Abstract
Fluoroquinolones exhibit immunomodulatory effects on monocytes and macrophages, in addition to their bactericidal activities. It remains unknown even whether the quinolones act directly on the prostate. This study was based on the understanding of the molecular mechanisms of the actions of the fluoroquinolones that can be used for the treatment of chronic prostatitis/chronic pelvic pain syndrome. We investigated whether the 6-fluroro-8-methoxy quinolone gatifloxacin (GFLX) affected the production and secretion of interleukin-8 (IL-8) in the prostate cell line PC-3. GFLX decreased the level of IL-8 release from unstimulated PC-3 cells. GFLX also attenuated IL-8 secretion from PC-3 cells stimulated with peptidoglycan, Mycoplasma hominis, phorbol ester, and tumor necrosis factor alpha (TNF-α), indicating that GFLX exhibits an anti-inflammatory effect on the prostate cell line. However, GFLX failed to alter activation of the NF-κB and AP-1 elicited by these stimulants. GFLX significantly attenuated the expression of IL-8 mRNA in TNF-α-stimulated PC-3 cells and down-regulated the transcriptional activity of the 5′-flanking region of the IL-8 gene from −1481 to +44 bp. The deletion construct without the 5′-flanking region from −1481 to −170 bp but not the construct without the region from −1481 to −188 bp reversed the suppressive effect of GFLX on IL-8 promoter activity. These results demonstrate that GFLX suppresses IL-8 expression in the prostate cell line by decreasing the promoter activity of the IL-8 gene.
Fluoroquinolones exhibit bactericidal activity by inhibiting bacterial DNA gyrase and topoisomerase (6). The quinolones readily penetrate into cells and express intracellular antibacterial effects. In addition to the bactericidal activities, fluoroquinolones modulate the production and secretion of cytokines. Ciprofloxacin inhibits the synthesis of tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6 in human monocytes (5, 30). Moxifloxacin inhibits IL-1α, IL-1β, IL-8, and TNF-α from lipopolysaccharide (LPS)-stimulated monocytes but not pansorbin-stimulated monocytes (4, 32), whereas it does not affect the production of IL-4, IL-6, or IL-12. Grepafloxacin inhibits the synthesis of the IL-1α, TNF-α, and IL-8 proteins and the expression of their mRNAs in LPS-stimulated monocytes; but it stimulates the synthesis of IL-2 (10, 25). Levofloxacin augments IL-2 production by monocytes but decreases IL-1β production. IL-8 production is negligibly affected by levofloxacin (34). These studies indicate that fluoroquinolones exhibit immunomodulatory effects. Although the diverse effects of these quinolones have been described, the results vary between the different stimuli used and the in vitro methods applied. Monocytes and macrophages have been used in most of these previous studies.
Proinflammatory cytokines appear to be one of what are believed to be multiple etiologies in chronic prostatitis (2, 22). IL-8 levels have been shown to be frequently elevated in the prostatic secretions of men who have been diagnosed with NIH category III prostatitis compared with those in the prostatic secretions of healthy controls (12), suggesting that IL-8 could be responsible, at least in part, for triggering prostatitis. The balance between the effects of proinflammatory and anti-inflammatory cytokines determines the outcome of prostatitis (15). Fluoroquinolones have been used for the treatment of prostatitis (3, 23), since these drugs are highly transferable from plasma to prostate tissue (1, 26). Fluoroquinolones are clinically effective even in patients with nonbacterial prostatitis (33), presumably because of their immunomodulatory activities. However, the mechanism by which these drugs modulate the production and secretion of cytokines is not completely understood. In addition, it remains unknown even whether the fluoroquinolones act directly on the prostate.
In this study we investigated whether the 6-fluoro-8-methoxy quinolone gatifloxacin (GFLX) affects the production and secretion of IL-8 in the prostate cell line PC-3. This is the first report to show that a fluoroquinolone functions as an inhibitor of IL-8 secretion from the prostate cell line.
MATERIALS AND METHODS
Cells and reagents.
Human prostate cancer cell line PC-3 was obtained from ATCC. The cells were maintained in RPMI 1640 medium (Nissui) containing 10% fetal calf serum, 100 U/ml penicillin, and 100 U/ml streptomycin (Gibco BRL/Life Technologies, Inc., Gaithersburg, MD) at 37°C in a humidified incubator containing 5% CO2 in air. It was proven that the cell line was negative for Mycoplasma infection when it was analyzed with a Mycoplasma detection kit (Roche). The 6-fluoro-8-methoxy quinolone GFLX was kindly provided by Kyorin Pharmaceutical Co., Tokyo, Japan. Peptidoglycan (PGN) from Staphylococcus aureus was obtained from Fluka. Human recombinant TNF-α was obtained from Peprotech EC Ltd. (London, United Kingdom). The membrane fraction of Mycoplasma hominis was prepared as described previously (14). Phorbol 12-myristate 13-acetate (PMA) was obtained from Sigma.
GFLX preparation.
GFLX was dissolved in 0.1 N NaOH at a concentration of 2 mg/ml. The very small amount of the GFLX solution (diluted from 125 to 1,000 times) was added into the culture. The same amount of 0.1 N NaOH was added as a control.
Cell viability.
To examine the cytotoxicity of GFLX on the prostate cell line, the cells were plated at 5 × 104 cells per well in 96-well plates. After 24 h of incubation, GFLX was added at the indicated concentrations and the cells were incubated for 48 h. A modified method with 3-(4,5-dimethylthiazol-2-thiazoly)-2,5-diphenyltetrazolium bromide was finally performed by using a cell counting kit (cell counting kit 8; Dojindo, Kumamoto, Japan), according to the manufacturer's instructions.
Determination of IL-8 protein levels.
To measure IL-8 protein levels in culture supernatants, PC-3 cells were plated at 2 × 105 cells per well in 24-well plates. After 24 h of incubation, the supernatants were exchanged for serum-free RPMI 1640 medium after the cells were washed once with phosphate-buffered saline. GFLX was added at the indicated concentrations. After further incubation for 24 h, the concentrations of IL-8 in the medium were determined by an enzyme-linked immunosorbent assay by using an OptEIA human IL-8 set (Pharmingen, San Diego, CA), according to the manufacturer's instructions. TNF-α and IL-6 levels were also determined by an L929 cell bioassay (7) and with a human IL-6 chemiluminescent enzyme immunoassay kit (Fujirebio), respectively.
Reporter gene assay.
PC-3 cells were plated at 4 × 104 cells/well in 24-well plates. After 24 h of incubation, the cells were transiently transfected by use of the FuGENE 6 transfection reagent (Roche Molecular Biochemicals, Basel, Switzerland) with 179.1 ng of an NF-κB reporter construct (pNF-κB-Luc; Stratagene, La Jolla, CA) or an AP-1 reporter construct and 20.8 ng of a construct that directed the expression of the Renilla luciferase under the control of the constitutively active thymidine kinase promoter (pRL-TK; Promega, Madison, WI). Twenty-four hours after transfection, GFLX was added at a concentration of 16 μg/ml and the cells were incubated for 24 h. The medium was then exchanged for serum-free RPMI 1640 medium and GFLX was added again at the same concentration. The cells were then stimulated with M. hominis, PGN, PMA, or TNF-α. The stimulation with M. hominis, PGN, or PMA was performed for 6 h; and the supernatants were collected to measure the concentration of IL-8 protein. The stimulation with TNF-α was carried out for 2 h. Luciferase activity was measured with the dual luciferase reporter assay system (Promega), according to the manufacturer's instructions.
RT-PCR.
Reverse transcription-PCR (RT-PCR) was performed to examine the effect of GFLX on the expression of IL-8 mRNA. PC-3 cells were plated at 4 × 105 cells per well in 35-mm2 dishes. After 24 h of incubation, GFLX was added at 16 μg/ml and the cells were incubated for an additional 48 h. The medium and GFLX were refreshed every 24 h. The supernatants were then exchanged for serum-free RPMI 1640 medium after the cells were washed once with phosphate-buffered saline. GFLX was added again at the same concentration, and the cells were stimulated with 10 ng/ml TNF-α for 4 h. After the incubation, total cellular RNA was isolated from the cells by using the TRIzol reagent (Life Technologies, Grand Island, NY). A cDNA pool was obtained from 200 ng of RNA by using 200 units of Superscript reverse transcriptase (Life Technologies) and 0.5 μg of oligo(dT)12-18 primer (Life Technologies). PCR amplification was performed with Taq polymerase (Promega). For the expression of IL-8 mRNA, 23 cycles of amplification were performed at a denaturing temperature of 95°C for 1 min and with annealing at 60°C for 1 min and extension at 72°C for 2 min, after incubation at 95°C for 5 min. For β-actin, 23 cycles of amplification were carried out at a denaturing temperature of 94°C for 1 min and with annealing at 54°C for 1 min and extension at 72°C for 2 min. The oligonucleotide primers used for IL-8 were sense primer 5′-CTTGGCAGCCTTCCTGATTT-3′ and antisense primer 5′-TCAAAAACTTCTCCCGACTC-3′. The primers used for β-actin were sense primer 5′-CTGTCTGGCGGCACCACCAT-3′ and antisense primer 5′-GCAACTAAGTCATAGTCCGC-3′. The PCR products were electrophoressed and visualized on a 1% agarose gel after they were stained with ethidium bromide. The expected sizes of the PCR products for IL-8 and β-actin were 265 bp and 285 bp, respectively. The UV-illuminated gels were photographed, and the densitometric analysis was performed by using Luminous 2.0.
Promoter activity of the 5′-flanking region of IL-8 gene in PC-3 cells.
Transfection vectors containing fusion genes of the 5′-flanking sequences of the IL-8 gene and a luciferase reporter gene were constructed from a pGL3-Basic vector (Promega). The 5′-flanking region of the IL-8 promoter, a 1,525-bp EcoRI-HindIII fragment spanning from −1481 to +44 bp (the numbering is based on the sequence of the IL-8 gene reported by Mukaida et al. [20]) or its sequentially deleted fragments (starting from −415, −240, −188, −170, −129, and −62 to +44 bp), was prepared by PCR and cloned into luciferase expression vectors between unique XhoI and HindIII sites of the pGL3-Basic vector (Promega). PC-3 cells were plated at 4 × 104 cells/well in 24-well plates. After 24 h of incubation, the cells were transiently transfected with the FuGENE 6 transfection reagent (Roche Molecular Biochemicals) with 179.1 ng of the IL-8 gene constructed in the pGL3-Basic vector and 20.8 ng of pRL-TK. Twenty-four hours after transfection, the cells were preincubated with GFLX at a concentration of 16 μg/ml for 24 h. After the preincubation, the medium was exchanged for serum-free RPMI 1640 medium. GFLX was then added again at the same concentration and the cells were stimulated with 10 ng/ml of TNF-α for 2 h. Finally, luciferase activity was measured as described above.
Statistical test.
Statistical analysis was performed by Fisher's protected least significant difference test. P values of <0.05 were considered statistically significant.
RESULTS
Cell viability.
We first examined the effect of GFLX on the viability of PC-3 cells. When GFLX at 2, 4, and 16 μg/ml was incubated with PC-3 cells for 48 h, the cell viabilities determined were 97.6% ± 11.7%, 100% ± 13.3%, and 96.6% ± 7.5%, respectively (the values are mean ± standard deviation [SD; n = 3] percentage of the viability of the controls not treated with GFLX). These results indicate that GFLX at 2 to 16 μg/ml does not exhibit cytotoxicity on PC-3 cells.
GFLX decreases IL-8 release from unstimulated PC-3 cells.
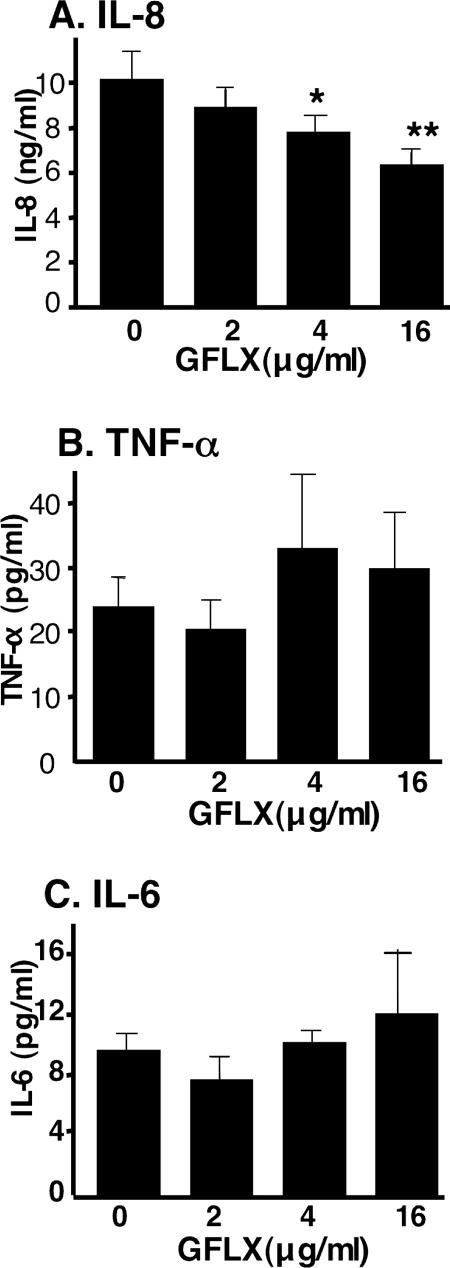
PC-3 cells continuously secrete low levels of IL-8 under unstimulated conditions (Fig. 1A). We first determined whether GFLX affected the basal level of secretion of IL-8 from PC-3 cells. GFLX decreased the basal level of secretion of IL-8 in a concentration-dependent manner. When 4 μg/ml and 16 μg/ml of GFLX were incubated with the cells, the levels of IL-8 secretion were significantly suppressed by 22% and 38%, respectively. We also examined whether GFLX affected the secretion of other cytokines. GFLX did not significantly reduce the basal level of secretion of TNF-α or IL-6 from PC-3 cells (Fig. 1B and C). Thus, the inhibitory effect of GFLX is suggested to be specific for IL-8 secretion.
FIG. 1.
GFLX attenuates the basal level of secretion of IL-8 from unstimulated PC-3 cells. PC-3 cells were plated at 2 × 105 cells/well in 24-well plates. After 24 h of incubation, the culture medium was refreshed with serum-free RPMI 1640 medium; GFLX was then added at the indicated concentrations, and the cells were incubated for 24 h. After the incubation, the culture medium was collected and the concentrations of IL-8 (A), TNF-α (B), and IL-6 (C) in the medium were determined as described in Materials and Methods. The data shown are the means ± SDs for three experiments. *, P < 0.05 compared with the results for cells not incubated with GFLX; **, P < 0.005 compared with the results for cells not incubated with GFLX.
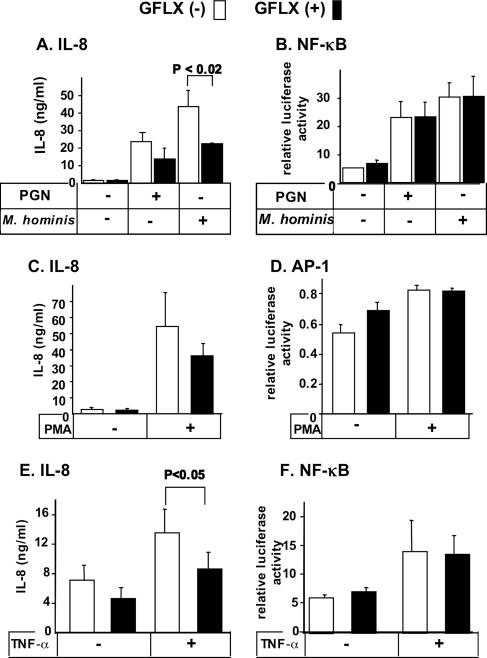
GFLX attenuates IL-8 secretion from PC-3 cells stimulated with peptidoglycan, Mycoplasma hominis, PMA, and TNF-α.
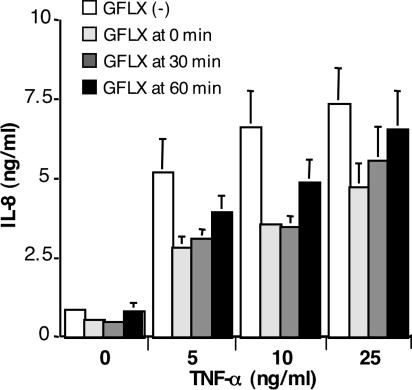
We next examined whether GFLX attenuated the secretion of IL-8 from stimulated PC-3 cells. PGN derived from S. aureus and the membrane fraction of M. hominis dramatically increased the level of IL-8 secretion from PC-3 cells (Fig. 2A). Addition of GFLX to the cells incubated with PGN and M. hominis resulted in reductions in the levels of IL-8 release by 42% and 52%, respectively. PMA is a potent stimulant of IL-8 secretion in PC-3 cells, and the PMA-stimulated IL-8 release was also suppressed by 34% (Fig. 2C). TNF-α increased the level of IL-8 secretion only twofold, but GFLX significantly attenuated the level of TNF-α-induced IL-8 secretion (Fig. 2E). We also determined whether GFLX affected the level of LPS-induced IL-8 secretion from PC-3 cells. When PC-3 cells were stimulated with LPS (0.1 and 1 ng/ml), the levels of LPS-elicited IL-8 secretion were significantly decreased by 19% and 16%, respectively (P < 0.02 and P < 0.05, respectively), in the presence of GFLX; the levels of IL-8 secretion stimulated by 0.1 and 1 ng/ml LPS were 69.5 ± 3.4 ng/ml and 63.2 ± 2.1 ng/ml (means ± SDs; n = 3), respectively. Taken together, these results clearly demonstrate that GFLX suppresses the level of IL-8 secretion from PC-3 cells under unstimulated and stimulated conditions and indicate that GFLX possesses an immunomodulatory effect, in addition to antimicrobial activity. In these experiments, GFLX was preincubated with the cells before stimulation with agonists. Thus, we examined the effects of simultaneous treatment or posttreatment with GFLX on the level of IL-8 secretion from PC-3 cells. The simultaneous addition of GFLX with TNF-α (5 to 25 ng/ml) reduced the levels of IL-8 secretion by 37 to 47% (Fig. 3). Addition of GFLX 30 min after TNF-α stimulation also inhibited the levels of IL-8 secretion by 25 to 47%. Addition of GFLX 60 min after TNF-α stimulation tended to decrease the level of IL-8 secretion, although the inhibitory effects became weaker. Taken together, GFLX appears to be effective in decreasing the IL-8 secretion even after stimulation of the cells.
FIG. 2.
GFLX suppresses stimulated IL-8 secretion from PC-3 cells but fails to alter the activation of NF-κB and AP-1. PC-3 cells were plated at 5 × 104 cells per well in 24-well plates. After 24 h of incubation, the cells were transiently transfected by use of the FuGENE 6 transfection reagent with 179.1 ng of an NF-κB or an AP-1 reporter construct and 20.8 ng of pRL-TK. GFLX was added at 16 μg/ml after 24 h of incubation. Forty-eight hours after transfection, the cells were stimulated with 1 μg/ml PGN (A and B), 0.1 μg/ml M. hominis (A and B), or 50 μg/ml PMA (C and D) for 6 h or with 10 ng/ml TNF-α (E and F) for 2 h. The concentrations of IL-8 secreted into the medium (A, C, and E) were determined by an enzyme-linked immunosorbent assay, and the luciferase activities for NF-κB (B and F) and AP-1 (D) were measured by the dual luciferase reporter assay described in Materials and Methods. Open and closed bars, incubation without and with GFLX, respectively. The data shown are the means ± SDs for three experiments. P < 0.02 and P < 0.05 indicate the P values for the results for incubation with GFLX compared with the results for incubation without GFLX.
FIG. 3.
Effects of simultaneous treatment or posttreatment with GFLX on IL-8 secretion from PC-3 cells. PC-3 cells were plated at 2 × 105 cells in a 24-well plate. After 24 h of incubation, GFLX (16 μg/ml) was added simultaneously (GFLX at 0 min) with TNF-α stimulation (0, 5, 10, and 25 ng/ml) or 30 min or 60 min after TNF-α stimulation (GFLX at 30 min and GFLX at 60 min, respectively). The cells were incubated with TNF-α for 120 min. The concentrations of IL-8 secreted into the medium were determined by an enzyme-linked immunosorbent assay, as described in Materials and Methods. The data shown are the means ± SDs for three experiments.
GFLX does not alter activation of NF-κB or AP-1.
Since the increased levels of IL-8 secretion produced by PGN, M. hominis, PMA, and TNF-α are accompanied by the activation of transcription factors, including NF-κB and AP-1, we examined the effects of GFLX on the activities of these transcription factors. NF-κB was activated when the cells were stimulated with PGN, M. hominis, and TNF-α (Fig. 2B and F). Inclusion of GFLX in the cell culture did not alter the level of activation of NF-κB compared with that by cells cultured without GFLX. PMA increased the level of AP-1 activation very slightly, but GFLX exhibited almost no effect on the level of AP-1 activation in cells with or without PMA activation (Fig. 2D). These results indicate that GFLX does not alter the activation of NF-κB or AP-1.
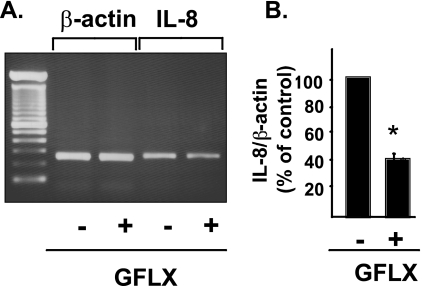
GFLX reduces the expression of IL-8 mRNA.
Since GFLX attenuates the release of the IL-8 protein from PC-3 cells, we next determined whether GFLX altered IL-8 expression at the mRNA level. When the cells were stimulated with TNF-α, a significant band of the PCR product for IL-8 mRNA was observed (Fig. 4A). When the cells were coincubated with GFLX and TNF-α, the band for IL-8 mRNA became thinner, although the bands for β-actin mRNAs did not appear to be different. The ratio of the IL-8/β-actin band intensities significantly decreased for cells incubated with GFLX compared with the ratio for cells not incubated with GFLX (Fig. 4B). These results demonstrate that GFLX suppresses IL-8 mRNA expression.
FIG. 4.
GFLX decreases IL-8 mRNA expression. (A) PC-3 cells were plated at 4 × 105 cells in a 35-mm dish. After 24 h of incubation, the cells were treated with 16 μg/ml GFLX for 48 h. TNF-α at 10 ng/ml was then added, and the cells were incubated for an additional 4 h. Total RNA was isolated, and RT-PCR was performed as described in Materials and Methods. After electrophoresis, the PCR products were visualized by ethidium bromide staining. (B) Densitometric analysis of the bands of PCR products shown as the ratio of IL-8/β-actin. P was <0.001 for the results for cells incubated with GFLX compared with the results for cells incubated without GFLX.
GFLX down-regulates IL-8 gene promoter activity.
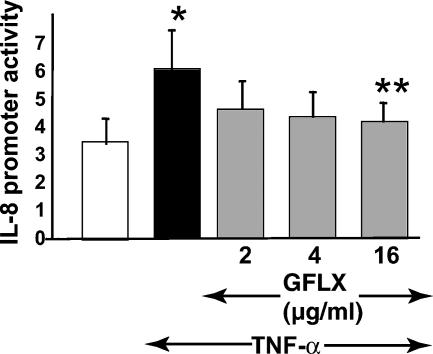
To examine the effect of GFLX on the transcriptional activity of the IL-8 gene, we transfected the fusion genes consisting of the 5′-flanking region (the sequence from −1481 to +44 bp) of the IL-8 gene and a luciferase reporter gene into PC-3 cells stimulated with TNF-α in the presence or the absence of GFLX (Fig. 5). When the cells were stimulated with TNF-α, the reporter gene construct of the IL-8 promoter, including the binding sites for HNF-1, GRE, AP-1, and NF-κB, exhibited a significantly increased level of promoter activity compared with that of the cells not stimulated (Fig. 5 and 6). Addition of GFLX to the cells stimulated with TNF-α decreased the level of IL-8 promoter activity. When the cells were incubated with 16 μg/ml of GFLX, IL-8 promoter activity was significantly attenuated. The results correlate well with those showing that GFLX suppresses IL-8 mRNA expression. Taken together, these results support the idea that GFLX suppresses IL-8 mRNA expression and attenuates IL-8 release from the cells by decreasing the level of IL-8 promoter activity.
FIG. 5.
GFLX suppresses IL-8 gene promoter activity. PC-3 cells were plated at 4 × 104 cells/well in 24-well plates. After 24 h of incubation, the cells were transiently transfected with 179.1 ng of the 5′-flanking region (sequence from −1481 to +44 bp) of the IL-8 gene in a pGL3-Basic vector and 20.8 ng of pRL-TK. Twenty-four hours after transfection, GFLX was added at 0, 2, 4, or 16 μg/ml and the cells were further incubated for 24 h. The medium was then exchanged with serum-free RPMI 1640 medium. GFLX at the same concentration and TNF-α at 10 ng/ml were added, and the cells were incubated for 2 h. After the incubation, the IL-8 gene promoter activity was finally determined by the dual luciferase reporter assay described in Materials and Methods. White and black bars, incubation stimulated without and with TNF-α, respectively; gray bars, incubations in the presence of GFLX and TNF-α. The data shown are the means ± SDs for three experiments. *, P < 0.05 compared with the results for incubation in the absence of TNF-α; **, P < 0.05 compared with the results for incubation without GFLX in the presence of TNF-α.
FIG. 6.
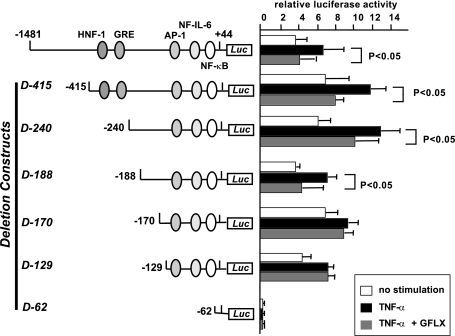
The deletion construct without the 5′-flanking region from −1481 to −170 bp reverses the suppressive effect of GFLX on IL-8 promoter activity. PC-3 cells were plated at 4 × 104 cells/well in 24-well plates. After 24 h of incubation, the cells were transiently transfected with 179.1 ng of a luciferase reporter gene construct containing sequentially deleted 5′-flanking regions of the IL-8 gene and 20.8 ng of pRL-TK. The constructs of the deletion mutants (D-415, D-240, D-188, D-170, D-129, and D-62) are shown to the left. Twenty-four hours after transfection, GFLX was added at 16 μg/ml and the cells were further incubated for 24 h. The medium was then exchanged for serum-free RPMI 1640 medium. GFLX at the same concentration and TNF-α at 10 ng/ml were added, and the cells were incubated for 2 h. After the incubation, the IL-8 gene promoter activity was finally determined by the dual luciferase reporter assay described in Materials and Methods. White bars, control without stimulation; black bars, incubation in the presence of TNF-α; gray bars, incubation in the presence of TNF-α and GFLX. The data shown are the means ± SDs of three experiments. *, P < 0.05 compared with the results for the luciferase activity of TNF-α-stimulated cells.
We next constructed six deletion mutants of the IL-8 promoter and examined the promoter activity in the presence or the absence of GFLX (Fig. 6). The deletion of the 5′-flanking region from −1481 to −129 bp (D-129) still increased the level of IL-8 promoter activity in the presence of TNF-α, but the construct without the region from −1481 to −62 bp (D-62) did not respond to TNF-α. Since this region contains the binding sites for AP-1, NF-IL-6, and NF-κΒ, the results indicate that these binding sites are important for the increase in TNF-α-induced IL-8 promoter activity.
The deletion of the 5′-flanking region from −1481 to −415 bp (D-415) did not alter the ability of GFLX to suppress TNF-α-induced IL-8 promoter activity (Fig. 6). The deletion constructs D-240 and D-188 also exhibited attenuated IL-8 promoter activity in the presence of GFLX, indicating that the 5′-flanking region from −1481 to −188 bp is not essential for the inhibitory effect of GFLX on IL-8 promoter activity. However, a deletion construct without the region from −1481 to −170 bp (D-170) reversed the inhibitory effect of GFLX on TNF-α-stimulated IL-8 promoter activity.
DISCUSSION
This study shows that GFLX down-regulates IL-8 secretion in stimulated and unstimulated PC-3 cells. Although the reason for the different effects of ciprofloxacin and GFLX on IL-8 expression remains unclear, it is probable that the low concentrations (2 to 16 μg/ml) of GFLX used in this study caused the difference. We chose these concentrations because concentrations of GFLX greater than 20 μg/ml exhibited cytotoxicity for PC-3 cells. In addition, the increased level of IL-8 expression caused by ciprofloxacin has been speculated to be due to increased mRNA stability in a human endothelial cell line (8), although no evidence has been provided. In this study we did not examine the mRNA stability but found that GFLX at 16 μg/ml suppresses IL-8 promoter activity in PC-3 cells. There is also a possibility that the actions of the fluoroquinolones are cell-type specific.
This study shows that GFLX inhibits IL-8 secretion from stimulated and unstimulated PC-3 cells. Since PC-3 is a human prostate cancer cell line, we also performed the experiments with a noncancerous cell line, a human prostatic myofibroblast cell line (11). GFLX at 4 and 16 μg/ml decreased the basal levels of secretion of IL-8 from prostatic myofibroblasts by 19% and 50%, respectively (the values are the means of two experiments), indicating that GFLX appears to exhibit an inhibitory effect on IL-8 secretion from stromal cells as well as cancerous cells. However, it remains unknown whether GFLX directly affects the healthy prostate. Thus, the results obtained in this study are applicable only to prostate cell lines.
Most of the experiments in this study were performed with 16 μg/ml GFLX, since the effect of GFLX in attenuating IL-8 secretion was the most prominent at this concentration. This concentration seems to be higher than those of GFLX in plasma and the extracellular fluids in the prostate (21), but oral administration of 200 mg GFLX has been reported to increase its concentration in prostatic tissue to up to 4.93 μg/g tissue (16). In this study 4 μg/ml GFLX also significantly decreased the level of IL-8 secretion from PC-3 cells (Fig. 1A).
This study demonstrates that GFLX exhibits an inhibitory effect on IL-8 production in the prostate cell line PC-3, indicating that fluoroquinolones can act directly on prostate cells. In addition, this study shows that GFLX down-regulates IL-8 promoter activity. In the present study we have shown that the deletion of the IL-8 promoter region from −1481 to −170 bp but not from −1481 to −188 bp abolishes the GFLX-mediated suppression of IL-8 promoter activity (Fig. 6). This suggests that the region from −188 to −170 bp may be important for the inhibitory effect of GFLX on TNF-α-induced IL-8 promoter activity. However, the relationship between the action of GFLX and the IL-8 promoter remains unclear. How the fluoroquinolone acts on the IL-8 promoter should be elucidated in the future.
We examined whether ciprofloxacin also down-regulated IL-8 expression in PC-3 cells. Unlike GFLX, ciprofloxacin did not significantly attenuate the IL-8 secretion elicited with peptidoglycan and Mycoplasma hominis in PC-3 cells (data not shown). It is unclear why GFLX and ciprofloxacin affected differently IL-8 secretion from PC-3 cells, but the actions of these fluoroquinolones may be cell type specific. Ciprofloxacin decreases the accumulation of IL-6 protein from a human endothelial cell line, while IL-8 protein production is decreased at low concentrations of ciprofloxacin but is increased at high concentrations (∼100 μg/ml) (9).
Chronic prostatitis is a common disease, although a basic mechanism of the disease has not been yet clarified. Various factors, including dysfunction of the immune system, define the symptoms of chronic prostatitis (27). The numbers of white blood cells and the bacterial counts in prostatic fluid have usually been used as diagnostic markers. However, one recent report suggests that these markers do not correlate with clinical symptoms (29). Previous studies of proinflammatory cytokines have shown that IL-1β, TNF-α, IL-6, gamma interferon, IL-2, IL-10, and IL-8 exhibit significant correlations with the clinical conditions that patients with chronic prostatitis develop (2, 12, 18, 22). Since IL-8 possesses a potent inflammatory function and has been also recognized as an inflammatory marker of urinary tract infection (19, 24, 28), IL-8 could be one of the reliable markers that can be used to evaluate the severity of prostatic inflammation. GFLX is a newly developed fluoroquinolone with broad-spectrum and extended antibacterial activity (13). Because of its good penetration into prostatic and seminal fluids (21), GFLX appears to be ideal for the treatment of chronic prostatitis. Because the proportion of cases of bacterial prostatitis among patients with chronic prostatitis is relatively low (31), the conclusions from this study provide a meaningful rationale for the use of this fluoroquinolone for the treatment of patients with nonbacterial prostatitis. However, it should be emphasized that the justification for the use of this fluoroquinolone for the treatment of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is dependent on the results of randomized clinical trials.
The levels of IL-8 and neutrophil-activating peptide 78 have been shown to be frequently elevated in the expressed prostatic secretions of men with CP/CPPS (12), suggesting that these cytokines may be responsible, in part, for the presence of an inflammatory reaction in the prostate. Since prostatic secretions come from the prostate epithelium, it is possible to assume that the prostate epithelium is the source of the seminal cytokines that may be important in CP/CPPS. In addition, inflammatory cells, including lymphocytes, neutrophils, and macrophages, may also be involved in the secretion of cytokines because these cells exist at the site of inflammation in the prostate gland (17).
In conclusion, this study demonstrates that GFLX suppresses IL-8 expression in the prostate cell line by decreasing the promoter activity of the IL-8 gene.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Aagaard, J., J. Knes, and P. O. Madsen. 1991. Prostatic tissue levels of ofloxacin. Urology 38:380-382. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, R. B., S. Ponniah, J. Hasday, and J. R. Hebel. 1998. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology 52:744-749. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, R. B., K. J. Propert, A. J. Schaeffer, R. Landis, J. C. Nickel, M. P. O'Leary, M. A. Pontari, M. McNaughton-Collins, D. A. Shosken, C. V. Comiter, N. S. Datta, J. E. Fowler, R. B. Nadler, S. Zeitlin, J. S. Knauss, Y. Wang, J. W. Kusek, L. M. Nyberg, M. S. Litwin, and C. P. R. Network. 2004. Ciprofloxacin or tamsulosin in men with chronic prostatitis/chronic pelvic pain syndrome: a randomized, double-blind trial. Ann. Intern. Med. 141:581-589. [DOI] [PubMed] [Google Scholar]
- 4.Araujo, F. G., T. R. Slifer, and J. S. Remington. 2002. Effect of moxifloxacin on secretion of cytokines by human monocytes stimulated with lipopolysaccharide. Clin. Microbiol. Infect. 8:26-30. [DOI] [PubMed] [Google Scholar]
- 5.Bailly, S., Y. Mahe, B. Ferrua, M. Fay, T. Tursz, H. Wakasugi, and M. A. Gougerot-Pocidalo. 1990. Quinolone-induced differential modification of interleukin 1α and interleukin 1β production by lipopolysaccharide-stimulated human monocytes. Cell. Immunol. 128:277-288. [DOI] [PubMed] [Google Scholar]
- 6.Dalhoff, A., and I. Shalit. 2003. Immunomodulatory effects of quinolones. Lancet Infect. Dis. 3:359-371. [DOI] [PubMed] [Google Scholar]
- 7.Flick, D. A., and G. E. Gifford. 1984. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J. Immunol. Methods 68:167-175. [DOI] [PubMed] [Google Scholar]
- 8.Galley, H. F., J. K. Dhillon, R. L. Paterson, and N. R. Webster. 2000. Effect of ciprofloxacin on the activation of the transcription factors nuclear factor κB, activator protein-1 and nuclear factor-interleukin-6, and interleukin-6 and interleukin-8 mRNA expression in a human endothelial cell line. Clin. Sci. (London) 99:405-410. [PubMed] [Google Scholar]
- 9.Galley, H. F., S. J. Nelson, A. M. Dubbels, and N. R. Webster. 1997. Effect of ciproxacin on the accumulation of interleukin-6, interleukin-8, and nitrite from a human endothelial model of sepsis. Crit. Care Med. 25:1392-1395. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto, S., K. Matsumoto, Y. Gon, S. Maruoka, S. Hayashi, Y. Asai, T. Machino, and T. Horie. 2000. Grepafloxacin inhibits tumor necrosis factor-alpha-induced interleukin-8 expression in human airway epithelial cells. Life Sci. 66:PL77-PL82. [DOI] [PubMed] [Google Scholar]
- 11.Hisataki, T., N. Itoh, K. Suzuki, A. Takahashi, N. Masumori, N. Tohse, Y. Ohmori, S. Yamada, and T. Tsukamoto. 2004. Modulation of phenotype of human prostatic stromal cells by trasnforming growth facor-beta. Prostate 58:174-182. [DOI] [PubMed] [Google Scholar]
- 12.Hochreiter, W. W., R. B. Nalder, A. E. Koch, P. L. Campbell, M. Ludwig, W. Weidner, and A. J. Schaeffer. 2000. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology 56:1025-1029. [DOI] [PubMed] [Google Scholar]
- 13.Hosaka, M., T. Yasue, H. Fukuda, H. Tomizawa, H. Aoyama, and K. Hirai. 1992. In vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinolone. Antimicrob. Agents Chemother. 36:2108-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Into, T., Y. Nodasaka, A. Hasebe, T. Okuzawa, J. Nakamura, N. Ohata, and K. Shibata. 2002. Mycoplasmal lipoproteins induce Toll-like receptor 2- and caspase-mediated cell death in lymphocytes and monocytes. Microbiol. Immunol. 46:265-276. [DOI] [PubMed] [Google Scholar]
- 15.Jang, T. L., and A. J. Schaeffer. 2003. The role of cytokines in prostatitis. World J. Urol. 21:95-99. [DOI] [PubMed] [Google Scholar]
- 16.Kawada, Y., Y. Kumamoto, S. Orikasa, Y. Aso, T. Machida, I. Saito, N. Kawamura, K. Suzuki, K. Kawabe, K. Okada, Y. Naide, S. Kamidono, H. Ohmori, T. Usui, J. Kumazawa, and Y. Ohi. 1999. Clinical early phase II study of gatifloxacin in urinary tract infection. Jpn. J. Chemother. 47(Suppl. 2):292-307. [Google Scholar]
- 17.Kohnen, P. W., and G. W. Drach. 1979. Patterns of inflammation in prostatic hyperplasia: a histologic and bacteriologic study. J. Urol. 121:755-760. [DOI] [PubMed] [Google Scholar]
- 18.Miller, L. J., K. A. Fischer, M. L. Goralnick, J. A. Burleson, P. Albertsen, and D. Kreutzer. 2002. Interleukin-10 levels in seminal plasma: implications for chronic prostatitis-chronic pelvic pain syndrome. J. Urol. 167:753-756. [DOI] [PubMed] [Google Scholar]
- 19.Mukaida, N., A. Harada, K. Yasumoto, and K. Matsushima. 1992. Properties of pro-inflammatory cell type-specific leukocyte chemotactic cytokines, interleukin 8 (IL-8) and monocyte chemotactic and activating factor (MCAF). Microbiol. Immunol. 36:773-789. [DOI] [PubMed] [Google Scholar]
- 20.Mukaida, N., M. Shiroo, and K. Matsushima. 1989. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J. Immunol. 143:1366-1371. [PubMed] [Google Scholar]
- 21.Naber, C. K., M. Steghafner, M. Kinzig-Schippers, C. Sauber, F. Sörgel, H. J. Stahlberg, and K. G. Naber. 2001. Concentration of gatifloxacin in plasma and urine and penetration into prostatic and seminal fluid, ejaculate, and sperm cells after single oral administrations of 400 milligrams to volunteers. Antimicrob. Agents Chemother. 45:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadler, R. B., A. E. Koch, E. A. Calhoun, P. L. Campbell, D. L. Pruden, C. L. Bennett, P. R. Yarnold, and A. J. Schaeffer. 2000. IL-1β and TNF-α in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J. Urol. 164:214-218. [PubMed] [Google Scholar]
- 23.Nickel, J. C., J. Downey, B. Johnston, J. Clark, and the Canadian Prostatitis Research Group. 2001. Predictors of patient response to antibiotic therapy for the chronic prostatitis/chronic pelvic pain syndrome: a prospective multicenter clinical trial. J. Urol. 165:1539-1544. [PubMed] [Google Scholar]
- 24.Olszyna, D. P., H. Vermeulen, A. H. Baan, P. Speelman, S. J. H. van Deventer, D. J. Gouma, and T. van der Poll. 2001. Urine interleukin-8 is a marker for urinary tract infection in postoperative patients. Infection 29:274-277. [DOI] [PubMed] [Google Scholar]
- 25.Ono, Y., Y. Ohmoto, K. Ouo, Y. Sakata, and K. Murata. 2000. Effect of grepafloxacin on cytokine production in vitro. J. Antimicrob. Chemother. 46:91-94. [DOI] [PubMed] [Google Scholar]
- 26.Png, J. C. D., E. Tan, K. T. Foo, M. K. Li, C. Cheng, and I. R. Rekhraj. 1997. A comparative study of the distribution of floxacin and ciproxacin in prostatic tissues after simultaneous oral ingestion. Br. J. Urol. 79:781-784. [DOI] [PubMed] [Google Scholar]
- 27.Pontari, M. A., and M. R. Ruggieri. 2004. Mechanisms in prostatitis/chronic pelvic pain syndrome. J. Urol. 172:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao, W. H., G. S. Evans, and A. Finn. 2001. The significance of interleukin 8 in urine. Arch. Dis. Child. 85:256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaeffer, A. J., J. S. Knauss, R. Landis, K. J. Propert, R. B. Alexander, M. S. Litwin, J. C. Nickel, M. P. O'Leary, R. B. Nadler, M. A. Pontari, D. A. Shoskes, S. Zeitlin, J. E. Fowler, C. A. Mazurick, J. W. Kusek, L. M. Nyberg, and the Chronic Prostatitis Collaborative Research Network Study Group. 2002. Leukocyte and bacterial counts do not correlate with severity of symptoms in men with chronic prostatitis: the National Institutes of Health Chronic Prostatitis Cohort Study. J. Urol. 168:1048-1053. [DOI] [PubMed] [Google Scholar]
- 30.Stunkel, K. G. E., G. Hewlett, and H. J. Zeiler. 1991. Ciprofloxacin enhances T cell function by modulating interleukin activities. Clin. Exp. Immunol. 86:525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidner, W., H. G. Schiefer, H. Krauss, C. Jantos, H. J. Friedrich, and M. Altmannsberger. 1991. Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1,461 patients. Infection 19:S119-S125. [DOI] [PubMed] [Google Scholar]
- 32.Williams, A. C., H. F. Galley, and N. R. Webster. 2001. The effect of moxifloxacin on release of interleukin-8 from human neutrophils. Br. J. Anaesth. 87:671-672. [Google Scholar]
- 33.Yasumoto, R., M. Kawano, T. Tsujino, Y. Iwai, S. Hayashi, N. Nishisaka, A. Horii, and T. Kishimoto. 1995. Seminal plasma cytokines in nonbacterial prostatitis: changes following sparfloxacin treatment. Acta Urol. Jpn. 41:771-774. [PubMed] [Google Scholar]
- 34.Yoshimura, T., C. Kurita, E. Usami, T. Nakao, S. Watanabe, J. Kobayashi, F. Yamazaki, and H. Nagai. 1996. Immunomodulatory action of levofloxacin on cytokine production by human peripheral blood mononuclear cells. Chemotherapy 42:459-464. [DOI] [PubMed] [Google Scholar]