Abstract
Placental sequestration of Plasmodium falciparum in pregnancy may impair the usefulness of molecular markers of sulfadoxine-pyrimethamine resistance. In 300 infected, delivering women, the concordance of PCR-restriction fragment length polymorphism-derived parasite resistance alleles in matched samples from placenta and circulation was 83 to 98%. Sulfadoxine-pyrimethamine resistance typing in peripheral blood is reasonably representative of P. falciparum infecting pregnant women.
Malaria in pregnancy is a serious public health problem in sub-Saharan Africa. Although commonly asymptomatic, its clinical consequences involve anemia, low birth weight, preterm delivery, and an estimated annual 75,000 to 200,000 attributable infant deaths (11).
Lately, intermittent preventive treatment (IPT) with sulfadoxine-pyrimethamine (SP) has been used for malaria control in pregnancy (13). IPT involves the administration of SP treatment three times during the second and third trimesters, irrespective of parasitemia. Monitoring SP resistance is essential for estimating the effectiveness of this policy, and molecular markers are increasingly applied for this purpose. SP resistance is associated with specific mutations in the Plasmodium falciparum dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) genes (8). In Ghana, SP failure was recently observed in 28% of treated children; the dhfr triple mutation (Ile51+Arg59+Asn108) increased the risk of treatment failure 10-fold (6). Due to preexisting immunity, antimalarial treatment commonly is more effective during pregnancy than it is in children (3). Moreover, pregnancy may influence the value of resistance markers because, due to specific ligand expression, P. falciparum sequesters in the placental intervillous space. The absence of microscopically visible parasites in peripheral blood despite placental parasitemia is one common consequence. Although the sensitivity of PCR in detecting placental infection in peripheral blood approaches 100% (7), it is unclear whether circulating parasite genotypes in pregnant women represent the actual parasite population or only part of it. Extensive discordance between placental and peripheral polymorphic merozoite surface protein (msp) genotypes has been reported, but rather homogenous parasite populations have been reported as well (2, 4, 10). So far, it is unknown whether and to what extent parasite genotype discordance in pregnant women affects the value of SP resistance typing. Here, we compared peripheral blood and placental dhfr alleles in 300 delivering Ghanaian women with microscopically proven placental malaria.
Between January 2000 and January 2001, 889 delivering and consenting women were recruited at the district hospital in Agogo, southern Ghana, an area of holoendemicity. The study protocol was approved by the Ethics Committee, University of Science and Technology, Kumasi, Ghana. The diagnostic procedures used for and the malariologic characteristics of the majority of the women have been described previously (7). For the present study, all 300 available, matched samples of placental and peripheral blood of women with microscopically confirmed placental parasitemia were examined. DNA was extracted (QIAmp; QIAGEN), and stored at −80°C until the dhfr mutations Ser108Asn, Asn51Ile, and Cys59Arg were determined by PCR-restriction fragment length polymorphism (RFLP) in 2006 (1). Mixed alleles (wild type and with mutation present) were considered mutations. Plasma concentrations of pyrimethamine, at the time of study conduct recommended for the chemoprophylaxis of malaria in pregnancy, were measured by enzyme-linked immunosorbent assay (limit of detection, 10 ng/ml) (12).
The characteristics of the 300 women are shown in Table 1. Successful dhfr typing of all three alleles was achieved in 294 (98%) placental and 297 (99%) peripheral samples. dhfr mutations were frequent: only 5% of circulating genotypes were of the wild type, while 4%, 37%, and 54% comprised one, two, and three dhfr mutations, respectively (Table 2) . Comparing the 297 peripheral genotypes to placental alleles, complete concordance was observed in 83.2% (247/297) of matched samples. The corresponding figures were 80.0% (128/160), 85.2% (75/88), and 89.9% (44/49) for placental samples with less than 1, between 1 and 10, and 10 or more parasites/high-power field (P = 0.2), respectively, and it was 73.3% (11/15) in febrile and 84.4% (234/277) in afebrile women (P = 0.3). Setting placental alleles as the reference, peripheral genotyping correctly identified 46.2% (6/13), 80.0% (88/110), and 91.6% (141/154) of isolates with one, two, and three dhfr mutations, respectively (P < 0.0001).
TABLE 1.
Characteristics of 300 delivering women with placental malaria
| Parameter | Value |
|---|---|
| Median age (yr) (range) | 23 (15-45) |
| % (Proportion) of primiparae | 48.3 (144/298) |
| % (Proportion) febrile (>37.4°C axillary temp) | 5.1 (15/295) |
| % (Proportion) anemic (hemoglobin <11 g/dl) | 48.3 (145/300) |
| % (Proportion) with single live delivery of low birth wt (<2,500 g) | 19.6 (56/286) |
| % (Proportion) with preterm single live delivery (<37 wk of gestation) | 20.9 (59/282) |
| % (Proportion) with peripheral parasitemia by microscopy | 50.3 (151/300) |
| Geometric mean parasite density (95% CIa) of peripheral parasitemia | 733/μl (525-1023) |
| Geometric mean parasite density (95% CI) of placental parasitemia | 0.84/HPFb (0.62-1.13) |
| % (Proportion) with pyrimethamine in plasma | 32.5 (95/292) |
CI, confidence interval.
HPF, high-power field.
TABLE 2.
Comparison of peripheral blood and placental Plasmodium falciparum dihydrofolate reductase genotypes
| Placental dhfr genotype of samplea | No. of samples with indicated peripheral blood dhfr genotypea
|
Total (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 51 59 108 | 51 59 108 | 51 59 108 | 51 59 108 | 51 59 108 | 51 59 108 | 51 59 108 | Nontypeable | ||
| 51 59 108 | 12 | 2 | 2 | 1 | 17 (5.7) | ||||
| 51 59 108 | 5 | 2 | 2 | 9 (3.0) | |||||
| 51 59 108 | 1 | 1 (0.3) | |||||||
| 51 59 108 | 2 | 1 | 3 (1.0) | ||||||
| 51 59 108 | 7 | 3 | 10 (3.3) | ||||||
| 51 59 108 | 1 | 1 | 1 | 81 | 14 | 2 | 100 (33.3) | ||
| 51 59 108 | 1 | 5 | 6 | 141 | 1 | 154 (51.3) | |||
| Nontypeable | 4 | 2 | 6 (2.0) | ||||||
| Total (%) | 15 (5.0) | 7 (2.3) | 3 (1.0) | 2 (0.7) | 16 (5.3) | 93 (31.0) | 161 (53.7) | 3 (1.0) | |
Mutated codons are displayed in bold. The concordance of alleles between matched placental and peripheral samples was high for codon 108 (97.6%; 284/291), lower for codon 59 (94.2%; 274/291), and lowest for codon 51 (90%; 262/291) (P = 0.0007). Of 20 discordant peripheral isolates with the dhfr triple mutation, 17 matching placental genotypes exhibited double mutations, and 1 was of the wild type. Contrariwise, of the 13 placental isolates with dhfr triple mutation not identified as such by peripheral genotyping, 11 showed double dhfr mutations.
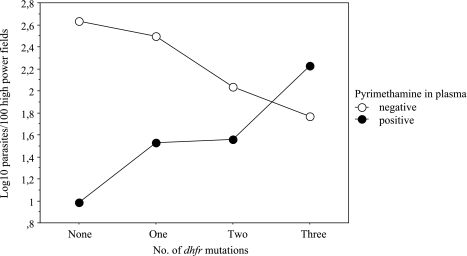
Pyrimethamine in plasma appeared to select for dhfr mutations. In women with and without pyrimethamine levels, placental wild-type isolates were seen in 2.1% (2/94) and 7.8% (15/193, P = 0.06), and isolates with two or three mutations were seen in 95.7% (90/94) and 87.6% (169/193, P = 0.03), respectively. Irrespective of plasma pyrimethamine, no influence of dhfr alleles on the clinical manifestation of malaria was observed (data not shown). However, the suppressive effect of pyrimethamine on placental parasite density decreased with the number of dhfr mutations (Fig. 1) (F = 5.1; P = 0.002).
FIG. 1.
Geometric mean placental parasite densities according to the presence of pyrimethamine in plasma and the dihydrofolate reductase genotype.
In Ghana, the P. falciparum dhfr triple mutation is highly predictive for SP treatment failure in children, whereas dhps alleles are not (and were thus not assessed here) (6). In the present study, the differential effect—depending on dhfr alleles—of plasma pyrimethamine on parasite density is suggestive of resistance. One major advantage of SP in IPT lies in its single-dose administration, but resistance is anticipated to spread and intensify. Here, we show that 93% and >50% of the women harbored parasites with the dhfr core mutation Asn108 and the high-resistance triple mutation, respectively. In 1998, these figures were 81% and 36% among infected antenatal care attendees (5), pointing to a rapid rise of SP resistance. Moreover, we report complete concordance between peripheral and placental dhfr genotypes obtained by PCR-RFLP in 83% of matched samples. Concordance was lower for the small group of wild-type and single-mutation parasites. Most discordant circulating genotypes differed from the matching placental ones in that double mutations were considered triple or vice versa. In both cases, SP resistance must be expected (6, 8). The concordance of placental and peripheral dhfr alleles was higher than was reported for msp genotypes (4, 10); less polymorphism in the former is one likely explanation. In practice, molecular markers need to be simple; we therefore grouped mixed dhfr alleles as mutations. When analyzed separately, concordance was still high (73% [data not shown]). PCR-RFLP is the most common technique for dhfr typing, and the assay used is highly specific. However, sensitivity might drop at very low parasitemia, particularly in polyclonal infections, and results occasionally differ from those obtained by other techniques (9). Consequently, our findings are only restrictedly transferable. Resistance markers are not routinely assessed in sub-Saharan Africa, and this is unlikely to change soon. Nevertheless, for epidemiological purposes we consider peripheral blood dhfr genotyping both useful and sufficiently precise to give an estimate of SP resistance in parasites infecting pregnant women. This should allow examination of the dynamics of SP resistance in infections occurring despite IPT. Once the predictive value of the dhfr mutations for IPT with SP has been established, monitoring these markers will provide valuable and rather easily accessible information, which is of basic importance to guide drug policy on the prevention and treatment of malaria in pregnancy.
Acknowledgments
We thank the mothers who participated in this study and the midwifes at Agogo Hospital.
This study was supported by Charité (grants 99-640, 2000-512, and 2001-613) and Merck Sharp & Dohme, Germany (grant “Infectious Diseases 1999”) and forms part of the doctoral thesis of L.H. We do not have a commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Duraisingh, M. T., J. Curtis, and D. C. Warhurst. 1998. Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp. Parasitol. 89:1-8. [DOI] [PubMed] [Google Scholar]
- 2.Jafari-Guemouri, S., N. T. Ndam, G. Bertin, E. Renart, S. Sow, J. Y. Le Hesran, and P. Deloron. 2005. Demonstration of a high level of parasite population homology by quantification of Plasmodium falciparum alleles in matched peripheral, placental, and umbilical cord blood samples. J. Clin. Microbiol. 43:2980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalanda, G. C., J. Hill, F. H. Verhoeff, and B. J. Brabin. 2006. Comparative efficacy of chloroquine and sulphadoxine-pyrimethamine in pregnant women and children: a meta-analysis. Trop. Med. Int. Health 11:569-577. [DOI] [PubMed] [Google Scholar]
- 4.Kamwendo, D. D., F. K. Dzinjalamala, G. Snounou, M. C. Kanjala, C. G. Mhango, M. E. Molyneux, and S. J. Rogerson. 2002. Plasmodium falciparum: PCR detection and genotyping of isolates from peripheral, placental, and cord blood of pregnant Malawian women and their infants. Trans. R. Soc. Trop. Med. Hyg. 96:145-149. [DOI] [PubMed] [Google Scholar]
- 5.Mockenhaupt, F. P., T. A. Eggelte, T. Böhme, W. N. Thompson, and U. Bienzle. 2001. Plasmodium falciparum dihydrofolate reductase alleles and pyrimethamine use in pregnant Ghanaian women. Am. J. Trop. Med. Hyg. 65:21-26. [DOI] [PubMed] [Google Scholar]
- 6.Mockenhaupt, F. P., T. J. Bousema, T. A. Eggelte, J. Schreiber, S. Ehrhardt, N. Wassilew, R. N. Otchwemah, R. W. Sauerwein, and U. Bienzle. 2005. Plasmodium falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Trop. Med. Int. Health 10:901-908. [DOI] [PubMed] [Google Scholar]
- 7.Mockenhaupt, F. P., U. Ulmen, C. von Gaertner, G. Bedu-Addo, and U. Bienzle. 2002. Diagnosis of placental malaria. J. Clin. Microbiol. 40:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nzila, A. M., E. K. Mberu, J. Sulo, H. Dayo, P. A. Winstanley, C. H. Sibley, and W. M. Watkins. 2000. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob. Agents Chemother. 44:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranford-Cartwright, L. C., K. L. Johnston, A. M. Abdel-Muhsin, B. K. Khan, and H. A. Babiker. 2002. Critical comparison of molecular genotyping methods for detection of drug-resistant Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 96:568-572. [DOI] [PubMed] [Google Scholar]
- 10.Schleiermacher, D., J. Y. Le Hesran, J. L. Ndiaye, R. Perraut, A. Gaye, and O. Mercereau-Puijalon. 2002. Hidden Plasmodium falciparum parasites in human infections: different genotype distribution in the peripheral circulation and in the placenta. Infect. Genet. Evol. 2:97-105. [DOI] [PubMed] [Google Scholar]
- 11.Steketee, R. W., B. L. Nahlen, M. E. Parise, and C. Menendez. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64(Suppl. 1-2):28-35. [DOI] [PubMed] [Google Scholar]
- 12.Witte, A. M., H. J. Klever, B. J. Brabin, T. A. Eggelte, H. J. van der Kaay, and M. P. Alpers. 1990. Field evaluation of the use of an ELISA to detect chloroquine and its metabolites in blood, urine and breast-milk. Trans. R. Soc. Trop. Med. Hyg. 84:521-525. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. 2004. A strategic framework for malaria control and prevention during pregnancy in the African region. AFR/MAL/04/01. WHO Regional Office for Africa, Brazzaville, Congo.