two “amitochondriate” protists.
An emetine-resistant E. histolytica strain was developed in vitro (21, 276, 277). This emetine-resistant E. histolytica line overexpressed P-glycoprotein and showed some features of the multidrug-resistant phenotype (21). The accumulation of emetine by the mutant amoebae was 50% lower than in wild-type amoebae (21). However, the rate of drug entry and efflux from the parasite per se was not examined in that study. Emetine resistance was reversed by the calcium channel blocker verapamil (21, 276). It was hypothesized that E. histolytica actively expelled hydrophobic drugs, including emetine, by P-glycoprotein as described for multidrug-resistant tumor cells (97), because the emetine-resistant E. histolytica line was cross-resistant to other hydrophobic drugs such as iodoquinol and diloxanide but not to nonpolar drugs such as chloroquine and metronidazole (277). Also, the resistant strain released radiolabeled emetine more rapidly than the susceptible strain, suggesting that P-glycoprotein overexpression was responsible for emetine resistance in E. histolytica (277).
Drug Resistance in G. intestinalis
The prevalence of clinical metronidazole-resistant cases of giardiasis is reported to be up to 20% (30, 95), with recurrence rates of up to 90% (339). Resistant organisms have been isolated from patients and characterized in various laboratories (1, 6, 7, 95, 150, 191, 310, 312, 314). Cross-resistance to tinidazole has also been demonstrated in metronidazole-resistant strains (310, 312, 314). Furanidazole-resistant G. intestinalis strains produced in vitro adapted more readily to quinacrine (311). In vitro development of albendazole resistance was also reported (180, 309). Albendazole resistance developed more easily in a furazolidone-resistant strain, giving rise to a multidrug-resistant phenotype (309).
PFOR expression was down-regulated fivefold in a metronidazole-resistant G. intestinalis line (308), which is consistent with the premise that PFOR is the primary target of metronidazole and that a decreased amount of the target is the mode of resistance in G. intestinalis. Recently, antisense inhibition of PFOR caused metronidazole resistance in G. intestinalis (64). In contrast to the case for metronidazole-resistant lines, PFOR did not change in a furazolidone- and quinacrine-cross-resistant G. intestinalis line (314). The activity of the next electron acceptor in the transport chain, ferredoxin I, decreased by sevenfold, while the amount of ferredoxin I decreased by only twofold (181, 314), suggesting that another layer of regulation influenced the acceptor activity of ferredoxin. In an independent study, a furazolidone-resistant line showed increased activity of NADH oxidase, which activates the drug to its free radical state (35, 37). That study concluded that furazolidone resistance is due to the reduced expression of ferredoxin and increased NADH oxidase activity, which differs from metronidazole resistance. In contrast, albendazole-resistant G. intestinalis strains revealed changes in the cytoskeleton, especially for β-tubulin (309), suggesting that qualitative changes in β-tubulin lead to decreased sensitivity to albendazole (191). In that study, benzimidazole analogues showed higher affinity for monomeric β- and heterodimeric αβ-tubulin derived from benzimidazole-sensitive parasites than for those from benzimidazole-insensitive organisms (191).
Drug Resistance in Trichomonas
It was proposed that metronidazole resistance be categorized into “aerobic” and “anaerobic” mechanisms (62). In “aerobic resistance,” ferredoxin and other components of the antioxidative system seem to play a major role. In “anaerobic resistance,” reduction of hydrogenosome functions in general and of PFOR and hydrogenase activities in particular are apparently responsible for resistance. Most clinical metronidazole-resistant strains show biochemical alterations, which is consistent with the “aerobic resistance” phenotype. It is important to note that aerobic resistance can develop in vivo with therapeutic levels of metronidazole and does not require exposure to incremental doses of metronidazole during prolonged treatment, as shown for the in vitro development of anaerobic metronidazole resistance by a stepwise increase in drug concentrations. There are a number of reports of clinical cases of resistance against metronidazole or 5-nitroimidazole derivatives (61, 225, 319, 336).
Because oxygen is an efficient electron acceptor, increased levels of cellular oxygen in hydrogenosomes result in the impaired reduction and activation of metronidazole. Oxygen concentrations in resistant strains were much higher than those in susceptible strains (82). The high oxygen concentrations likely inhibit accumulation of the drug, because oxygen competes with metronidazole for electrons from reduced ferredoxin. If metronidazole is not reduced, there is no concentration gradient of the drug across the plasma membrane to allow extracellular metronidazole to enter the cell. In addition, the reduced nitro free radical is oxidized back to the original compound by oxygen and in turn produces a superoxide anion (62, 245). This process is known as futile cycling and results in only limited damage to the cells via superoxide anions in comparison to cell death due to reactive nitro radicals. While “aerobic resistance” phenotypes were frequently observed in clinical isolates, anaerobic resistance is found mostly in in vitro-induced metronidazole-resistant lines. Metronidazole-resistant T. vaginalis strains artificially developed by increasing the drug concentration in vitro showed either reduced or absent PFOR and hydrogenase activities (169). Unfortunately, the current view of metabolic mechanisms giving rise to metronidazole-resistant trichomoniasis, both in clinical settings and in vitro, is not completely elucidated by the “aerobic” versus “anaerobic” models.
Clinical drug-resistant T. vaginalis isolates show various biochemical changes, e.g., decreased expression of PFOR, ferredoxin, and hydrogenase activities (62, 73, 168, 253); oxygen resistance (80, 336); and decreased oxidase activity (336). In addition, highly resistant T. vaginalis clinical isolates possess neither detectable PFOR activity nor PFOR and ferredoxin mRNAs (34, 73, 169, 175, 253). Earlier work suggested that hydrogenosomes could be lost in drug-resistant parasites. However, it was reported later that the organelle remained, but in a modified form (168). Although structural changes of hydrogenosomes were reported to occur in a metronidazole-resistant T. vaginalis strain, it is unclear whether these are primary events or are secondary to the advent of resistance (153). Also, metronidazole-resistant strains simultaneously lost multiple hydrogenosomal proteins, including ferredoxin, PFOR, malic enzyme, and hydrogenase, due to inactivation of hydrogenosomes (140, 254). The metronidazole-resistant T. vaginalis trophozoites showed enhanced lactate fermentation as they lost PFOR activity, PFOR mRNA, and ferredoxin mRNA and thus the pyruvate-oxidizing pathway in the hydrogenosomes (34, 73). Hrdy et al. (140) demonstrated that metronidazole is activated by electrons from ferredoxin that originate not from PFOR but from malate in this metronidazole-resistant strain. These data support the notion that trichomonads acquire high-level metronidazole resistance only after both pyruvate- and malate-dependent pathways of metronidazole activation are eliminated from hydrogenosomes. These lines of evidence also suggested that “aerobic” and “anaerobic” resistance mechanisms are not mutually exclusive.
One previous study provided contradictory evidence that there was no significant change in PFOR activity, anaerobic fermentation, and intracellular accumulation of metronidazole between metronidazole-resistant and -susceptible isolates (220). However, accumulation of [14C]metronidazole was more inhibited under aerobic conditions in resistant isolates than in susceptible strains. Thus, they concluded that the production of electrons was not hampered but that the activation of metronidazole was reduced in the resistant isolates (220). This observation was also consistent with the premise that the metronidazole-resistant strain had reduced electron transport ability, and this therefore was classified as “aerobic resistance.”
One line of evidence suggests that ferredoxin plays a major role in metronidazole resistance. The major ferredoxin purified from T. vaginalis trophozoites was indeed reduced by PFOR in vitro, as detected by electron paramagnetic resonance spectroscopy (116). This ferredoxin could interact with metronidazole and accept electrons from PFOR (116). Changes in the upstream transcriptional regulatory regions (nucleotide −239 upstream of the transcription initiation site) of the ferredoxin gene were also demonstrated in metronidazole-resistant strains (253). The mRNA and protein levels of ferredoxin decreased by ∼50% in these strains. A recent study showed that ferredoxin gene replacement in T. vaginalis did not lead to in vitro metronidazole resistance (176). Thus, it remains unclear to what extent ferredoxins are involved in metronidazole resistance. The T. vaginalis genome project indicates that the genome is 160 to 180 Mb in size and highly repetitive. These data indicate that knockout of a single gene may not be deleterious for the parasite and that an alternative ferredoxin or flavodoxin might also be responsible for metronidazole activation in T. vaginalis.
Cross-resistance between different nitroimidazoles was also reported (208, 225). For instance, metronidazole-resistant clinical isolates showed partial cross-resistance to tinidazole (61, 319). However, metronidazole-resistant isolates do not always coexist with cross-resistance against other nitroimidazole compounds (61). Metronidazole-resistant isolates described by Narcisi and Secor were sensitive to a nonnitroimidazole nitrofuran, furazolidone (225), which is consistent with the notion that the mechanism of metronidazole resistance in T. vaginalis differs from that in G. intestinalis (314). Further studies need to be conducted to clarify molecular mechanisms of cross-resistance.
SULFUR-CONTAINING-AMINO-ACID METABOLISM AS A NOVEL DRUG TARGET
Metabolic Pathways in Protozoan Parasites under Investigation To Explore as Targets for Drug Development
Unique metabolic pathways that are present in pathogens but absent or divergent in their hosts are always potential rational targets for drug development. Among a number of pathways, some of which are listed here, the unique pathways in parasitic protists include fatty acid, isoprenoid, phospholipid, sterol, and heme biosynthesis in Plasmodia, Toxoplasma gondii, Trypanosoma, and Leishmania (32, 40, 60, 266, 291, 329); polyamine metabolism in Trypanosoma (259); aspartic acid proteases (plasmepsins) and cysteine proteases (falcipains) from Plasmodium (52); trypanothione metabolism in Trypanosoma (93); thioredoxin metabolism in Plasmodium and T. vaginalis (221); protein kinases from Plasmodium, Toxoplasma, and Eimeria (68); the hexose transporter from Plasmodium (151); and the dihydroorotate dehydrogenase (23) and the mitochondrial cyanide-insensitive terminal oxidase from Trypanosoma brucei (159).
The number of targets in “amitochondriate” parasites currently under investigation is not sufficient considering the rapid emergence of drug resistance described above. Potential targets for the development of antiamoebic drugs include the glycolytic pathway (namely, pyrophosphate-dependent phosphofructokinase and pyruvate kinase) (103, 200), alcohol dehydrogenase 2 (90, 337), cysteine proteases (157, 252), isoprenyltransferases (170, 195), and sulfur-containing-amino-acid metabolism (233). For the development of new chemotherapeutics against giardiasis, several candidates have been investigated, including guanine phosphoribosyltransferase, a key enzyme in the purine salvage pathway (222), and biosynthesis of a novel β-(1,3)-N-acetyl-d-galactosamine homopolymer [including 4′-epimerase and β-(1,3)-N-acetyl-d-galactosamine transferase] (102, 148, 285). Encystation and excystation are unique cellular processes that fulfill the criteria for rational drug targets. In particular, the biosyntheses of mannoproteins, chitin, and β-1,3-glucan are suggested as targets for antifungal drugs against Entamoeba and Giardia (148). Possible targets of T. vaginalis that have been investigated include a thioredoxin-linked peroxiredoxin antioxidant system (54, 221) and sulfur-containing-amino-acid metabolism (53, 326) (see below).
Among the handful of possible targets, we propose sulfur-containing-amino-acid metabolism as one of the rational and promising pathways for the development of new chemotherapeutic agents against “amitochondriate” parasites, particularly those causing amoebiasis and trichomoniasis. First, sulfur-containing-amino-acid metabolism plays a pivotal role in virtually all organisms (233). Namely, methionine and cysteine are building blocks of proteins; S-adenosylmethionine is the precursor for polyamine biosynthesis and the essential methyl donor for many methyl transfer reactions, including the DNA methylation involved in the regulation of gene expression; and cysteine is a precursor for the biosynthesis of glutathione, which is an essential antioxidant. Second, there are remarkable qualitative, not quantitative, differences between the parasites and their hosts. In most of the drug targets described below, suitable targets are selectively present only in “amitochondriate” parasites and are absent in their hosts. Finally, sulfur-containing-amino-acid metabolism has also been viewed as a reasonable target for the development of drugs against infection caused by other parasitic protists, including Plasmodium and Trypanosoma, and their physiological and biological significance has been well studied (233).
Physiological Importance of Cysteine and Fe-S Cluster Biosynthesis
Cysteine and its intermediates are essential for survival of virtually all living organisms from bacteria to higher eukaryotes (see reference 233). Cysteine have various important function, in E. histolytica, G. intestinalis, and T. vaginalis and is the major thiol in E. histolytica (92) and G. intestinalis (36, 108, 109), where it is present in a reduced form. Thus, cysteine plays an important role in maintaining the redox balance of thiols in these organisms. In addition, cysteine provides an inorganic sulfur atom for the biosynthesis of Fe-S clusters, which are important in various proteins, including PFOR, ferredoxin, and hydrogenase. Fe-S clusters have various important functions, including oxidative phosphorylation, electron transfer, and regulation of gene expression and of enzyme activities including substrate binding and activation, sulfur donation, and iron storage (27, 152).
Heterogeneity of Fe-S Cluster Biosynthesis
Three independent systems are known for the biosynthesis of Fe-S clusters: the ISC, sulfur utilization factor (SUF), and NIF systems (15). While the ISC machinery has a ubiquitous housekeeping function in most organisms, the SUF machinery is involved in the stress response under iron-deficient and oxidative stress conditions in a range of organisms from archaebacteria to certain protists (223, 242, 302). In contrast to the ISC and SUF systems, the NIF machinery is present in only a limited number of organisms, especially anaerobic or microaerophilic bacteria such as nitrogen-fixing bacteria and nondiazotropic Epsilonprotobacteria, including Campylobacter jejuni, Helicobacter pylori (240), and E. histolytica (15, 315). There is no precedent for the NIF system in any other eukaryotes.
In contrast to E. histolytica, which possesses only the NIF system and lacks both the ISC and SUF systems, T. vaginalis and G. intestinalis (69, 294, 295, 304), together with Cryptosporidium parvum (174), exclusively contain an ISC system, while Plasmodium falciparum has both the ISC and SUF systems (83, 284, 331). While the NIF system in E. histolytica is localized mainly, if not exclusively, in the cytoplasm (V. Ali and T. Nozaki, unpublished data), the ISC system is localized in the hydrogenosomes and the mitosomes in T. vaginalis and G. intestinalis, respectively (69, 294, 304), similar to the mitochondrial localization of the ISC system in aerobic mitochondrion-containing eukaryotes. The major Fe-S cluster-containing proteins, ferredoxins and PFOR, play an important role in energy metabolism, electron transfer, and redox regulation and participate in the activation of chemotherapeutics, including metronidazole, in these three “amitochondriate” protists, as described above. Therefore, differences in the biochemical properties and intracellular localization of Fe-S biosynthesis between the “amitochondriate” protists should strongly influence strategies for future drug development.
Unique Aspects of Sulfur-Containing-Amino-Acid Metabolism
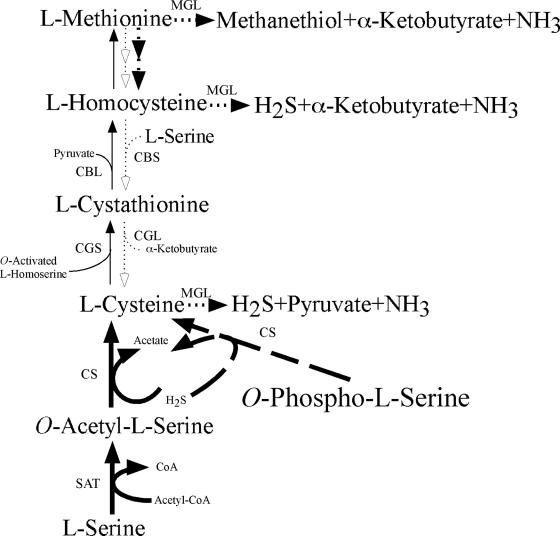
Sulfur-containing-amino-acid metabolism in E. histolytica and that in T. vaginalis have many striking similarities including (i) the presence of a sulfur-assimilatory de novo cysteine biosynthetic pathway (233, 235, 326); (ii) the presence of a unique enzyme, methionine γ-lyase (MGL), for degradation of sulfur-containing amino acids (184, 202, 301); and (iii) the presence of both phosphorylated and nonphosphorylated serine metabolic pathways upstream of cysteine metabolism (12-14, 233, 326). Since MGL and two enzymes involved in the cysteine biosynthetic pathway, serine O-acetyltransferase and cysteine synthase (CS), are absent in mammals, they are potentially ideal targets for new chemotherapeutic agents against these protozoan parasites. Methionine and cysteine metabolism in protozoan parasites was recently reviewed in detail (233), where we described genome-wide comparisons of pathways and individual enzymes involved in the pathways in representative parasitic protozoa that infect humans. Here, we highlight only two aspects of unique amino acid metabolism: degradation of sulfur-containing amino acids and cysteine biosynthesis.
Degradation of Sulfur-Containing Amino Acids
MGL (EC 4.4.1.11), which is present in both E. histolytica and T. vaginalis, catalyzes the decomposition of methionine, homocysteine, cysteine, and some other substituted serine or homoserine analogues, including O-acetylserine and O-acetyl-l-homoserine, by α- and β-elimination or by α- and γ-elimination (Fig. 3). MGL belongs to the α family of pyridoxal 5′-phosphate (PLP)-dependent enzymes and requires PLP as a cofactor for its activity (206). Both E. histolytica and T. vaginalis possess two isotypes of MGL which differ in substrate specificities, overall charge (i.e., pI), and other enzymological properties (202, 301). The biochemical properties of MGLs from Pseudomonas putida (147), an Aeromonas sp. (224), and Clostridium sporogenes (165) were also characterized. The MGLs from E. histolytica and T. vaginalis lack activity toward cystathionine (184, 301), whereas the P. putida and Aeromonas MGLs utilize cystathionine as a substrate (89). The lack of cystathionine lyase activity in E. histolytica and T. vaginalis MGLs seems reasonable, since these protozoa lack both forward and reverse transsulfuration pathways (Fig. 3) and thus do not produce cystathionine as an intermediate (202). The biological significance of MGL includes the production of propionic acid for energy metabolism (18) and the degradation of toxic sulfur-containing amino acids (233). Sulfur-containing amino acids, particularly homocysteine, are implicated in cardiovascular diseases, Alzheimer's disease, and dementia (305).
FIG. 3.
General scheme of transsulfuration, cysteine biosynthesis, and sulfur amino acid degradation. The schematic diagram shows all pathways present in bacteria. Open arrows with thin dotted lines depict pathways present in mammals; arrows with thick dotted lines represent pathways present in both E. histolytica and T. vaginalis; and arrows with thick unbroken lines or thick broken lines represent pathways present only in E. histolytica or T. vaginalis, respectively. CBS, cystathionine β-synthase; CBL, cystathionine β-lyase.
Specific functions for each MGL isotype and the significance of the apparent redundancy of the two isotypes are not understood for either E. histolytica or T. vaginalis. Our recent DNA microarray analysis showed that mRNA expression of MGL1 and that of MGL2 were comparable (unpublished data), supporting the previous observation that the two MGL isotypes were expressed in comparable amounts (301). Interestingly, the expression of MGL1, but not that of MGL2, was up-regulated >14-fold at 1 day after the amoebae were inoculated into the mouse intestine (105), suggesting an in vivo role for this MGL isotype for adaptation to the intestinal environment or, alternatively, MGL1 overexpression being part of the stress response.
MGL activity was not detected in crude extracts from the other anaerobic protozoan parasites (e.g., the related reptilian species E. invadens, T. foetus, Trichomitus batrachorum, and G. intestinalis) (184, 299), suggesting that the presence of MGL is not directly associated with anaerobic metabolism. Since MGL was also found in the closely related but nonpathogenic Entamoeba species E. dispar, MGL is unlikely to be involved in the virulence mechanism but rather is likely to be associated with commensal adaptation. Phylogenetic analysis suggested that MGL isotypes of E. histolytica or T. vaginalis were most likely obtained from an ancestral archaeal or bacterial organism, respectively, by lateral gene transfer (301).
Structural Differences between Protozoan MGL and Mammalian CGL
A number of PLP-dependent enzymes are involved in the metabolism of cysteine, homocysteine, and methionine. These enzymes include cystathionine γ-lyase (CGL), cystathionine γ-synthase (CGS), cystathionine β-lyase, MGL, and CS. Mammals lack the forward transsulfuration pathway (cysteine to methionine) and possess only the reverse transsulfuration pathway (methionine to cysteine) (233). One should be cautious that drugs targeting MGL are designed in such a way that they do not affect mammalian CGL. Recently, a crystal structure of yeast CGL, which has >50% amino acid identity to human CGL, has been resolved (210). We and other groups have also determined the tertiary structures of MGL and CGL by X-ray crystallography (unpublished data). Such structural comparisons as well as amino acid alignments (210) revealed conserved amino acids that participate in the catalytic activity of γ-elimination. MGLs from E. histolytica (301), T. vaginalis (202) (PDB accession no. 1E5F), and P. putida (214); CGL from yeast (210); and CGS from E. coli (50) are homotetramers, and their overall crystal structures are similar. The tetramer consists of two active dimers (subunits A/C and B/D) related by a twofold axis. While both CGL and MGL catalyze γ-elimination reactions, they have differential substrate preferences. Methionine, which contains a hydrophobic end group, is the preferred substrate of MGL, whereas cystathionine, which has a peptide group instead of a methyl moiety, is the preferred substrate of CGL. The overlay of the active sites of yeast CGL (210) and T. vaginalis MGL revealed structural explanations for the substrate preference. The conserved amino acids of E. histolytica MGL1 (Phe-44, Met-84, Cys-110, and Val-331) were present in all known MGLs and generally missing in other PLP-dependent enzymes (301). The conserved Glu-333 of yeast CGL, which binds to cystathionine, is replaced with Val-331 in all known MGLs (301). Similarly, the conserved Phe-44 is absent in CGL, and Cys-110 is replaced by glycine, which affects the substrate specificity (210). The phenyl group of Phe-44 of MGL forms a hydrophobic contact area together with the side chains of Leu-55 and Ile-59 to bind the methyl group of methionine. Structural comparisons also provide an explanation for the remarkable differences in cystathionine utilization between MGL and cystathionine β-lyase, CGS, and CGL (210). The three latter enzymes have a conserved glutamate (E333 in yeast CGL) which binds to the peptide group of cystathionine or the amino group of cysteine. The active sites of CGLs from yeast or human are virtually identical to that of E. coli CGS. Both CGL and bacterial CGS have γ-synthase and γ-lyase activities, depending on their position in metabolic pathways and the available substrate. Remarkable differences in the structure of the active site of protozoan MGL compared with that of mammalian CGL reinforce the premise that substrate analogues that specifically target parasite MGL, but not human CGL, can be designed. It should also be noted, however, that many important residues, including the ones involved in PLP binding, are shared by protozoan MGL and the related enzymes from human and bacteria. For instance, hydrophobic residues (Ile-55 and Leu-59) in the active site are shared by both yeast and human CGLs, similar to the case for MGLs. A helical structure located at the amino terminus of MGL was shown to cover the cysteine-binding pocket in E. coli CGS and CGL in a manner similar to that found in MGL (210). The crystal structure studies on E. histolytica MGL isotypes currently being conducted should give insights into detailed structural differences between amoebic MGL and human CGL and between the two MGL isotypes, which could lead to the design of new inhibitors and prodrugs.
Drug Development Targeting Methionine γ-Lyase
As explained above, MGL represents an ideal target for the development of new drugs against E. histolytica and T. vaginalis. A fluorinated derivative of methionine, l-trifluoromethionine or l-S-(trifluoromethyl)homocysteine (TFM), was reported to be a microbial growth inhibitor 40 years ago (342). TFM has good activity against a variety of bacteria, including Porphyromonas gingivalis (338), Clostridium, and Bacteroides (96). TFM is decomposed into α-ketobutyrate, ammonia, and trifluoromethanethiol (CF3SH) by MGL. The last compound is unstable under physiological conditions and nonenzymatically breaks down to carbonothionic difluoride (CSF2), which is a potent cross-linker of primary amine groups (16, 262). Thus, TFM or its analogues are highly effective against many anaerobic bacteria that cause botulism (Clostridium botulinum), colitis (Clostridium difficile), tooth decay (Porphyromonas species), and intra-abdominal infections (Bacteroides species) (96). Complete inhibition of growth occurred at 1 mM TFM in P. gingivalis. TFM also showed significant in vivo efficacy against peritoneal P. gingivalis infection in mice (338).
TFM was recently shown to be effective against T. vaginalis (53) and E. histolytica in vitro (301). TFM effectively killed T. vaginalis trophozoites within 24 h at 5 μg/ml in an in vitro culture. TFM also killed E. histolytica trophozoites after 48 h with an IC50 of 18 μM in an axenic culture. In addition, peritoneal administration of TFM (40 mg/kg of body weight) cured peritoneal infection by T. vaginalis in mice within 24 h (53). Five of six mice treated with TFM at 40 mg/kg had no lesions, while a lower dose of 12 mg/kg resulted in a cure rate of 70% (53). TFM also successfully cured experimental amoebic infection. Either intraperitoneal or subcutaneous administration of TFM (36 mg/kg of body weight) successfully cured liver abscesses in hamsters, without notable side effects (unpublished data). In addition, intraperitoneal administration of TFM also partially cured intestinal amoebiasis in a C3H/HeJ mouse model (139) of infection with a direct inoculation of trophozoites into the cecum (unpublished data). The major side effect in the treated mice was weight loss, suggesting that there may be species-dependent side effects with TFM. We are currently synthesizing and testing a variety of TFM derivatives to increase the efficacy as well as to decrease the side effects. We have found amide derivatives of TFM that are highly effective against in vitro cultures of E. histolytica within 24 to 48 h, with an IC50 of 1.5 to 2 μM. These derivatives are likely lead compounds for the development of new drugs against E. histolytica and T. vaginalis (unpublished data).
A compound antagonistic to MGL, propargylglycine, was not deleterious to amoebic trophozoites in axenic cultures (301). Propargylglycine at 20 μM did not affect E. histolytica growth, although it did completely inhibit MGL activity after 24 h. In addition, propargylglycine did not kill the amoebae in polyxenic cultures (unpublished data). These data indicate that MGL is not essential for E. histolytica survival and growth under these in vitro conditions. However, this does not necessarily rule out the possibility that MGL may be involved in the in vivo survival and virulence of these pathogens in mammalian hosts. In contrast, G. intestinalis possesses a rather distinct metabolism of sulfur-containing amino acids and does not contain MGL. TFM showed no effect on the growth of G. intestinalis, which is consistent with the absence of MGL in G. intestinalis (53).
Sulfur-Assimilatory De Novo Cysteine Biosynthesis
The cysteine biosynthetic pathway plays an important role in the incorporation of inorganic sulfur into organic compounds in bacteria, plants, and parasitic protists, including E. histolytica, T. vaginalis, and Trypanosoma cruzi, which is the etiological agent of Chagas' disease (American trypanosomiasis) (233-235, 237, 238, 326). The pathway has been extensively studied in bacteria and plants (126, 239, 270). In plants, the pathway is differentially localized in at least three compartments: the cytosol, mitochondria, and chloroplasts (127, 128, 130, 230, 270, 272, 332). In E. histolytica, where neither conventional mitochondria nor chloroplasts are present, the pathway exists exclusively in the cytosol. The pathway is also likely present in the cytosol in T. vaginalis and T. cruzi, because neither of the two enzymes in the pathway contains organelle-targeting sequences.
In bacteria and plants, which can reduce incorporated sulfate to sulfide via sulfite, extracellular sulfate is first imported by sulfate transporters (270-272). After sulfate activation and reduction, incorporated sulfate (+6) is derivatized to sulfide (−2), which serves as an acceptor for the alanyl moiety of the donor molecule. Serine O-acetyltransferase (SAT) (EC 2.3.1.30) catalyzes the formation of the alanyl donor O-acetylserine from serine and acetyl-CoA (235) (Fig. 3). CS [O-acetyl-l-serine (thiol)-lyase] (EC 4.2.99.8) then catalyzes the production of l-cysteine by the transfer of the alanyl moiety of O-acetylserine to sulfide (234, 238). Since E. histolytica and T. vaginalis apparently lack a sulfate reduction pathway, they likely utilize sulfide derived from the iron-sulfur proteins from ingested bacteria in the intestine or vagina, respectively. In contrast to these parasites, animals lack the sulfur-assimilatory pathway and thus require exogenous methionine as a sulfur source.
Although it was previously shown that E. histolytica possesses one SAT and two allelic isotypes of CS (234, 235), our recent survey of CS and SAT in the E. histolytica genome database (185) revealed a third CS isotype and two additional SAT isotypes (unpublished data). There are several unique features of the amoebic SAT and CS which are remarkably different from those of other organisms. It has been shown that SAT1 (previously named SAT) is a regulated key enzyme of the cysteine biosynthetic pathway in E. histolytica (235). SAT1 is regulated by allosteric negative feedback by cysteine and cystine. Negative regulation by l-cystine has no precedent, highlighting the amoebic SAT1 as a unique enzyme. Another unique aspect of the amoebic SAT1 is a lack of protein-protein interaction with CS. In both bacteria and plants, CS and SAT form a heteromeric complex with a molecular mass of several hundred kilodaltons (70, 71, 164). However, both CS1 (and CS2) and SAT1 form a homodimer, and they do not interact with each other under physiological conditions. The lack of CS-SAT interaction was unequivocally demonstrated by three biochemical and genetic methods: (i) separation by conventional chromatography during purification from the crude cell lysate, (ii) an inability to coimmunoaffinity purify the proteins, and (iii) the yeast two-hybrid system (235). The second and third SATs (SAT2 and SAT3, respectively) showed 73% and 48% amino acid identity to SAT1, respectively. E. histolytica SAT3 possesses a 25- to 30-amino-acid extension at the carboxyl terminus and a low isoelectric point (5.7) compared to E. histolytica SAT1 and E. histolytica SAT2. These features may favor an interaction with E. histolytica CS, in particular with E. histolytica CS3, which possesses the highest pI (8.17) among the three isotypes (unpublished data).
Among the three CS isotypes (CS1 to -3), two CS proteins (CS1 and CS2) are identical except for two conservative amino acid changes (234). CS3 is divergent from the other two isotypes, with ∼83% amino acid identity to CS1 and CS2 (unpublished data). While both CS1 and CS2 are localized in the cytoplasm of E. histolytica, similar to prokaryotic CysK and CysM, the intracellular distribution of CS3 remains unknown. In plants, three isotypes, i.e., CS-A, CS-B, and CS-C, are localized to the cytoplasm, chloroplasts, and mitochondria, respectively (130, 230, 270-273). CS1, CS2, and CS3 rescued the growth defect of a CysK-deficient E. coli strain, suggesting that all these isoforms of CS are functional as CysK in a heterologous organism (234; unpublished data). E. dispar also possesses two CS isotypes, CS1 and CS2 (82 to 83% mutual identity) (238). E. dispar CS1 and CS2 correspond to E. histolytica CS1/2 and 3, respectively. Taken together, this information indicates that Entamoeba possesses at least two classes of CS isotypes, each with a distinct pI. All of these CS isotypes lack signal sequences or organelle-targeting sequences, suggesting a cytosolic location. The presence of multiple cytosolic CS isotypes in the nonpathogenic E. dispar species suggests that this enzyme is not directly associated with the pathogenicity of the amoeba but that it plays an important housekeeping role in Entamoeba.
Recently, sulfur-assimilatory cysteine biosynthesis was characterized in T. vaginalis (326). The genomic analysis of T. vaginalis revealed six copies of CS but no SAT. Enzymological characterization of CS indicates that T. vaginalis CS is able to utilize O-phosphoserine as well as O-acetylserine as an alanyl donor. Although T. vaginalis lacks SAT and is thus unable to produce O-acetylserine, T. vaginalis also possesses three copies each of 3-phosphoglycerate dehydrogenase and phosphoserine aminotransferase and presumably is able to form O-phosphoserine from 3-phosphoglycerate derived from glycolysis.
The biological significance of cysteine synthesis in “amitochondriate” protists is still not yet fully understood. It was shown that cysteine is a major intracellular thiol in these organisms (36, 92, 106, 107, 110). l-Cysteine could not be replaced by any other thiols or reducing agents in the growth medium (109), suggesting that cysteine is essential for growth and survival of E. histolytica and G. intestinalis (36, 108, 109). In addition, cysteine also plays a role in the antioxidative defense of E. histolytica (235). Overexpression of CS (2- to 3-fold) but not of SAT (13-fold) by episomal introduction of multicopy plasmids resulted in only a 2-fold increase in the intracellular thiol content and the hydrogen peroxide resistance (235). These data indicate that the intracellular concentration of CS1 (or CS2) but not SAT1 mainly affects the thiol content. One of the major unsolved questions related to the pathway is why these protists possess apparently redundant systems, while they have lost many other metabolic pathways by reductive evolution.
Cysteine Synthase as a Drug Target
CS from E. histolytica utilizes a variety of alanyl acceptors, including some thiols or N-heterocyclic compounds besides sulfide (234), similar to the case for plant CS (144). The specificity of these alanyl acceptors varies between the organisms from which the CS is derived. The spectrum of the alanyl acceptors for CS was most extensively studied for the plant enzymes. For instance, spinach CS utilizes a wide range of alanyl acceptors to form the corresponding β-substituted alanines. These products include l-quisqualic acid, l-mimosine, l-willardiine, l-isowillardiine, β-(pyrazol-1-yl)-l-alanine, β-(1,2,4-triazol-1-yl)-l-alanine, and β-(3-amino-1,2,4-triazol-1-yl)-l-alanine (144, 145, 201). Some of these amino acids are toxic to or physiologically active in organisms in which they do not normally occur. For example, l-quisqualic acid, present in Quisqualis spp., is a neuroexcitory amino acid and is utilized as a vermicide in Chinese medicine. l-Mimosine is present in Mimosa and Leucaena spp. and is a thyrotoxic amino acid that causes loss of hair in growing animals. β-(3-Isoxazolin-5-on-2-yl)-l-alanine from Pisum and Lathyrus spp. showed antimitotic activity in Saccharomyces cerevisiae. Commonly used herbicides (1,2,4-triazole and 3-hydroxy-5-methylisoxazole) are alanylated by CS (146).
E. histolytica CS also uses sulfide, 1,2,4-triazol, isoxazolin-5-one, pyrazole, and cyanide as alanyl acceptors. The specific activity against these acceptors is, however, 9- to 4,000-fold less than that for sulfide (234). As seen for triazole, pyrazole, tetrazole, and their derivatives, which have been used as herbicides targeting plant CS, these compounds showed toxic effects on E. histolytica in vitro (unpublished data), necessitating a further screening of CS substrates with amoebacidal activity. Thiol derivatives of tetrazole and triazole are highly cytotoxic against E. histolytica cultures in vitro (unpublished data). Recently, it was also demonstrated that reduced forms of pyrazole, pyrazolines, and their derivatives are highly effective against amoebiasis (3-5, 41). The mode of action and toxicity of these derivatives against mammalian cells have not been studied. Similarly, 1,2,4-triazine and triazine derivatives were also cytotoxic to African trypanosomes and were selective between host cells and parasites (24, 66). Triazine-substituted polyamines have also been viewed as excellent chemotherapeutics against trypanosomiasis based on an in vitro study (160). The triazine derivatives showed amoebacidal activity in vitro, with IC50s lower than that of metronidazole (287). However, neither their target in E. histolytica trophozoites nor their mode of action has been elucidated. None of these compounds has been tested in vivo. It is conceivable that some of these derivatives target sulfur-containing-amino-acid metabolism and in particular CS or MGL.
CONCLUSIONS
Due to an immediate necessity for new drugs against parasitic infections, parasite-specific targets have been exploited. Sulfur-containing-amino-acid metabolism represents one promising source of such targets. The exclusive existence of sulfur-assimilatory de novo cysteine biosynthesis and the unique enzyme for degradation of sulfur-containing amino acids in E. histolytica and T. vaginalis verified that these pathways are ideal targets for drug development. Sulfur-containing-amino-acid metabolism and its intermediates play various pivotal roles in parasitic protozoa. Two proteins, MGL and CS, have most often been exploited to develop antiprotozoan drugs. Currently, l-S-(trifluoromethyl)homocysteine and its derivatives are being extensively synthesized and tested for in vitro and in vivo efficacy against liver and intestinal amoebiasis in animals. Once the tertiary structures of the substrate-binding pockets of MGL and mammalian CGL are determined, the rational design of compounds targeting MGL without adverse effects on the host CGL will become possible.
REFERENCES
- 1.Abboud, P., V. Lemee, G. Gargala, P. Brasseur, J. J. Ballet, F. Borsa-Lebas, F. Caron, and L. Favennec. 2001. Successful treatment of metronidazole- and albendazole-resistant giardiasis with nitazoxanide in a patient with acquired immunodeficiency syndrome. Clin. Infect. Dis. 32:1792-1794. [DOI] [PubMed] [Google Scholar]
- 2.Abe, N., Y. Nishikawa, A. Yasukawa, and K. Haruki. 1999. Entamoeba histolytica outbreaks in institutions for the mentally retarded. Jpn. J. Infect. Dis. 52:135-136. [PubMed] [Google Scholar]
- 3.Abid, M., and A. Azam. 2005. 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazolines: synthesis and in vitro antiamoebic activities. Eur. J. Med. Chem. 40:935-942. [DOI] [PubMed] [Google Scholar]
- 4.Abid, M., and A. Azam. 2005. Synthesis and antiamoebic activities of 1-N-substituted cyclised pyrazoline analogues of thiosemicarbazones. Bioorg. Med. Chem. 13:2213-2220. [DOI] [PubMed] [Google Scholar]
- 5.Abid, M., and A. Azam. 2006. Synthesis, characterization and antiamoebic activity of 1-(thiazolo[4,5-b]quinoxaline-2-yl)-3-phenyl-2-pyrazoline derivatives. Bioorg. Med. Chem. Lett. 16:2812-2816. [DOI] [PubMed] [Google Scholar]
- 6.Adagu, I. S., D. Nolder, D. C. Warhurst, and J. F. Rossignol. 2002. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob. Chemother. 49:103-111. [DOI] [PubMed] [Google Scholar]
- 7.Adam, R. D. 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev. 14:447-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam, R. D. 1991. The biology of Giardia spp. Microbiol. Rev. 55:706-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alderete, J. F., R. Arroyo, and M. W. Lehker. 1995. Analysis for adhesins and specific cytoadhesion of Trichomonas vaginalis. Methods Enzymol. 253:407-414. [DOI] [PubMed] [Google Scholar]
- 10.Aldritt, S. M., P. Tien, and C. C. Wang. 1985. Pyrimidine salvage in Giardia lamblia. J. Exp. Med. 161:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali, I. K., M. Zaki, and C. G. Clark. 2005. Use of PCR amplification of tRNA gene-linked short tandem repeats for genotyping Entamoeba histolytica. J. Clin. Microbiol. 43:5842-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali, V., T. Hashimoto, Y. Shigeta, and T. Nozaki. 2004. Molecular and biochemical characterization of d-phosphoglycerate dehydrogenase from Entamoeba histolytica. A unique enteric protozoan parasite that possesses both phosphorylated and nonphosphorylated serine metabolic pathways. Eur. J. Biochem. 271:2670-2681. [DOI] [PubMed] [Google Scholar]
- 13.Ali, V., and T. Nozaki. 2006. Biochemical and functional characterization of phosphoserine aminotransferase from Entamoeba histolytica, which possesses both phosphorylated and non-phosphorylated serine metabolic pathways. Mol. Biochem. Parasitol. 145:71-83. [DOI] [PubMed] [Google Scholar]
- 14.Ali, V., Y. Shigeta, and T. Nozaki. 2003. Molecular and structural characterization of NADPH-dependent d-glycerate dehydrogenase from the enteric parasitic protist Entamoeba histolytica. Biochem. J. 375:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali, V., Y. Shigeta, U. Tokumoto, Y. Takahashi, and T. Nozaki. 2004. An intestinal parasitic protist, Entamoeba histolytica, possesses a non-redundant nitrogen fixation-like system for iron-sulfur cluster assembly under anaerobic conditions. J. Biol. Chem. 279:16863-16874. [DOI] [PubMed] [Google Scholar]
- 16.Alston, T. A., and H. J. Bright. 1983. Conversion of trifluoromethionine to a cross-linking agent by gamma-cystathionase. Biochem. Pharmacol. 32:947-950. [DOI] [PubMed] [Google Scholar]
- 17.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson, I. J., and B. J. Loftus. 2005. Entamoeba histolytica: observations on metabolism based on the genome sequence. Exp. Parasitol. 110:173-177. [DOI] [PubMed] [Google Scholar]
- 19.Arroyo, R., A. Gonzalez-Robles, A. Martinez-Palomo, and J. F. Alderete. 1993. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol. Microbiol. 7:299-309. [DOI] [PubMed] [Google Scholar]
- 20.Avron, B., T. Stolarsky, A. Chayen, and D. Mirelman. 1986. Encystation of Entamoeba invadens IP-1 is induced by lowering the osmotic pressure and depletion of nutrients from the medium. J. Protozool. 33:522-525. [DOI] [PubMed] [Google Scholar]
- 21.Ayala, P., J. Samuelson, D. Wirth, and E. Orozco. 1990. Entamoeba histolytica: physiology of multidrug resistance. Exp. Parasitol. 71:169-175. [DOI] [PubMed] [Google Scholar]
- 22.Ayeh-Kumi, P. F., I. M. Ali, L. A. Lockhart, C. A. Gilchrist, W. A. Petri, Jr., and R. Haque. 2001. Entamoeba histolytica: genetic diversity of clinical isolates from Bangladesh as demonstrated by polymorphisms in the serine-rich gene. Exp. Parasitol. 99:80-88. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin, J., A. M. Farajallah, N. A. Malmquist, P. K. Rathod, and M. A. Phillips. 2002. Malarial dihydroorotate dehydrogenase. Substrate and inhibitor specificity. J. Biol. Chem. 277:41827-41834. [DOI] [PubMed] [Google Scholar]
- 24.Barrett, M. P., and A. H. Fairlamb. 1999. The biochemical basis of arsenical-diamidine crossresistance in African trypanosomes. Parasitol. Today 15:136-140. [DOI] [PubMed] [Google Scholar]
- 25.Barwick, R. S., D. A. Levy, G. F. Craun, M. J. Beach, and R. L. Calderon. 2000. Surveillance for waterborne-disease outbreaks—United States, 1997-1998. Morb. Mortal. Wkly. Rep. 49:1-21. [PubMed] [Google Scholar]
- 26.Bassily, S., Z. Farid, N. A. el Masry, and E. M. Mikhail. 1987. Treatment of intestinal E. histolytica and G. lamblia with metronidazole, tinidazole and ornidazole: a comparative study. J. Trop. Med. Hyg. 90:9-12. [PubMed] [Google Scholar]
- 27.Beinert, H., R. H. Holm, and E. Munck. 1997. Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277:653-659. [DOI] [PubMed] [Google Scholar]
- 28.Bendesky, A., D. Menendez, and P. Ostrosky-Wegman. 2002. Is metronidazole carcinogenic? Mutat. Res. 511:133-144. [DOI] [PubMed] [Google Scholar]
- 29.Blamey, J. M., and M. W. Adams. 1994. Characterization of an ancestral type of pyruvate ferredoxin oxidoreductase from the hyperthermophilic bacterium, Thermotoga maritima. Biochemistry 33:1000-1007. [DOI] [PubMed] [Google Scholar]
- 30.Boreham, P. F., R. E. Phillips, and R. W. Shepherd. 1988. Altered uptake of metronidazole in vitro by stocks of Giardia intestinalis with different drug sensitivities. Trans. R. Soc. Trop. Med. Hyg. 82:104-106. [DOI] [PubMed] [Google Scholar]
- 31.Boreham, P. F. L. 1994. The current status of chemotherapy for giardiasis, p. 317-328. In R. C. A. Thompson, J. A. Reynoldson, and A. J. Lymberg (ed.), Giardia: from molecules to disease. CAB International, Cambridge, United Kingdom.
- 32.Brady, R. L., and A. Cameron. 2004. Structure-based approaches to the development of novel anti-malarials. Curr. Drug Targets 5:137-149. [DOI] [PubMed] [Google Scholar]
- 33.Brodsky, R. E., H. C. Spencer, Jr., and M. G. Schultz. 1974. Giardiasis in American travelers to the Soviet Union. J. Infect. Dis. 130:319-323. [DOI] [PubMed] [Google Scholar]
- 34.Brown, D. M., J. A. Upcroft, H. N. Dodd, N. Chen, and P. Upcroft. 1999. Alternative 2-keto acid oxidoreductase activities in Trichomonas vaginalis. Mol. Biochem. Parasitol. 98:203-214. [DOI] [PubMed] [Google Scholar]
- 35.Brown, D. M., J. A. Upcroft, M. R. Edwards, and P. Upcroft. 1998. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int. J. Parasitol. 28:149-164. [DOI] [PubMed] [Google Scholar]
- 36.Brown, D. M., J. A. Upcroft, and P. Upcroft. 1993. Cysteine is the major low-molecular weight thiol in Giardia duodenalis. Mol. Biochem. Parasitol. 61:155-158. [DOI] [PubMed] [Google Scholar]
- 37.Brown, D. M., J. A. Upcroft, and P. Upcroft. 1996. A H2O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. Eur. J. Biochem. 241:155-161. [DOI] [PubMed] [Google Scholar]
- 38.Bruchhaus, I., S. Richter, and E. Tannich. 1998. Recombinant expression and biochemical characterization of an NADPH:flavin oxidoreductase from Entamoeba histolytica. Biochem. J. 330:1217-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruchhaus, I., and E. Tannich. 1994. Induction of the iron-containing superoxide dismutase in Entamoeba histolytica by a superoxide anion-generating system or by iron chelation. Mol. Biochem. Parasitol. 67:281-288. [DOI] [PubMed] [Google Scholar]
- 40.Buckner, F. S., R. T. Eastman, K. Yokoyama, M. H. Gelb, and W. C. Van Voorhis. 2005. Protein farnesyl transferase inhibitors for the treatment of malaria and African trypanosomiasis. Curr. Opin. Investig. Drugs 6:791-797. [PubMed] [Google Scholar]
- 41.Budakoti, A., M. Abid, and A. Azam. 2006. Synthesis and antiamoebic activity of new 1-N-substituted thiocarbamoyl-3,5-diphenyl-2-pyrazoline derivatives and their Pd(II) complexes. Eur. J. Med. Chem. 41:63-70. [DOI] [PubMed] [Google Scholar]
- 42.Bui, E. T., and P. J. Johnson. 1996. Identification and characterization of [Fe]-hydrogenases in the hydrogenosome of Trichomonas vaginalis. Mol. Biochem. Parasitol. 76:305-310. [DOI] [PubMed] [Google Scholar]
- 43.Buret, A. 1994. Pathogenesis—how does Giardia cause disease?, p. 293-315. In R. C. A. Thompson, J. A. Reynoldson, and A. J. Lymberg (ed.), Giardia: from molecules to disease. CAB International, Cambridge, United Kingdom.
- 44.Cantelli-Forti, G., G. Aicardi, M. C. Guerra, A. M. Barbaro, and G. L. Biagi. 1983. Mutagenicity of a series of 25 nitroimidazoles and two nitrothiazoles in Salmonella typhimurium. Teratog. Carcinog. Mutagen. 3: 51-63. [DOI] [PubMed] [Google Scholar]
- 45.Caro-Paton, T., A. Carvajal, I. Martin de Diego, L. H. Martin-Arias, A. Alvarez Requejo, and E. Rodriguez Pinilla. 1997. Is metronidazole teratogenic? A meta-analysis. Br. J. Clin. Pharmacol. 44:179-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casalot, L., and M. Rousset. 2001. Maturation of the [NiFe] hydrogenases. Trends Microbiol. 9:228-237. [DOI] [PubMed] [Google Scholar]
- 47.Cavaliere, A., M. Bacci, A. Amorosi, M. Del Gaudio, and R. Vitali. 1983. Induction of lung tumors and lymphomas in BALB/c mice by metronidazole. Tumori 69:379-382. [DOI] [PubMed] [Google Scholar]
- 48.Cedillo-Rivera, R., B. Chavez, A. Gonzalez-Robles, A. Tapia, and L. Yepez-Mulia. 2002. In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites. J. Eukaryot. Microbiol. 49:201-208. [DOI] [PubMed] [Google Scholar]
- 49.Clark, C. G., and A. J. Roger. 1995. Direct evidence for secondary loss of mitochondria in Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 92:6518-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clausen, T., R. Huber, B. Laber, H. D. Pohlenz, and A. Messerschmidt. 1996. Crystal structure of the pyridoxal-5′-phosphate dependent cystathionine beta-lyase from Escherichia coli at 1.83 A. J. Mol. Biol. 262:202-224. [DOI] [PubMed] [Google Scholar]
- 51.Connor, T. H., M. Stoeckel, J. Evrard, and M. S. Legator. 1977. The contribution of metronidazole and two metabolites to the mutagenic activity detected in urine of treated humans and mice. Cancer Res. 37:629-633. [PubMed] [Google Scholar]
- 52.Coombs, G. H., D. E. Goldberg, M. Klemba, C. Berry, J. Kay, and J. C. Mottram. 2001. Aspartic proteases of Plasmodium falciparum and other parasitic protozoa as drug targets. Trends Parasitol. 17:532-537. [DOI] [PubMed] [Google Scholar]
- 53.Coombs, G. H., and J. C. Mottram. 2001. Trifluoromethionine, a prodrug designed against methionine gamma-lyase-containing pathogens, has efficacy in vitro and in vivo against Trichomonas vaginalis. Antimicrob. Agents Chemother. 45:1743-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coombs, G. H., G. D. Westrop, P. Suchan, G. Puzova, R. P. Hirt, T. M. Embley, J. C. Mottram, and S. Muller. 2004. The amitochondriate eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. J. Biol. Chem. 279:5249-5256. [DOI] [PubMed] [Google Scholar]
- 55.Corbeil, L. B. 1994. Vaccination strategies against Tritrichomonas foetus. Parasitol. Today 10:103-106. [DOI] [PubMed] [Google Scholar]
- 56.Cosar, C., and L. Julou. 1959. The activity of 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole (R. P. 8823) against experimental Trichomonas vaginalis infections. Ann. Inst. Pasteur (Paris) 96:238-241. [PubMed] [Google Scholar]
- 57.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, G. G. Rhoads, et al. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 58.Coustan, D. R. 1999. Use of metronidazole during pregnancy. Pediatr. Infect. Dis. J. 18:79. [DOI] [PubMed] [Google Scholar]
- 59.Coustan, D. R., and T. R. Mochizuki. 1998. Metronidazole, p. 297-298. In Handbook for prescribing medications during pregnancy, 3rd ed. Lippincott-Raven, Philadelphia, PA.
- 60.Croft, S. L., M. P. Barrett, and J. A. Urbina. 2005. Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol. 21:508-512. [DOI] [PubMed] [Google Scholar]
- 61.Crowell, A. L., K. A. Sanders-Lewis, and W. E. Secor. 2003. In vitro metronidazole and tinidazole activities against metronidazole-resistant strains of Trichomonas vaginalis. Antimicrob. Agents Chemother. 47:1407-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cudmore, S. L., K. L. Delgaty, S. F. Hayward-McClelland, D. P. Petrin, and G. E. Garber. 2004. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin. Microbiol. Rev. 17:783-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czeizel, A. E., and M. Rockenbauer. 1998. A population based case-control teratologic study of oral metronidazole treatment during pregnancy. Br. J. Obstet. Gynaecol. 105:322-327. [DOI] [PubMed] [Google Scholar]
- 64.Dan, M., A. L. Wang, and C. C. Wang. 2000. Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol. Microbiol. 36:447-456. [DOI] [PubMed] [Google Scholar]
- 65.Declerck, P. J., and C. J. De Ranter. 1986. In vitro reductive activation of nitroimidazoles. Biochem. Pharmacol. 35:59-61. [DOI] [PubMed] [Google Scholar]
- 66.de Koning, H. P., and S. M. Jarvis. 1999. Adenosine transporters in bloodstream forms of Trypanosoma brucei brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 56:1162-1170. [DOI] [PubMed] [Google Scholar]
- 67.Diamond, L. S., and C. G. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 40:340-344. [DOI] [PubMed] [Google Scholar]
- 68.Doerig, C. 2004. Protein kinases as targets for anti-parasitic chemotherapy. Biochim. Biophys. Acta 1697:155-168. [DOI] [PubMed] [Google Scholar]
- 69.Dolezal, P., O. Smid, P. Rada, Z. Zubacova, D. Bursac, R. Sutak, J. Nebesarova, T. Lithgow, and J. Tachezy. 2005. Giardia mitosomes and trichomonad hydrogenosomes share a common mode of protein targeting. Proc. Natl. Acad. Sci. USA 102:10924-10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Droux, M., M. L. Ruffet, R. Douce, and D. Job. 1998. Interactions between serine acetyltransferase and O-acetylserine (thiol) lyase in higher plants—structural and kinetic properties of the free and bound enzymes. Eur. J. Biochem. 255:235-245. [DOI] [PubMed] [Google Scholar]
- 71.Droux, M., P. Sajus, and R. Douce. 1992. Purification and characterization of O-acetylserine (thiol) lyase from spinach chloroplasts. Arch. Biochem. Biophys. 295:379-390. [DOI] [PubMed] [Google Scholar]
- 72.Duggal, P., R. Haque, S. Roy, D. Mondal, R. B. Sack, B. M. Farr, T. H. Beaty, and W. A. Petri, Jr. 2004. Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. J. Infect. Dis. 189:520-526. [DOI] [PubMed] [Google Scholar]
- 73.Dunne, R. L., L. A. Dunn, P. Upcroft, P. J. O'Donoghue, and J. A. Upcroft. 2003. Drug resistance in the sexually transmitted protozoan Trichomonas vaginalis. Cell Res. 13:239-249. [DOI] [PubMed] [Google Scholar]
- 74.Dyall, S. D., C. M. Koehler, M. G. Delgadillo-Correa, P. J. Bradley, E. Plumper, D. Leuenberger, C. W. Turck, and P. J. Johnson. 2000. Presence of a member of the mitochondrial carrier family in hydrogenosomes: conservation of membrane-targeting pathways between hydrogenosomes and mitochondria. Mol. Cell. Biol. 20:2488-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dyall, S. D., W. Yan, M. G. Delgadillo-Correa, A. Lunceford, J. A. Loo, C. F. Clarke, and P. J. Johnson. 2004. Non-mitochondrial complex I proteins in a hydrogenosomal oxidoreductase complex. Nature 431:1103-1107. [DOI] [PubMed] [Google Scholar]
- 76.Edwards, D. I. 1993. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J. Antimicrob. Chemother. 31:9-20. [DOI] [PubMed] [Google Scholar]
- 77.Edwards, D. I. 1993. Nitroimidazole drugs—action and resistance mechanisms. II. Mechanisms of resistance. J. Antimicrob. Chemother. 31:201-210. [DOI] [PubMed] [Google Scholar]
- 78.Eichinger, D. 2001. Encystation in parasitic protozoa. Curr. Opin. Microbiol. 4:421-426. [DOI] [PubMed] [Google Scholar]
- 79.Eichinger, D. 2001. A role for a galactose lectin and its ligands during encystment of Entamoeba. J. Eukaryot. Microbiol. 48:17-21. [DOI] [PubMed] [Google Scholar]
- 80.Ellis, J. E., D. Cole, and D. Lloyd. 1992. Influence of oxygen on the fermentative metabolism of metronidazole-sensitive and resistant strains of Trichomonas vaginalis. Mol. Biochem. Parasitol. 56:79-88. [DOI] [PubMed] [Google Scholar]
- 81.Ellis, J. E., R. Williams, D. Cole, R. Cammack, and D. Lloyd. 1993. Electron transport components of the parasitic protozoon Giardia lamblia. FEBS Lett. 325:196-200. [DOI] [PubMed] [Google Scholar]
- 82.Ellis, J. E., N. Yarlett, D. Cole, M. J. Humphreys, and D. Lloyd. 1994. Antioxidant defences in the microaerophilic protozoan Trichomonas vaginalis: comparison of metronidazole-resistant and sensitive strains. Microbiology 140:2489-2494. [DOI] [PubMed] [Google Scholar]
- 83.Ellis, K. E., B. Clough, J. W. Saldanha, and R. J. Wilson. 2001. Nifs and Sufs in malaria. Mol. Microbiol. 41:973-981. [DOI] [PubMed] [Google Scholar]
- 84.Embley, T. M., and W. Martin. 2006. Eukaryotic evolution, changes and challenges. Nature 440:623-630. [DOI] [PubMed] [Google Scholar]
- 85.Embley, T. M., and W. Martin. 1998. A hydrogen-producing mitochondrion. Nature 396:517-519. [DOI] [PubMed] [Google Scholar]
- 86.Embley, T. M., M. van der Giezen, D. S. Horner, P. L. Dyal, S. Bell, and P. G. Foster. 2003. Hydrogenosomes, mitochondria and early eukaryotic evolution. IUBMB Life 55:387-395. [DOI] [PubMed] [Google Scholar]
- 87.Embley, T. M., M. van der Giezen, D. S. Horner, P. L. Dyal, and P. Foster. 2003. Mitochondria and hydrogenosomes are two forms of the same fundamental organelle. Philos. Trans. R. Soc. London B 358:191-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Erlandsen, S. L., and E. A. Meyer (ed.). 1984. Giardia and giardiasis: biology, pathogenesis, and epidemiology, p. 33-63. Plenum Press, New York, NY.
- 89.Esaki, N., and K. Soda. 1987. l-Methionine gamma-lyase from Pseudomonas putida and Aeromonas. Methods Enzymol. 143:459-465. [DOI] [PubMed] [Google Scholar]
- 90.Espinosa, A., D. Clark, and S. L. Stanley, Jr. 2004. Entamoeba histolytica alcohol dehydrogenase 2 (EhADH2) as a target for anti-amoebic agents. J. Antimicrob. Chemother. 54:56-59. [DOI] [PubMed] [Google Scholar]
- 91.Estambale, B. B., and R. Knight. 1992. Protozoan infections and HIV-1 infection: a review. East Afr. Med. J. 69:373-377. [PubMed] [Google Scholar]
- 92.Fahey, R. C., G. L. Newton, B. Arrick, T. Overdank-Bogart, and S. B. Aley. 1984. Entamoeba histolytica: a eukaryote without glutathione metabolism. Science 224:70-72. [DOI] [PubMed] [Google Scholar]
- 93.Fairlamb, A. H. 2002. Metabolic pathway analysis in trypanosomes and malaria parasites. Philos. Trans. R. Soc. London B 357:101-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Falagas, M. E., A. M. Walker, H. Jick, R. Ruthazer, J. Griffith, and D. R. Snydman. 1998. Late incidence of cancer after metronidazole use: a matched metronidazole user/nonuser study. Clin. Infect. Dis. 26:384-388. [DOI] [PubMed] [Google Scholar]
- 95.Farthing, M. J. 1996. Giardiasis. Gastroenterol. Clin. N. Am. 25:493-515. [DOI] [PubMed] [Google Scholar]
- 96.Finegold, S. M., and H. M. Wexler. 1996. Present studies of therapy for anaerobic infections. Clin. Infect. Dis. 23(Suppl. 1):S9-S14. [DOI] [PubMed] [Google Scholar]
- 97.Fojo, A., S. Akiyama, M. M. Gottesman, and I. Pastan. 1985. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 45:3002-3007. [PubMed] [Google Scholar]
- 98.Frey, M. 2002. Hydrogenases: hydrogen-activating enzymes. Chem. Biochem. 3:153-160. [DOI] [PubMed] [Google Scholar]
- 99.Gardner, T. B., and D. R. Hill. 2001. Treatment of giardiasis. Clin. Microbiol. Rev. 14:114-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gargala, G., A. Delaunay, X. Li, P. Brasseur, L. Favennec, and J. J. Ballet. 2000. Efficacy of nitazoxanide, tizoxanide and tizoxanide glucuronide against Cryptosporidium parvum development in sporozoite-infected HCT-8 enterocytic cells. J. Antimicrob. Chemother. 46:57-60. [DOI] [PubMed] [Google Scholar]
- 101.Gault, M. J., D. S. Reiner, and F. D. Gillin. 1985. Tolerance of axenically cultured Entamoeba histolytica and Giardia lamblia to a variety of antimicrobial agents. Trans. R. Soc. Trop. Med. Hyg. 79:60-62. [DOI] [PubMed] [Google Scholar]
- 102.Gerwig, G. J., J. A. van Kuik, B. R. Leeflang, J. P. Kamerling, J. F. Vliegenthart, C. D. Karr, and E. L. Jarroll. 2002. The Giardia intestinalis filamentous cyst wall contains a novel beta(1-3)-N-acetyl-d-galactosamine polymer: a structural and conformational study. Glycobiology 12:499-505. [DOI] [PubMed] [Google Scholar]
- 103.Ghosh, S., J. M. Chan, C. R. Lea, G. A. Meints, J. C. Lewis, Z. S. Tovian, R. M. Flessner, T. C. Loftus, I. Bruchhaus, H. Kendrick, S. L. Croft, R. G. Kemp, S. Kobayashi, T. Nozaki, and E. Oldfield. 2004. Effects of bisphosphonates on the growth of Entamoeba histolytica and Plasmodium species in vitro and in vivo. J. Med. Chem. 47:175-187. [DOI] [PubMed] [Google Scholar]
- 104.Ghosh, S., J. Field, R. Rogers, M. Hickman, and J. Samuelson. 2000. The Entamoeba histolytica mitochondrion-derived organelle (crypton) contains double-stranded DNA and appears to be bound by a double membrane. Infect. Immun. 68:4319-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gilchrist, C. A., E. Houpt, N. Trapaidze, Z. Fei, O. Crasta, A. Asgharpour, C. Evans, S. Martino-Catt, D. J. Baba, S. Stroup, S. Hamano, G. Ehrenkaufer, M. Okada, U. Singh, T. Nozaki, B. J. Mann, and W. A. Petri, Jr. 2006. Impact of intestinal colonization and invasion on the Entamoeba histolytica transcriptome. Mol. Biochem. Parasitol. 147:163-176. [DOI] [PubMed] [Google Scholar]
- 106.Gillin, F. D., and L. S. Diamond. 1980. Attachment and short-term maintenance of motility and viability of Entamoeba histolytica in a defined medium. J. Protozool. 27:220-225. [DOI] [PubMed] [Google Scholar]
- 107.Gillin, F. D., and L. S. Diamond. 1980. Attachment of Entamoeba histolytica to glass in a defined maintenance medium: specific requirement for cysteine and ascorbic acid. J. Protozool. 27:474-478. [DOI] [PubMed] [Google Scholar]
- 108.Gillin, F. D., and L. S. Diamond. 1981. Entamoeba histolytica and Giardia lamblia: effects of cysteine and oxygen tension on trophozoite attachment to glass and survival in culture media. Exp. Parasitol. 52:9-17. [DOI] [PubMed] [Google Scholar]
- 109.Gillin, F. D., and L. S. Diamond. 1981. Entamoeba histolytica and Giardia lamblia: growth responses to reducing agents. Exp. Parasitol. 51:382-391. [DOI] [PubMed] [Google Scholar]
- 110.Gillin, F. D., D. S. Reiner, R. B. Levy, and P. A. Henkart. 1984. Thiol groups on the surface of anaerobic parasitic protozoa. Mol. Biochem. Parasitol. 13:1-12. [DOI] [PubMed] [Google Scholar]
- 111.Gillin, F. D., D. S. Reiner, and J. M. McCaffery. 1996. Cell biology of the primitive eukaryote Giardia lamblia. Annu. Rev. Microbiol. 50:679-705. [DOI] [PubMed] [Google Scholar]
- 112.Ginger, M. L. 2006. Niche metabolism in parasitic protozoa. Philos. Trans. R. Soc. London B 361:101-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glick, B. R., W. G. Martin, and S. M. Martin. 1980. Purification and properties of the periplasmic hydrogenase from Desulfovibrio desulfuricans. Can. J. Microbiol. 26:1214-1223. [DOI] [PubMed] [Google Scholar]
- 114.Reference deleted.
- 115.Goodwin, A., D. Kersulyte, G. Sisson, S. J. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 116.Gorrell, T. E., N. Yarlett, and M. Muller. 1984. Isolation and characterization of Trichomonas vaginalis ferredoxin. Carlsberg Res. Commun. 49:259-268. [Google Scholar]
- 117.Gupta, P. K., and J. K. Frost. 1990. Cytopathology and histopathology of the female genital tract in Trichomonas vaginalis infection, p. 274-290. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, NY.
- 118.Haghighi, A., S. Kobayashi, T. Takeuchi, G. Masuda, and T. Nozaki. 2002. Remarkable genetic polymorphism among Entamoeba histolytica isolates from a limited geographic area. J. Clin. Microbiol. 40:4081-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haghighi, A., S. Kobayashi, T. Takeuchi, N. Thammapalerd, and T. Nozaki. 2003. Geographic diversity of genotypes among Entamoeba histolytica field isolates. J. Clin. Microbiol. 41:3748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hall, A., and Q. Nahar. 1993. Albendazole as a treatment for infections with Giardia duodenalis in children in Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 87:84-86. [DOI] [PubMed] [Google Scholar]
- 121.Hammerschlag, M. R. 1998. Sexually transmitted diseases in sexually abused children: medical and legal implications. Sex. Transm. Infect. 74:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Haque, R., C. D. Huston, M. Hughes, E. Houpt, and W. A. Petri, Jr. 2003. Amebiasis. N. Engl. J. Med. 348:1565-1573. [DOI] [PubMed] [Google Scholar]
- 123.Hardy, P. H., J. B. Hardy, E. E. Nell, D. A. Graham, M. R. Spence, and R. C. Rosenbaum. 1984. Prevalence of six sexually transmitted disease agents among pregnant inner-city adolescents and pregnancy outcome. Lancet ii:333-337. [DOI] [PubMed] [Google Scholar]
- 124.Heath, J. P. 1981. Behaviour and pathogenicity of Trichomonas vaginalis in epithelial cell cultures: a study by light and scanning electron microscopy. Br. J. Vener. Dis. 57:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heddi, A., A. M. Grenier, C. Khatchadourian, H. Charles, and P. Nardon. 1999. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc. Natl. Acad. Sci. USA 96:6814-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hell, R., C. Bork, N. Bogdanova, I. Frolov, and R. Hauschild. 1994. Isolation and characterization of two cDNAs encoding for compartment specific isoforms of O-acetylserine (thiol) lyase from Arabidopsis thaliana. FEBS Lett. 351:257-262. [DOI] [PubMed] [Google Scholar]
- 127.Hell, R., and H. Hillebrand. 2001. Plant concepts for mineral acquisition and allocation. Curr. Opin. Biotechnol. 12:161-168. [DOI] [PubMed] [Google Scholar]
- 128.Hell, R., R. Jost, O. Berkowitz, and M. Wirtz. 2002. Molecular and biochemical analysis of the enzymes of cysteine biosynthesis in the plant Arabidopsis thaliana. Amino Acids 22:245-257. [DOI] [PubMed] [Google Scholar]
- 129.Heyworth, M. F. 1996. Giardiasis, p. 272-280. In D. J. Weatherall, J. G. G. Ledingham, and D. A. Warrell (ed.), Oxford textbook of medicine. Oxford University Press, Oxford, United Kingdom.
- 130.Hofgen, R., O. Kreft, L. Willmitzer, and H. Hesse. 2001. Manipulation of thiol contents in plants. Amino Acids 20:291-299. [DOI] [PubMed] [Google Scholar]
- 131.Holmes, K. K., H. H. Handsfield, S. P. Wang, B. B. Wentworth, M. Turck, J. B. Anderson, and E. R. Alexander. 1975. Etiology of nongonococcal urethritis. N. Engl. J. Med. 292:1199-1205. [DOI] [PubMed] [Google Scholar]
- 132.Honigberg, B. M. 1990. Trichomonads found outside the urogenital tract of humans, p. 342-393. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, NY.
- 133.Honigberg, B. M. 1978. Trichomonads of verterinary importance, p. 163-273. In J. P. Kreier (ed.), Parasitic protozoa, vol. II. Academic Press, New York, NY. [Google Scholar]
- 134.Honigberg, B. M., and V. M. King. 1964. Structure of Trichomonas vaginalis Donn'e. J. Parasitol. 50:345-364. [PubMed] [Google Scholar]
- 135.Hoosen, A. A., S. M. Ross, M. J. Mulla, and M. Patel. 1981. The incidence of selected vaginal infections among pregnant urban blacks. S. Afr. Med. J. 59:827-829. [PubMed] [Google Scholar]
- 136.Horner, D. S., P. G. Foster, and T. M. Embley. 2000. Iron hydrogenases and the evolution of anaerobic eukaryotes. Mol. Biol. Evol. 17:1695-1709. [DOI] [PubMed] [Google Scholar]
- 137.Horner, D. S., B. Heil, T. Happe, and T. M. Embley. 2002. Iron hydrogenases—ancient enzymes in modern eukaryotes. Trends Biochem. Sci. 27:148-153. [DOI] [PubMed] [Google Scholar]
- 138.Horner, D. S., R. P. Hirt, and T. M. Embley. 1999. A single eubacterial origin of eukaryotic pyruvate: ferredoxin oxidoreductase genes: implications for the evolution of anaerobic eukaryotes. Mol. Biol. Evol. 16:1280-1291. [DOI] [PubMed] [Google Scholar]
- 139.Houpt, E. R., D. J. Glembocki, T. G. Obrig, C. A. Moskaluk, L. A. Lockhart, R. L. Wright, R. M. Seaner, T. R. Keepers, T. D. Wilkins, and W. A. Petri, Jr. 2002. The mouse model of amebic colitis reveals mouse strain susceptibility to infection and exacerbation of disease by CD4+ T cells. J. Immunol. 169:4496-4503. [DOI] [PubMed] [Google Scholar]
- 140.Hrdy, I., R. Cammack, P. Stopka, J. Kulda, and J. Tachezy. 2005. Alternative pathway of metronidazole activation in Trichomonas vaginalis hydrogenosomes. Antimicrob. Agents Chemother. 49:5033-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hrdy, I., R. P. Hirt, P. Dolezal, L. Bardonova, P. G. Foster, J. Tachezy, and T. M. Embley. 2004. Trichomonas hydrogenosomes contain the NADH dehydrogenase module of mitochondrial complex I. Nature 432:618-622. [DOI] [PubMed] [Google Scholar]
- 142.Hrdy, I., and M. Muller. 1995. Primary structure and eubacterial relationships of the pyruvate:ferredoxin oxidoreductase of the amitochondriate eukaryote Trichomonas vaginalis. J. Mol. Evol. 41:388-396. [PubMed] [Google Scholar]
- 143.Huber, M., L. Garfinkel, C. Gitler, D. Mirelman, M. Revel, and S. Rozenblatt. 1988. Nucleotide sequence analysis of an Entamoeba histolytica ferredoxin gene. Mol. Biochem. Parasitol. 31:27-33. [DOI] [PubMed] [Google Scholar]
- 144.Ikegami, F., M. Kamiya, Y. H. Kuo, F. Lambein, and I. Murakoshi. 1996. Enzymatic synthesis of two isoxazolylalanine isomers by cysteine synthases in Lathyrus species. Biol. Pharm. Bull. 19:1214-1215. [DOI] [PubMed] [Google Scholar]
- 145.Ikegami, F., and I. Murakoshi. 1993. Heterocyclic beta-substituted alanines. Methods Plant Biochem. 9:133-151. [Google Scholar]
- 146.Ikegami, F., M. Mizuno, M. Kihara, and I. Murakoshi. 1990. Enzymatic synthesis of the thyrotoxic amino acid mimosine by cysteine synthase. Phytochemistry 29:3461-3465. [Google Scholar]
- 147.Ito, S., T. Nakamura, and Y. Eguchi. 1976. Purification and characterization of methioninase from Pseudomonas putida. J. Biochem. (Tokyo) 79:1263-1272. [DOI] [PubMed] [Google Scholar]
- 148.Jarroll, E. L., and K. Sener. 2003. Potential drug targets in cyst-wall biosynthesis by intestinal protozoa. Drug Resist. Update 6:239-246. [DOI] [PubMed] [Google Scholar]
- 149.Jernigan, J. A., and R. D. Pearson. 1995. Antiparasitic agents, p. 458-492. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas and Bennett's principles and practice of infectious diseases. Churchill Livingstone, New York, NY.
- 150.Jimenez-Cardoso, E., A. Flores-Luna, and J. Perez-Urizar. 2004. In vitro activity of two phenyl-carbamate derivatives, singly and in combination with albendazole against albendazole-resistant Giardia intestinalis. Acta Trop. 92:237-244. [DOI] [PubMed] [Google Scholar]
- 151.Joet, T., C. Morin, J. Fischbarg, A. I. Louw, U. Eckstein-Ludwig, C. Woodrow, and S. Krishna. 2003. Why is the Plasmodium falciparum hexose transporter a promising new drug target? Expert Opin. Ther. Targets 7:593-602. [DOI] [PubMed] [Google Scholar]
- 152.Johnson, D. C., D. R. Dean, A. D. Smith, and M. K. Johnson. 2005. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 74:247-281. [DOI] [PubMed] [Google Scholar]
- 153.Johnson, P. J. 1993. Metronidazole and drug resistance. Parasitol. Today 9:183-186. [DOI] [PubMed] [Google Scholar]
- 154.Johnson, P. J., C. E. d'Oliveira, T. E. Gorrell, and M. Muller. 1990. Molecular analysis of the hydrogenosomal ferredoxin of the anaerobic protist Trichomonas vaginalis. Proc. Natl. Acad. Sci. USA 87:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Karabay, O., A. Tamer, H. Gunduz, D. Kayas, H. Arinc, and H. Celebi. 2004. Albendazole versus metronidazole treatment of adult giardiasis: an open randomized clinical study. World J. Gastroenterol. 10:1215-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Keeling, P. J., G. Burger, D. G. Durnford, B. F. Lang, R. W. Lee, R. E. Pearlman, A. J. Roger, and M. W. Gray. 2005. The tree of eukaryotes. Trends Ecol. Evol. 20:670-676. [DOI] [PubMed] [Google Scholar]
- 157.Keene, W. E., M. G. Petitt, S. Allen, and J. H. McKerrow. 1986. The major neutral proteinase of Entamoeba histolytica. J. Exp. Med. 163:536-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kerekes, A., A. Tege, K. Szarka, and P. Temesvari. 2000. Neonatal respiratory insufficiency caused by maternal infection with Trichomonas vaginalis. Orv. Hetil. 141:1821-1822. [PubMed] [Google Scholar]
- 159.Kita, K., C. Nihei, and E. Tomitsuka. 2003. Parasite mitochondria as drug target: diversity and dynamic changes during the life cycle. Curr. Med. Chem. 10:2535-2548. [DOI] [PubMed] [Google Scholar]
- 160.Klenke, B., M. Stewart, M. P. Barrett, R. Brun, and I. H. Gilbert. 2001. Synthesis and biological evaluation of s-triazine substituted polyamines as potential new anti-trypanosomal drugs. J. Med. Chem. 44:3440-3452. [DOI] [PubMed] [Google Scholar]
- 161.Kletzin, A., and M. W. Adams. 1996. Molecular and phylogenetic characterization of pyruvate and 2-ketoisovalerate ferredoxin oxidoreductases from Pyrococcus furiosus and pyruvate ferredoxin oxidoreductase from Thermotoga maritima. J. Bacteriol. 178:248-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Knight, R. 1980. The chemotherapy of amoebiasis. J. Antimicrob. Chemother. 6:577-593. [DOI] [PubMed] [Google Scholar]
- 163.Kobori, T., H. Sasaki, W. C. Lee, S. Zenno, K. Saigo, M. E. Murphy, and M. Tanokura. 2001. Structure and site-directed mutagenesis of a flavoprotein from Escherichia coli that reduces nitrocompounds: alteration of pyridine nucleotide binding by a single amino acid substitution. J. Biol. Chem. 276:2816-2823. [DOI] [PubMed] [Google Scholar]
- 164.Kredich, N. M., and G. M. Tomkins. 1966. The enzymic synthesis of l-cysteine in Escherichia coli and Salmonella typhimurium. J. Biol. Chem. 241:4955-4965. [PubMed] [Google Scholar]
- 165.Kreis, W., and C. Hession. 1973. Isolation and purification of l-methionine-alpha-deamino-gamma-mercaptomethane-lyase (l-methioninase) from Clostridium sporogenes. Cancer Res. 33:1862-1865. [PubMed] [Google Scholar]
- 166.Krieger, J. N. 1990. Epidemiology and clinical manifestations of urogenital trichomoniasis in men, p. 235-245. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, NY.
- 167.Krieger, J. N. 1984. Prostatitis syndromes: pathophysiology, differential diagnosis, and treatment. Sex. Transm. Dis. 11:100-112. [PubMed] [Google Scholar]
- 168.Kulda, J. 1999. Trichomonads, hydrogenosomes and drug resistance. Int. J. Parasitol. 29:199-212. [DOI] [PubMed] [Google Scholar]
- 169.Kulda, J., J. Tachezy, and A. Cerkasovova. 1993. In vitro induced anaerobic resistance to metronidazole in Trichomonas vaginalis. J. Eukaryot. Microbiol. 40:262-269. [DOI] [PubMed] [Google Scholar]
- 170.Kumagai, M., A. Makioka, T. Takeuchi, and T. Nozaki. 2004. Molecular cloning and characterization of a protein farnesyltransferase from the enteric protozoan parasite Entamoeba histolytica. J. Biol. Chem. 279:2316-2323. [DOI] [PubMed] [Google Scholar]
- 171.Kwa, M. S., J. G. Veenstra, M. Van Dijk, and M. H. Roos. 1995. Beta-tubulin genes from the parasitic nematode Haemonchus contortus modulate drug resistance in Caenorhabditis elegans. J. Mol. Biol. 246:500-510. [DOI] [PubMed] [Google Scholar]
- 172.Lafleur, M. V., E. J. Pluijmackers-Westmijze, and H. Loman. 1986. Effect of radiation-induced reduction of nitroimidazoles on biologically active DNA. Int. J. Radiat. Oncol. Biol. Phys. 12:1211-1214. [DOI] [PubMed] [Google Scholar]
- 173.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, M. Alary, et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95-102. [DOI] [PubMed] [Google Scholar]
- 174.LaGier, M. J., J. Tachezy, F. Stejskal, K. Kutisova, and J. S. Keithly. 2003. Mitochondrial-type iron-sulfur cluster biosynthesis genes (IscS and IscU) in the apicomplexan Cryptosporidium parvum. Microbiology 149:3519-3530. [DOI] [PubMed] [Google Scholar]
- 175.Land, K. M., D. L. Clemens, and P. J. Johnson. 2001. Loss of multiple hydrogenosomal proteins associated with organelle metabolism and high-level drug resistance in trichomonads. Exp. Parasitol. 97:102-110. [DOI] [PubMed] [Google Scholar]
- 176.Land, K. M., M. G. Delgadillo-Correa, J. Tachezy, S. Vanacova, C. L. Hsieh, R. Sutak, and P. J. Johnson. 2004. Targeted gene replacement of a ferredoxin gene in Trichomonas vaginalis does not lead to metronidazole resistance. Mol. Microbiol. 51:115-122. [DOI] [PubMed] [Google Scholar]
- 177.Lau, A. H., N. P. Lam, S. C. Piscitelli, L. Wilkes, and L. H. Danziger. 1992. Clinical pharmacokinetics of metronidazole and other nitroimidazole anti-infectives. Clin. Pharmacokinet. 23:328-364. [DOI] [PubMed] [Google Scholar]
- 178.Lindmark, D. G. 1980. Energy metabolism of the anaerobic protozoon Giardia lamblia. Mol. Biochem. Parasitol. 1:1-12. [DOI] [PubMed] [Google Scholar]
- 179.Lindmark, D. G., and M. Muller. 1973. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J. Biol. Chem. 248:7724-7728. [PubMed] [Google Scholar]
- 180.Lindquist, H. D. 1996. Induction of albendazole resistance in Giardia lamblia. Microb. Drug Resist. 2:433-434. [DOI] [PubMed] [Google Scholar]
- 181.Liu, S. M., D. M. Brown, P. O'Donoghue, P. Upcroft, and J. A. Upcroft. 2000. Ferredoxin involvement in metronidazole resistance of Giardia duodenalis. Mol. Biochem. Parasitol. 108:137-140. [DOI] [PubMed] [Google Scholar]
- 182.Lloyd, D., and B. Kristensen. 1985. Metronidazole inhibition of hydrogen production in vivo in drug-sensitive and resistant strains of Trichomonas vaginalis. J. Gen. Microbiol. 131:849-853. [DOI] [PubMed] [Google Scholar]
- 183.Lloyd, D., J. R. Ralphs, and J. C. Harris. 2002. Giardia intestinalis, a eukaryote without hydrogenosomes, produces hydrogen. Microbiology 148:727-733. [DOI] [PubMed] [Google Scholar]
- 184.Lockwood, B. C., and G. H. Coombs. 1991. Purification and characterization of methionine gamma-lyase from Trichomonas vaginalis. Biochem. J. 279:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Loftus, B., I. Anderson, R. Davies, U. C. Alsmark, J. Samuelson, P. Amedeo, P. Roncaglia, M. Berriman, R. P. Hirt, B. J. Mann, T. Nozaki, B. Suh, M. Pop, M. Duchene, J. Ackers, E. Tannich, M. Leippe, M. Hofer, I. Bruchhaus, U. Willhoeft, A. Bhattacharya, T. Chillingworth, C. Churcher, Z. Hance, B. Harris, D. Harris, K. Jagels, S. Moule, K. Mungall, D. Ormond, R. Squares, S. Whitehead, M. A. Quail, E. Rabbinowitsch, H. Norbertczak, C. Price, Z. Wang, N. Guillen, C. Gilchrist, S. E. Stroup, S. Bhattacharya, A. Lohia, P. G. Foster, T. Sicheritz-Ponten, C. Weber, U. Singh, C. Mukherjee, N. M. El-Sayed, W. A. Petri, Jr., C. G. Clark, T. M. Embley, B. Barrell, C. M. Fraser, and N. Hall. 2005. The genome of the protist parasite Entamoeba histolytica. Nature 433:865-868. [DOI] [PubMed] [Google Scholar]
- 186.Lossick, J. G. 1990. Treatment of sexually transmitted vaginosis/vaginitis. Rev. Infect. Dis. 12(Suppl. 6):S665-S681. [DOI] [PubMed] [Google Scholar]
- 187.Lubega, G. W., T. G. Geary, R. D. Klein, and R. K. Prichard. 1993. Expression of cloned beta-tubulin genes of Haemonchus contortus in Escherichia coli: interaction of recombinant beta-tubulin with native tubulin and mebendazole. Mol. Biochem. Parasitol. 62:281-292. [DOI] [PubMed] [Google Scholar]
- 188.Lubega, G. W., and R. K. Prichard. 1990. Specific interaction of benzimidazole anthelmintics with tubulin: high-affinity binding and benzimidazole resistance in Haemonchus contortus. Mol. Biochem. Parasitol. 38:221-232. [DOI] [PubMed] [Google Scholar]
- 189.Lujan, H. D., M. R. Mowatt, and T. E. Nash. 1997. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol. Mol. Biol. Rev. 61:294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lushbaugh, W. B., J. D. Cleary, and R. W. Finley. 1995. Cytotoxicity of hamycin for Trichomonas vaginalis, HeLa and BHK-21. J. Antimicrob. Chemother. 36:795-802. [DOI] [PubMed] [Google Scholar]
- 191.MacDonald, L. M., A. Armson, A. R. Thompson, and J. A. Reynoldson. 2004. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol. Biochem. Parasitol. 138:89-96. [DOI] [PubMed] [Google Scholar]
- 192.MacDonald, L. M., A. Armson, R. C. Thompson, and J. A. Reynoldson. 2003. Characterization of factors favoring the expression of soluble protozoan tubulin proteins in Escherichia coli. Protein Expr. Purif. 29:117-122. [DOI] [PubMed] [Google Scholar]
- 193.MacFarlane, R., D. Bhattacharya, and U. Singh. 2005. Genomic DNA microarrays for Entamoeba histolytica: applications for use in expression profiling and strain genotyping. Exp. Parasitol. 110:196-202. [DOI] [PubMed] [Google Scholar]
- 194.Mai, Z., S. Ghosh, M. Frisardi, B. Rosenthal, R. Rogers, and J. Samuelson. 1999. Hsp60 is targeted to a cryptic mitochondrion-derived organelle (“crypton”) in the microaerophilic protozoan parasite Entamoeba histolytica. Mol. Cell. Biol. 19:2198-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Makioka, A., M. Kumagai, T. Takeuchi, and T. Nozaki. 2006. Characterization of protein geranylgeranyltransferase I from the enteric protist Entamoeba histolytica. Mol. Biochem. Parasitol. 145:216-225. [DOI] [PubMed] [Google Scholar]
- 196.Malagoli, M., T. Rossi, A. Baggio, G. Zandomeneghi, A. Zanca, C. Casolari, and M. Castelli. 2002. ‘In vitro’ study of chemotherapeutic activity of sulphimidazole on some sensitive and metronidazole-resistant Trichomonas vaginalis strains. Pharmacol. Res. 46:469-472. [DOI] [PubMed] [Google Scholar]
- 197.Mardh, P. A., and S. Colleen. 1975. Search for uro-genital tract infections in patients with symptoms of prostatitis. Studies on aerobic and strictly anaerobic bacteria, mycoplasmas, fungi, trichomonads and viruses. Scand. J. Urol. Nephrol. 9:8-16. [DOI] [PubMed] [Google Scholar]
- 198.Marecki, M., K. Heeb, and W. Zitka. 1997. Protozoan infection in the perinatal period. J. Perinat. Neonat. Nurs. 11:21-33, 87-88. [DOI] [PubMed] [Google Scholar]
- 199.Marshall, M. M., D. Naumovitz, Y. Ortega, and C. R. Sterling. 1997. Waterborne protozoan pathogens. Clin. Microbiol. Rev. 10:67-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Martin, M. B., J. S. Grimley, J. C. Lewis, H. T. Heath III, B. N. Bailey, H. Kendrick, V. Yardley, A. Caldera, R. Lira, J. A. Urbina, S. N. Moreno, R. Docampo, S. L. Croft, and E. Oldfield. 2001. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: a potential route to chemotherapy. J. Med. Chem. 44:909-916. [DOI] [PubMed] [Google Scholar]
- 201.Maruyama, A., K. Ishizawa, T. Takagi, and Y. Esashi. 1998. Cytosolic beta-cyanoalanine synthase activity attributed to cysteine synthases in cocklebur seeds. Purification and characterization of cytosolic cysteine synthases. Plant Cell Physiol. 39:671-680. [DOI] [PubMed] [Google Scholar]
- 202.McKie, A. E., T. Edlind, J. Walker, J. C. Mottram, and G. H. Coombs. 1998. The primitive protozoon Trichomonas vaginalis contains two methionine gamma-lyase genes that encode members of the gamma-family of pyridoxal 5′-phosphate-dependent enzymes. J. Biol. Chem. 273:5549-5556. [DOI] [PubMed] [Google Scholar]
- 203.McLaughlin, J., and S. Aley. 1985. The biochemistry and functional morphology of the Entamoeba. J. Protozool. 32:221-240. [DOI] [PubMed] [Google Scholar]
- 204.McLellan, R., M. R. Spence, M. Brockman, L. Raffel, and J. L. Smith. 1982. The clinical diagnosis of trichomoniasis. Obstet. Gynecol. 60:30-34. [PubMed] [Google Scholar]
- 205.Mehlotra, R. K. 1996. Antioxidant defense mechanisms in parasitic protozoa. Crit. Rev. Microbiol. 22:295-314. [DOI] [PubMed] [Google Scholar]
- 206.Mehta, P. K., and P. Christen. 1998. The molecular evolution of pyridoxal-5′-phosphate-dependent enzymes. Adv. Enzymol. Relat. Areas Mol. Biol. 74:129-184. [DOI] [PubMed] [Google Scholar]
- 207.Meinecke, B., J. Bertram, and G. Gottschalk. 1989. Purification and characterization of the pyruvate-ferredoxin oxidoreductase from Clostridium acetobutylicum. Arch. Microbiol. 152:244-250. [DOI] [PubMed] [Google Scholar]
- 208.Meingassner, J. G., and H. Mieth. 1976. Cross-resistance of trichomonads to 5-nitroimidazole-derivatives. Experientia 32:183-184. [DOI] [PubMed] [Google Scholar]
- 209.Mertens, E., E. Van Schaftingen, and M. Muller. 1992. Pyruvate kinase from Trichomonas vaginalis, an allosteric enzyme stimulated by ribose 5-phosphate and glycerate 3-phosphate. Mol. Biochem. Parasitol. 54:13-20. [DOI] [PubMed] [Google Scholar]
- 210.Messerschmidt, A., M. Worbs, C. Steegborn, M. C. Wahl, R. Huber, B. Laber, and T. Clausen. 2003. Determinants of enzymatic specificity in the Cys-Met-metabolism PLP-dependent enzymes family: crystal structure of cystathionine gamma-lyase from yeast and intrafamiliar structure comparison. Biol. Chem. 384:373-386. [DOI] [PubMed] [Google Scholar]
- 211.Meysick, K., and G. E. Garber. 1995. Trichomonas vaginalis. Curr. Opin. Infect. Dis. 8:22-25. [Google Scholar]
- 212.Mintz, E. D., M. Hudson-Wragg, P. Mshar, M. L. Cartter, and J. L. Hadler. 1993. Foodborne giardiasis in a corporate office setting. J. Infect. Dis. 167:250-253. [DOI] [PubMed] [Google Scholar]
- 213.Moreno-Brito, V., C. Yanez-Gomez, P. Meza-Cervantez, L. Avila-Gonzalez, M. A. Rodriguez, J. Ortega-Lopez, A. Gonzalez-Robles, and R. Arroyo. 2005. A Trichomonas vaginalis 120 kDa protein with identity to hydrogenosome pyruvate:ferredoxin oxidoreductase is a surface adhesin induced by iron. Cell Microbiol. 7:245-258. [DOI] [PubMed] [Google Scholar]
- 214.Motoshima, H., K. Inagaki, T. Kumasaka, M. Furuichi, H. Inoue, T. Tamura, N. Esaki, K. Soda, N. Tanaka, M. Yamamoto, and H. Tanaka. 2000. Crystal structure of the pyridoxal 5′-phosphate dependent l-methionine gamma-lyase from Pseudomonas putida. J. Biochem. (Tokyo) 128:349-354. [DOI] [PubMed] [Google Scholar]
- 215.Muller, M. 1992. Energy metabolism of ancestral eukaryotes: a hypothesis based on the biochemistry of amitochondriate parasitic protists. Biosystems 28:33-40. [DOI] [PubMed] [Google Scholar]
- 216.Muller, M. 1988. Energy metabolism of protozoa without mitochondria. Annu. Rev. Microbiol. 42:465-488. [DOI] [PubMed] [Google Scholar]
- 217.Muller, M. 2003. Energy metabolism. I. Anaerobic protozoa, p. 125-139. In J. J. Marr, T. W. Nelsen, and R. W. Komuniecki (ed.), Molecular medical parasitology. Academic Press, London, United Kingdom.
- 218.Muller, M. 1993. The hydrogenosome. J. Gen. Microbiol. 139:2879-2889. [DOI] [PubMed] [Google Scholar]
- 219.Muller, M. 1986. Reductive activation of nitroimidazoles in anaerobic microorganisms. Biochem. Pharmacol. 35:37-41. [DOI] [PubMed] [Google Scholar]
- 220.Muller, M., and T. E. Gorrell. 1983. Metabolism and metronidazole uptake in Trichomonas vaginalis isolates with different metronidazole susceptibilities. Antimicrob. Agents Chemother. 24:667-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Muller, S., E. Liebau, R. D. Walter, and R. L. Krauth-Siegel. 2003. Thiol-based redox metabolism of protozoan parasites. Trends Parasitol. 19:320-328. [DOI] [PubMed] [Google Scholar]
- 222.Munagala, N., A. E. Sarver, and C. C. Wang. 2000. Converting the guanine phosphoribosyltransferase from Giardia lamblia to a hypoxanthine-guanine phosphoribosyltransferase. J. Biol. Chem. 275:37072-37077. [DOI] [PubMed] [Google Scholar]
- 223.Nachin, L., L. Loiseau, D. Expert, and F. Barras. 2003. SufC: an unorthodox cytoplasmic ABC/ATPase required for [Fe-S] biogenesis under oxidative stress. EMBO J. 22:427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nakayama, T., N. Esaki, K. Sugie, T. T. Beresov, H. Tanaka, and K. Soda. 1984. Purification of bacterial l-methionine gamma-lyase. Anal. Biochem. 138:421-424. [DOI] [PubMed] [Google Scholar]
- 225.Narcisi, E. M., and W. E. Secor. 1996. In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob. Agents Chemother. 40:1121-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nare, B., G. Lubega, R. K. Prichard, and E. Georges. 1996. p-Azidosalicyl-5-amino-6-phenoxybenzimidazole photolabels the N-terminal 63-103 amino acids of Haemonchus contortus beta-tubulin 1. J. Biol. Chem. 271:8575-8581. [DOI] [PubMed] [Google Scholar]
- 227.Nash, T. E., C. A. Ohl, E. Thomas, G. Subramanian, P. Keiser, and T. A. Moore. 2001. Treatment of patients with refractory giardiasis. Clin. Infect. Dis. 33:22-28. [DOI] [PubMed] [Google Scholar]
- 228.Nixon, J. E., J. Field, A. G. McArthur, M. L. Sogin, N. Yarlett, B. J. Loftus, and J. Samuelson. 2003. Iron-dependent hydrogenases of Entamoeba histolytica and Giardia lamblia: activity of the recombinant entamoebic enzyme and evidence for lateral gene transfer. Biol. Bull. 204:1-9. [DOI] [PubMed] [Google Scholar]
- 229.Nixon, J. E., A. Wang, J. Field, H. G. Morrison, A. G. McArthur, M. L. Sogin, B. J. Loftus, and J. Samuelson. 2002. Evidence for lateral transfer of genes encoding ferredoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica. Eukaryot. Cell 1:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Noji, M., and K. Saito. 2002. Molecular and biochemical analysis of serine acetyltransferase and cysteine synthase towards sulfur metabolic engineering in plants. Amino Acids 22:231-243. [DOI] [PubMed] [Google Scholar]
- 231.Nolling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nozaki, T. 2000. Current problems of amebiasis in Japan and recent advances in amebiasis research. Jpn. J. Infect. Dis. 53:229-237. [PubMed] [Google Scholar]
- 233.Nozaki, T., V. Ali, and M. Tokoro. 2005. Sulfur-containing amino acid metabolism in parasitic protozoa. Adv. Parasitol. 60C:1-99. [DOI] [PubMed] [Google Scholar]
- 234.Nozaki, T., T. Asai, S. Kobayashi, F. Ikegami, M. Noji, K. Saito, and T. Takeuchi. 1998. Molecular cloning and characterization of the genes encoding two isoforms of cysteine synthase in the enteric protozoan parasite Entamoeba histolytica. Mol. Biochem. Parasitol. 97:33-44. [DOI] [PubMed] [Google Scholar]
- 235.Nozaki, T., T. Asai, L. B. Sanchez, S. Kobayashi, M. Nakazawa, and T. Takeuchi. 1999. Characterization of the gene encoding serine acetyltransferase, a regulated enzyme of cysteine biosynthesis from the protist parasites Entamoeba histolytica and Entamoeba dispar. Regulation and possible function of the cysteine biosynthetic pathway in Entamoeba. J. Biol. Chem. 274:32445-32452. [DOI] [PubMed] [Google Scholar]
- 236.Nozaki, T., S. Kobayashi, T. Takeuchi, and A. Haghighi. 2006. Diversity of clinical isolates of Entamoeba histolytica in Japan. Arch. Med. Res. 37:277-279. [DOI] [PubMed] [Google Scholar]
- 237.Nozaki, T., Y. Shigeta, Y. Saito-Nakano, M. Imada, and W. D. Kruger. 2001. Characterization of transsulfuration and cysteine biosynthetic pathways in the protozoan hemoflagellate, Trypanosoma cruzi. Isolation and molecular characterization of cystathionine beta-synthase and serine acetyltransferase from Trypanosoma. J. Biol. Chem. 276:6516-6523. [DOI] [PubMed] [Google Scholar]
- 238.Nozaki, T., M. Tokoro, M. Imada, Y. Saito, Y. Abe, Y. Shigeta, and T. Takeuchi. 2000. Cloning and biochemical characterization of genes encoding two isozymes of cysteine synthase from Entamoeba dispar. Mol. Biochem. Parasitol. 107:129-133. [DOI] [PubMed] [Google Scholar]
- 239.Ogasawara, N., S. Nakai, and H. Yoshikawa. 1994. Systematic sequencing of the 180 kilobase region of the Bacillus subtilis chromosome containing the replication origin. DNA Res. 1:1-14. [DOI] [PubMed] [Google Scholar]
- 240.Olson, J. W., J. N. Agar, M. K. Johnson, and R. J. Maier. 2000. Characterization of the NifU and NifS Fe-S cluster formation proteins essential for viability in Helicobacter pylori. Biochemistry 39:16213-16219. [DOI] [PubMed] [Google Scholar]
- 241.Ortega, Y. R., and R. D. Adam. 1997. Giardia: overview and update. Clin. Infect. Dis. 25:545-550. [DOI] [PubMed] [Google Scholar]
- 242.Outten, F. W., O. Djaman, and G. Storz. 2004. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol. Microbiol. 52:861-872. [DOI] [PubMed] [Google Scholar]
- 243.Paget, T. A., M. L. Kelly, E. L. Jarroll, D. G. Lindmark, and D. Lloyd. 1993. The effects of oxygen on fermentation in Giardia lamblia. Mol. Biochem. Parasitol. 57:65-71. [DOI] [PubMed] [Google Scholar]
- 244.Paget, T. A., M. H. Raynor, D. W. Shipp, and D. Lloyd. 1990. Giardia lamblia produces alanine anaerobically but not in the presence of oxygen. Mol. Biochem. Parasitol. 42:63-67. [DOI] [PubMed] [Google Scholar]
- 245.Perez-Reyes, E., B. Kalyanaraman, and R. P. Mason. 1980. The reductive metabolism of metronidazole and ronidazole by aerobic liver microsomes. Mol. Pharmacol. 17:239-244. [PubMed] [Google Scholar]
- 246.Petri, W. A., Jr. 2003. Therapy of intestinal protozoa. Trends Parasitol. 19:523-526. [DOI] [PubMed] [Google Scholar]
- 247.Petrin, D., K. Delgaty, R. Bhatt, and G. Garber. 1998. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11:300-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pfanner, N., and A. Geissler. 2001. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell Biol. 2:339-349. [DOI] [PubMed] [Google Scholar]
- 249.Poppe, W. A. 2001. Nitroimidazole-resistant vaginal trichomoniasis treated with paromomycin. Eur. J. Obstet. Gynecol. Reprod. Biol. 96:119-120. [DOI] [PubMed] [Google Scholar]
- 250.Price, M. A., D. Zimba, I. F. Hoffman, S. C. Kaydos-Daniels, W. C. Miller, F. Martinson, D. Chilongozi, E. Kip, E. Msowoya, M. M. Hobbs, P. N. Kazembe, and M. S. Cohen. 2003. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial. Sex. Transm. Dis. 30:516-522. [DOI] [PubMed] [Google Scholar]
- 251.Putz, S., P. Dolezal, G. Gelius-Dietrich, L. Bohacova, J. Tachezy, and K. Henze. 2006. Fe-hydrogenase maturases in the hydrogenosomes of Trichomonas vaginalis. Eukaryot. Cell 5:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Que, X., and S. L. Reed. 2000. Cysteine proteinases and the pathogenesis of amebiasis. Clin. Microbiol. Rev. 13:196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Quon, D. V., C. E. d'Oliveira, and P. J. Johnson. 1992. Reduced transcription of the ferredoxin gene in metronidazole-resistant Trichomonas vaginalis. Proc. Natl. Acad. Sci. USA 89:4402-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rasoloson, D., E. Tomkova, R. Cammack, J. Kulda, and J. Tachezy. 2001. Metronidazole-resistant strains of Trichomonas vaginalis display increased susceptibility to oxygen. Parasitology 123:45-56. [DOI] [PubMed] [Google Scholar]
- 255.Reeves, R. E. 1984. Metabolism of Entamoeba histolytica Schaudinn, 1903. Adv. Parasitol. 23:105-142. [DOI] [PubMed] [Google Scholar]
- 256.Reeves, R. E., J. D. Guthrie, and P. Lobelle-Rich. 1980. Entamoeba histolytica: isolation of ferredoxin. Exp. Parasitol. 49:83-88. [DOI] [PubMed] [Google Scholar]
- 257.Reeves, R. E., L. G. Warren, B. Susskind, and H. S. Lo. 1977. An energy-conserving pyruvate-to-acetate pathway in Entamoeba histolytica. Pyruvate synthase and a new acetate thiokinase. J. Biol. Chem. 252:726-731. [PubMed] [Google Scholar]
- 258.Regoes, A., D. Zourmpanou, G. Leon-Avila, M. van der Giezen, J. Tovar, and A. B. Hehl. 2005. Protein import, replication, and inheritance of a vestigial mitochondrion. J. Biol. Chem. 280:30557-30563. [DOI] [PubMed] [Google Scholar]
- 259.Reguera, R. M., B. L. Tekwani, and R. Balana-Fouce. 2005. Polyamine transport in parasites: a potential target for new antiparasitic drug development. Comp. Biochem. Physiol. 140:151-164. [DOI] [PubMed] [Google Scholar]
- 260.Rein, M. F., and T. A. Chapel. 1975. Trichomoniasis, candidiasis, and the minor venereal diseases. Clin. Obstet. Gynecol. 18:73-88. [DOI] [PubMed] [Google Scholar]
- 261.Rein, M. F., and M. Muller. 1990. Trichomonas vaginalis and trichomoniasis, p. 481-492. In K. K. Holmes, P.-A. Mårdh, P. F. Sparling, and P. J. Wiesner (ed.), Sexually transmitted diseases. McGraw-Hill, New York, NY.
- 262.Riscoe, M. K., A. J. Ferro, and J. H. Fitchen. 1989. Methionine recycling as a target for antiprotozoal drug development. Parasitol. Today 5:330-333. [DOI] [PubMed] [Google Scholar]
- 263.Rodriguez, M. A., R. M. Garcia-Perez, L. Mendoza, T. Sanchez, N. Guillen, and E. Orozco. 1998. The pyruvate:ferredoxin oxidoreductase enzyme is located in the plasma membrane and in a cytoplasmic structure in Entamoeba. Microb. Pathog. 25:1-10. [DOI] [PubMed] [Google Scholar]
- 264.Rodriguez, M. A., M. E. Hidalgo, T. Sanchez, and E. Orozco. 1996. Cloning and characterization of the Entamoeba histolytica pyruvate: ferredoxin oxidoreductase gene. Mol. Biochem. Parasitol. 78:273-277. [DOI] [PubMed] [Google Scholar]
- 265.Roe, F. J. 1983. Toxicologic evaluation of metronidazole with particular reference to carcinogenic, mutagenic, and teratogenic potential. Surgery 93:158-164. [PubMed] [Google Scholar]
- 266.Rohmer, M., C. Grosdemange-Billiard, M. Seemann, and D. Tritsch. 2004. Isoprenoid biosynthesis as a novel target for antibacterial and antiparasitic drugs. Curr. Opin. Investig. Drugs 5:154-162. [PubMed] [Google Scholar]
- 267.Rossignol, J. F., A. Ayoub, and M. S. Ayers. 2001. Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double-blind, placebo-controlled study of nitazoxanide. J. Infect. Dis. 184:381-384. [DOI] [PubMed] [Google Scholar]
- 268.Ryu, J. S., and D. Lloyd. 1995. Cell cytotoxicity of sodium nitrite, sodium nitroprusside and Roussin's black salt against Trichomonas vaginalis. FEMS Microbiol. Lett. 130:183-187. [DOI] [PubMed] [Google Scholar]
- 269.Saavedra, E., A. Olivos, R. Encalada, and R. Moreno-Sanchez. 2004. Entamoeba histolytica: kinetic and molecular evidence of a previously unidentified pyruvate kinase. Exp. Parasitol. 106:11-21. [DOI] [PubMed] [Google Scholar]
- 270.Saito, K., N. Miura, M. Yamazaki, H. Hirano, and I. Murakoshi. 1992. Molecular cloning and bacterial expression of cDNA encoding a plant cysteine synthase. Proc. Natl. Acad. Sci. USA 89:8078-8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Saito, K., K. Tatsuguchi, I. Murakoshi, and H. Hirano. 1993. cDNA cloning and expression of cysteine synthase B localized in chloroplasts of Spinacia oleracea. FEBS Lett. 324:247-252. [DOI] [PubMed] [Google Scholar]
- 272.Saito, K., K. Tatsuguchi, Y. Takagi, and I. Murakoshi. 1994. Isolation and characterization of cDNA that encodes a putative mitochondrion-localizing isoform of cysteine synthase (O-acetylserine(thiol)-lyase) from Spinacia oleracea. J. Biol. Chem. 269:28187-28192. [PubMed] [Google Scholar]
- 273.Saito, K., H. Yokoyama, M. Noji, and I. Murakoshi. 1995. Molecular cloning and characterization of a plant serine acetyltransferase playing a regulatory role in cysteine biosynthesis from watermelon. J. Biol. Chem. 270:16321-16326. [DOI] [PubMed] [Google Scholar]
- 274.Samarawickrema, N. A., D. M. Brown, J. A. Upcroft, N. Thammapalerd, and P. Upcroft. 1997. Involvement of superoxide dismutase and pyruvate:ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J. Antimicrob. Chemother. 40:833-840. [DOI] [PubMed] [Google Scholar]
- 275.Samuelson, J. 1999. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 43:1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Samuelson, J., P. Ayala, E. Orozco, and D. Wirth. 1990. Emetine-resistant mutants of Entamoeba histolytica overexpress mRNAs for multidrug resistance. Mol. Biochem. Parasitol. 38:281-290. [DOI] [PubMed] [Google Scholar]
- 277.Samuelson, J. C., A. Burke, and J. M. Courval. 1992. Susceptibility of an emetine-resistant mutant of Entamoeba histolytica to multiple drugs and to channel blockers. Antimicrob. Agents Chemother. 36:2392-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sanchez, L., V. Enea, and D. Eichinger. 1994. Identification of a developmentally regulated transcript expressed during encystation of Entamoeba invadens. Mol. Biochem. Parasitol. 67:125-135. [DOI] [PubMed] [Google Scholar]
- 279.Saurina, G. R., and W. M. McCormack. 1997. Trichomoniasis in pregnancy. Sex. Transm. Dis. 24:361-362. [DOI] [PubMed] [Google Scholar]
- 280.Savioli, L., H. Smith, and A. Thompson. 2006. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 22:203-208. [DOI] [PubMed] [Google Scholar]
- 281.Schwebke, J. R., T. Aira, N. Jordan, P. E. Jolly, and S. H. Vermund. 1998. Sexually transmitted diseases in Ulaanbaatar, Mongolia. Int. J. STD AIDS 9:354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Schwebke, J. R., and D. Burgess. 2004. Trichomoniasis. Clin. Microbiol. Rev. 17:794-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Schwebke, J. R., and E. W. Hook III. 2003. High rates of Trichomonas vaginalis among men attending a sexually transmitted diseases clinic: implications for screening and urethritis management. J. Infect. Dis. 188:465-468. [DOI] [PubMed] [Google Scholar]
- 284.Seeber, F. 2002. Biogenesis of iron-sulphur clusters in amitochondriate and apicomplexan protists. Int. J. Parasitol. 32:1207-1217. [DOI] [PubMed] [Google Scholar]
- 285.Sener, K., Z. Shen, D. S. Newburg, and E. L. Jarroll. 2004. Amino sugar phosphate levels in Giardia change during cyst wall formation. Microbiology 150:1225-1230. [DOI] [PubMed] [Google Scholar]
- 286.Simjee, A. E., V. Gathiram, T. F. Jackson, and B. F. Khan. 1985. A comparative trial of metronidazole v. tinidazole in the treatment of amoebic liver abscess. S. Afr. Med. J. 68:923-924. [PubMed] [Google Scholar]
- 287.Singh, S., K. Husain, F. Athar, and A. Azam. 2005. Synthesis and antiamoebic activity of 3,7-dimethyl-pyrazolo[3,4-e][1,2,4] triazin-4-yl thiosemicarbazide derivatives. Eur. J. Pharm. Sci. 25:255-262. [DOI] [PubMed] [Google Scholar]
- 288.Slamovits, C. H., and P. J. Keeling. 2006. Pyruvate-phosphate dikinase of oxymonads and parabasalia and the evolution of pyrophosphate-dependent glycolysis in anaerobic eukaryotes. Eukaryot. Cell 5:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sobel, J. D., V. Nagappan, and P. Nyirjesy. 1999. Metronidazole-resistant vaginal trichomoniasis—an emerging problem. N. Engl. J. Med. 341:292-293. [DOI] [PubMed] [Google Scholar]
- 290.Sobel, J. D., P. Nyirjesy, and W. Brown. 2001. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin. Infect. Dis. 33:1341-1346. [DOI] [PubMed] [Google Scholar]
- 291.Sonda, S., and A. B. Hehl. 2006. Lipid biology of Apicomplexa: perspectives for new drug targets, particularly for Toxoplasma gondii. Trends Parasitol. 22:41-47. [DOI] [PubMed] [Google Scholar]
- 292.Sorvillo, F., and P. Kerndt. 1998. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet 351:213-214. [DOI] [PubMed] [Google Scholar]
- 293.Stanley, S. L., Jr. 2003. Amoebiasis. Lancet 361:1025-1034. [DOI] [PubMed] [Google Scholar]
- 294.Sutak, R., P. Dolezal, H. L. Fiumera, I. Hrdy, A. Dancis, M. Delgadillo-Correa, P. J. Johnson, M. Muller, and J. Tachezy. 2004. Mitochondrial-type assembly of FeS centers in the hydrogenosomes of the amitochondriate eukaryote Trichomonas vaginalis. Proc. Natl. Acad. Sci. USA 101:10368-10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Tachezy, J., L. B. Sanchez, and M. Muller. 2001. Mitochondrial type iron-sulfur cluster assembly in the amitochondriate eukaryotes Trichomonas vaginalis and Giardia intestinalis, as indicated by the phylogeny of IscS. Mol. Biol. Evol. 18:1919-1928. [DOI] [PubMed] [Google Scholar]
- 296.Takeuchi, T., E. C. Weinbach, M. Gottlieb, and L. S. Diamond. 1979. Mechanism of l-serine oxidation in Entamoeba histolytica. Comp. Biochem. Physiol. B 62:281-285. [DOI] [PubMed] [Google Scholar]
- 297.Tannich, E., I. Bruchhaus, R. D. Walter, and R. D. Horstmann. 1991. Pathogenic and nonpathogenic Entamoeba histolytica: identification and molecular cloning of an iron-containing superoxide dismutase. Mol. Biochem. Parasitol. 49:61-71. [DOI] [PubMed] [Google Scholar]
- 298.Terai, K., T. Masuda, and H. Miyamoto. 1999. Survey of amebiasis at an institution for the mentally retarded in Shizuoka Prefecture. Kansenshogaku Zasshi 73:626-627. [DOI] [PubMed] [Google Scholar]
- 299.Thong, K. W., G. H. Coombs, and B. E. Sanderson. 1987. l-Methionine catabolism in trichomonads. Mol. Biochem. Parasitol. 23:223-231. [DOI] [PubMed] [Google Scholar]
- 300.Reference deleted.
- 301.Tokoro, M., T. Asai, S. Kobayashi, T. Takeuchi, and T. Nozaki. 2003. Identification and characterization of two isoenzymes of methionine gamma-lyase from Entamoeba histolytica: a key enzyme of sulfur-amino acid degradation in an anaerobic parasitic protist that lacks forward and reverse trans-sulfuration pathways. J. Biol. Chem. 278:42717-42727. [DOI] [PubMed] [Google Scholar]
- 302.Tokumoto, U., S. Kitamura, K. Fukuyama, and Y. Takahashi. 2004. Interchangeability and distinct properties of bacterial Fe-S cluster assembly systems: functional replacement of the isc and suf operons in Escherichia coli with the nifSU-like operon from Helicobacter pylori. J. Biochem. (Tokyo) 136:199-209. [DOI] [PubMed] [Google Scholar]
- 303.Tovar, J., A. Fischer, and C. G. Clark. 1999. The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol. Microbiol. 32:1013-1021. [DOI] [PubMed] [Google Scholar]
- 304.Tovar, J., G. Leon-Avila, L. B. Sanchez, R. Sutak, J. Tachezy, M. Van Der Giezen, M. Hernandez, M. Muller, and J. M. Lucocq. 2003. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426:172-176. [DOI] [PubMed] [Google Scholar]
- 305.Townsend, D. M., K. D. Tew, and H. Tapiero. 2004. Sulfur containing amino acids and human disease. Biomed. Pharmacother. 58:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Townson, S. M., P. F. Boreham, P. Upcroft, and J. A. Upcroft. 1994. Resistance to the nitroheterocyclic drugs. Acta Trop. 56:173-194. [DOI] [PubMed] [Google Scholar]
- 307.Townson, S. M., G. R. Hanson, J. A. Upcroft, and P. Upcroft. 1994. A purified ferredoxin from Giardia duodenalis. Eur. J. Biochem. 220:439-446. [DOI] [PubMed] [Google Scholar]
- 308.Townson, S. M., J. A. Upcroft, and P. Upcroft. 1996. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol. Biochem. Parasitol. 79:183-193. [DOI] [PubMed] [Google Scholar]
- 309.Upcroft, J., R. Mitchell, N. Chen, and P. Upcroft. 1996. Albendazole resistance in Giardia is correlated with cytoskeletal changes but not with a mutation at amino acid 200 in beta-tubulin. Microb. Drug Resist. 2:303-308. [DOI] [PubMed] [Google Scholar]
- 310.Upcroft, J. A., R. W. Campbell, K. Benakli, P. Upcroft, and P. Vanelle. 1999. Efficacy of new 5-nitroimidazoles against metronidazole-susceptible and -resistant Giardia, Trichomonas, and Entamoeba spp. Antimicrob. Agents Chemother. 43:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Upcroft, J. A., R. W. Campbell, and P. Upcroft. 1996. Quinacrine-resistant Giardia duodenalis. Parasitology 112:309-313. [DOI] [PubMed] [Google Scholar]
- 312.Upcroft, J. A., and P. Upcroft. 1993. Drug resistance and Giardia. Parasitol. Today 9:187-190. [DOI] [PubMed] [Google Scholar]
- 313.Upcroft, J. A., and P. Upcroft. 1999. Keto-acid oxidoreductases in the anaerobic protozoa. J. Eukaryot. Microbiol. 46:447-449. [DOI] [PubMed] [Google Scholar]
- 314.Upcroft, P., and J. A. Upcroft. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14:150-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.van der Giezen, M., S. Cox, and J. Tovar. 2004. The iron-sulfur cluster assembly genes iscS and iscU of Entamoeba histolytica were acquired by horizontal gene transfer. BMC Evol. Biol. 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.van der Giezen, M., J. Tovar, and C. G. Clark. 2005. Mitochondrion-derived organelles in protists and fungi. Int. Rev. Cytol. 244:175-225. [DOI] [PubMed] [Google Scholar]
- 317.Vazquezdelara-Cisneros, L. G., and A. Arroyo-Begovich. 1984. Induction of encystation of Entamoeba invadens by removal of glucose from the culture medium. J. Parasitol. 70:629-633. [PubMed] [Google Scholar]
- 318.Verhagen, M. F., T. O'Rourke, and M. W. Adams. 1999. The hyperthermophilic bacterium, Thermotoga maritima, contains an unusually complex iron-hydrogenase: amino acid sequence analyses versus biochemical characterization. Biochim. Biophys. Acta 1412:212-229. [DOI] [PubMed] [Google Scholar]
- 319.Voolmann, T., and P. Boreham. 1993. Metronidazole resistant Trichomonas vaginalis in Brisbane. Med. J. Aust. 159:490. [DOI] [PubMed] [Google Scholar]
- 320.Wallis, P. M. 1994. Abiotic transmission—is water really significant?, p. 99-122. In R. C. A. Thompson, J. A. Reynoldson, and A. J. Lymberg (ed.), Giardia: from molecules to disease. CAB International, Wallingford, United Kingdom.
- 321.Walsh, J. A. 1986. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev. Infect. Dis. 8:228-238. [DOI] [PubMed] [Google Scholar]
- 322.Wang, C. C., R. Verham, S. F. Tzeng, S. Aldritt, and H. W. Cheng. 1983. Pyrimidine metabolism in Tritrichomonas foetus. Proc. Natl. Acad. Sci. USA 80:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wassmann, C., A. Hellberg, E. Tannich, and I. Bruchhaus. 1999. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J. Biol. Chem. 274:26051-26056. [DOI] [PubMed] [Google Scholar]
- 324.Weber, G., W. Mohr, K. Fleischer, and P. G. Sargeaunt. 1990. Entamoeba histolytica infections in flight personnel of an international airline. Trans. R. Soc. Trop. Med. Hyg. 84:803-805. [DOI] [PubMed] [Google Scholar]
- 325.Weinbach, E. C., and L. S. Diamond. 1974. Entamoeba histolytica. I. Aerobic metabolism. Exp. Parasitol. 35:232-243. [DOI] [PubMed] [Google Scholar]
- 326.Westrop, G. D., G. Goodall, J. C. Mottram, and G. H. Coombs. 2006. Cysteine biosynthesis in Trichomonas vaginalis involves cysteine synthase utilizing O-phosphoserine. J. Biol. Chem. 281:25062-25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Whiteway, J., P. Koziarz, J. Veall, N. Sandhu, P. Kumar, B. Hoecher, and I. B. Lambert. 1998. Oxygen-insensitive nitroreductases: analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J. Bacteriol. 180:5529-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Reference deleted.
- 329.Wiesner, J., and F. Seeber. 2005. The plastid-derived organelle of protozoan human parasites as a target of established and emerging drugs. Expert Opin. Ther. Targets 9:23-44. [DOI] [PubMed] [Google Scholar]
- 330.Williams, K., P. N. Lowe, and P. F. Leadlay. 1987. Purification and characterization of pyruvate:ferredoxin oxidoreductase from the anaerobic protozoon Trichomonas vaginalis. Biochem. J. 246:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wilson, R. J., K. Rangachari, J. W. Saldanha, L. Rickman, R. S. Buxton, and J. F. Eccleston. 2003. Parasite plastids: maintenance and functions. Philos. Trans. R. Soc. London B 358:155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wirtz, M., O. Berkowitz, M. Droux, and R. Hell. 2001. The cysteine synthase complex from plants. Mitochondrial serine acetyltransferase from Arabidopsis thaliana carries a bifunctional domain for catalysis and protein-protein interaction. Eur. J. Biochem. 268:686-693. [DOI] [PubMed] [Google Scholar]
- 333.World Health Organization. 1998. Intestinal parasites control: burden and trends. World Health Organization, Geneva, Switzerland.
- 334.Yarlett, N., H. Hof, and N. C. Yarlett. 1987. Activities of metronidazole and niridazole against Trichomonas vaginalis clinical isolates. J. Antimicrob. Chemother. 19:767-770. [DOI] [PubMed] [Google Scholar]
- 335.Yarlett, N., C. C. Rowlands, N. C. Yarlett, J. C. Evans, and D. Lloyd. 1987. Reduction of niridazole by metronidazole resistant and susceptible strains of Trichomonas vaginalis. Parasitology 94:93-99. [DOI] [PubMed] [Google Scholar]
- 336.Yarlett, N., N. C. Yarlett, and D. Lloyd. 1986. Metronidazole-resistant clinical isolates of Trichomonas vaginalis have lowered oxygen affinities. Mol. Biochem. Parasitol. 19:111-116. [DOI] [PubMed] [Google Scholar]
- 337.Yong, T. S., E. Li, D. Clark, and S. L. Stanley, Jr. 1996. Complementation of an Escherichia coli adhE mutant by the Entamoeba histolytica EhADH2 gene provides a method for the identification of new antiamebic drugs. Proc. Natl. Acad. Sci. USA 93:6464-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Yoshimura, M., Y. Nakano, and T. Koga. 2002. l-Methionine-gamma-lyase, as a target to inhibit malodorous bacterial growth by trifluoromethionine. Biochem. Biophys. Res. Commun. 292:964-968. [DOI] [PubMed] [Google Scholar]
- 339.Zaat, J. O., T. G. Mank, and W. J. Assendelft. 1997. A systematic review on the treatment of giardiasis. Trop. Med. Int. Health 2:63-82. [DOI] [PubMed] [Google Scholar]
- 340.Zhang, Y. Q., Z. Y. Wang, S. Q. Lu, M. L. Feng, J. F. Peng, and J. G. Wang. 1986. A familial infection of giardiasis. Chin. Med. J. 99:417-419. [PubMed] [Google Scholar]
- 341.Zhong, H. L., W. J. Cao, J. F. Rossignol, M. L. Feng, R. Y. Hu, S. B. Gan, and W. Tan. 1986. Albendazole in nematode, cestode, trematode and protozoan (Giardia) infections. Chin. Med. J. 99:912-915. [PubMed] [Google Scholar]
- 342.Zygmunt, W. A., and P. A. Tavormina. 1966. dl-S-Trifluoromethylhomocysteine, a novel inhibitor of microbial growth. Can. J. Microbiol. 12:143-148. [DOI] [PubMed] [Google Scholar]