Abstract
Osteomyelitis, osteitis, spondylodiscitis, septic arthritis, and prosthetic joint infections still represent the worst complications of orthopedic surgery and traumatology. Successful treatment requires, besides surgical débridement, long-term systemic and high-concentration local antibiotic therapy, with possible local antibiotic concentrations of 100 μg/ml and more. In this study, we investigated the effect of 20 different antibiotics on primary human osteoblasts (PHO), the osteosarcoma cell line MG63, and the epithelial cell line HeLa. High concentrations of fluoroquinolones, macrolides, clindamycin, chloramphenicol, rifampin, tetracycline, and linezolid during 48 h of incubation inhibited proliferation and metabolic activity, whereas aminoglycosides and inhibitors of bacterial cell wall synthesis did not. Twenty percent inhibitory concentrations for proliferation of PHO were determined as 20 to 40 μg/ml for macrolides, clindamycin, and rifampin, 60 to 80 μg/ml for chloramphenicol, tetracylin, and fluoroquinolones, and 240 μg/ml for linezolid. The proliferation of the cell lines was always less inhibited. We established the measurement of extracellular lactate concentration as an indicator of glycolysis using inhibitors of the respiratory chain (antimycin A, rotenone, and sodium azide) and glycolysis (iodoacetic acid) as reference compounds, whereas inhibition of the respiratory chain increased and inhibition of glycolysis decreased lactate production. The measurement of extracellular lactate concentration revealed that fluoroquinolones, macrolides, clindamycin, rifampin, tetracycline, and especially chloramphenicol and linezolid impaired mitochondrial energetics in high concentrations. This explains partly the observed inhibition of metabolic activity and proliferation in our experiments. Because of differences in the energy metabolism, PHO provided a more sensitive model for orthopedic antibiotic usage than stable cell lines.
The septic diseases osteomyelitis, osteitis, spondylodiscitis, septic arthritis, and prosthetic joint infection represent the worst complications of orthopedic surgery and traumatology. Successful treatment usually requires a combination of surgical débridement and antibiotic therapy. Only antibiotics with a high bioavailability in bone are suited for treatment, but they have to be administered in high dosage and for long terms, at least 4 to 6 weeks. Complementary antibiotics are often applied locally to achieve extremely high concentrations in bone tissue without high serum concentrations to avoid detrimental side effects. In 1970, Buchholz and Engelbrecht (6) reported that penicillin, erythromycin, and gentamicin incorporated into polymethylmethacrylate (PMMA) cement used to attach total hip joint prostheses diffused out into the surrounding tissues over a period of months, thereby providing prolonged concentrations of local antibiotic. As a therapy for osteomyelitis, Klemm (29) formed gentamicin-impregnated PMMA cement into beads and used them to temporarily fill in the dead space created after the débridement of infected bone. Adams et al. (1) measured the elution of several antibiotics (cefazolin, ciprofloxacin, clindamycin, ticarcillin, tobramycin, and vancomycin) from PMMA beads in mongrel dogs. Depending on the antibiotic, they found initial concentrations of active antibiotic in the space around the beads between 7.5 μg/ml (ciprofloxacin) and 1,516.7 μg/ml (clindamycin). With the exception of ticarcillin, antibiotic concentrations in the granulation tissue surrounding the beads exceeded 30 μg/ml even 28 days after implantation. In pharmacokinetic studies in patients after implantation of gentamicin-impregnated PMMA, concentrations of up to 150 μg/ml active gentamicin were observed in wound exudates derived directly from the vicinity of the implanted cement (49). Besides the nonbiodegradable PMMA, numerous biodegradable materials have been studied for use as local drug delivery systems for antibiotics (27). Commercially available are gentamicin collagen sponges that can create local concentrations of up to 1,000 μg/ml gentamicin over a short period of time (50).
In consideration of the fact that very high concentrations of antibiotics are achieved in bone tissue during local treatment, the influence of antibiotics, especially on bone cell function, seems most relevant. We investigated 20 antibiotics of different classes and antibacterial mechanisms in a cell culture model of primary human osteoblasts. For comparison, all experiments were carried out with the osteosarcoma cell line MG63 and the epithelial cell line HeLa as well. Different cell parameters have been investigated: cell proliferation, metabolic activity, cytotoxicity, and lactate production as an indicator of impaired mitochondrial energetics.
MATERIALS AND METHODS
Materials, reagents, and antibiotics.
The following antibiotics were investigated: cefazolin, chloramphenicol, clindamycin, erythromycin, gentamicin, lincomycin, penicillin G, rifampin, roxithromycin, streptomycin, tetracycline, tobramycin, and vancomycin (all from Sigma, Taufkirchen, Germany); amoxicillin and flucloxacillin (GlaxoSmithKline GmbH & Co. KG, Munich, Germany); ciprofloxacin (MP Biomedicals GmbH, Eschwege, Germany); fosfomycin (InfectoPharm, Heppenheim, Germany); moxifloxacin (Bayer AG, Leverkusen, Germany); and linezolid and azithromycin (Pfizer GmbH, Karlsruhe, Germany).
High-glucose Dulbecco's modified Eagle medium (DMEM), GlutaMAX-I, fetal calf serum (FCS), and sodium pyruvate were obtained from Invitrogen GmbH (Karlsruhe, Germany); Liberase Blendzyme 3, Cytotoxicity Detection Kit, and Cell Proliferation enzyme-linked immunosorbent assay (ELISA) were from Roche Applied Science (Mannheim, Germany); ITS+ (insulin-transferrin-selenium) was from BD Bioscience (Heidelberg, Germany); platelet-derived growth factor (PDGF-BB) was from R&D Systems GmbH (Wiesbaden, Germany); epidermal growth factor (EGF), dexamethasone, ascorbic acid 2-phosphate, β-glycerophosphate, and thiazolyl blue tetrazolium bromide (MTT) were from Sigma (Taufkirchen, Germany); Accutase was from PAA Laboratories GmbH (Cölbe, Germany); Ultroser G was from PALL GmbH (Dreieich, Germany); fibronectin was from Biochrom AG (Berlin, Germany); and Lactate Reagent was from Trinity Biotech (Bray, Ireland).
All cell culture plastic was obtained from BD Bioscience (Heidelberg, Germany) with the exception of 96-well microtiter plates, which were purchased from NUNC (Wiesbaden, Germany).
Primary human osteoblast culture.
Mesenchymal stem cells were isolated from trabecular bone obtained during total hip replacements from the femurs of patients aged 42 to 82 years (41, 46). The bone pieces were cut into fragments of 3 to 10 mm and incubated with Liberase Blendzyme 3 in a concentration of 70 μg/ml for 1.5 to 2 h at 37°C. The supernatant containing the mesenchymal stem cells, bone marrow, and blood cells was stored, the fragments were washed twice with phosphate-buffered saline (PBS), and the lavage was united with the supernatant. The cell suspension was centrifuged at 300 × g for 5 min. The pellet was resuspended in expansion medium (modified after Reyes et al. [38]) consisting of DMEM supplemented with 2% GlutaMAX-I, 1% FCS (embryonic stem cell tested), 1 mM sodium pyruvate, 1% ITS+, 25 μg/ml ciprofloxacin, 10 ng/ml human EGF, and 10 ng/ml human PDGF-BB and seeded in fibronectin-coated cell culture flasks (38). After 24 h of incubation at 37°C and 5% CO2, the supernatant containing nonadherent cells, like blood cells, was removed, the adherent cells were washed twice with PBS, and fresh medium was added. After nearly reaching confluence, the cultures were passaged in expansion medium without ciprofloxacin after 7 to 10 days. To gain mature osteoblasts, the cells were placed at passage two or three in differentiating medium consisting of DMEM supplemented with 2% Ultroser G serum substitute, 2% GlutaMAX-I, 1 mM sodium pyruvate, 100 nM dexamethansone, 50 μM ascorbic acid 2-phosphate, and 10 mM β-glycerophosphate for 5 to 7 days (26). We characterized the obtained cells on the basis of expression of osteocalcin, osteopontin, SPARC (secreted protein acidic and rich in cysteine), and alkaline phosphatase; the ability to build extracellular matrix (collagen I); and their mineralization as mature osteoblasts (5, 28, 40). The cells were detached with Accutase for passage or seeding. Cells from different subjects were cultivated separately and never pooled. For experiments, cells from one subject were seeded on 96-well microtiter plates at a density of 0.75 × 104 cells/cm2 for proliferation assays and 1.5 × 104 cells/cm2 for MTT assay, lactate dehydrogenase (LDH) release, and lactate determination in 100 μl differentiation medium per well. For time kinetic experiments, the cells were seeded in 25-cm2 cell culture flasks at a density of 1.5 × 104 cells/cm2 in 7 ml medium. All cultures were examined regularly for mycoplasma contamination. All experiments were performed in differentiating medium if not stated otherwise.
MG63 and HeLa cultures.
Cells were cultivated in DMEM supplemented with 2% GlutaMAX-I, 1 mM sodium pyruvate, and 10% FCS (standard medium) at 37°C and 5% CO2. They were passaged thrice a week. For experiments they were seeded on 96-well microtiter plates at a density of 0.5 × 104 cells/cm2 (MG63) or 0.75 × 104 cells/cm2 (HeLa) for proliferation assays and 1.5 × 104 cells/cm2 for MTT assay and lactate determination in 100 μl standard medium. For time kinetic experiments, the cells were seeded in 25-cm2 cell culture flasks at a density of 1.5 × 104 cells/cm2 in 7 ml medium. All cultures were examined regularly for mycoplasma contamination. Experiments to determine the proliferation in the presence of antibiotics were performed in standard medium containing 10% FCS. For experiments to determine metabolic activity and lactate production, the medium was changed 24 h after seeding with DMEM containing 2% of the serum supplement Ultroser G, if not stated otherwise.
Cytotoxicity.
Quantification of cell death and lysis of primary human osteoblasts was based on the measurement of LDH activity released from the cytosol of damaged cells into the supernatant. Twenty-four hours after seeding, the medium was replaced with fresh differentiating medium and the cells were incubated with the antibiotics. After 24 h of incubation, the LDH release in the supernatant and the intracellular LDH content of the corresponding adherent cells after lysis with 1% Triton X were determined via the Cytotoxicity Detection Kit following the manufacturers' instructions. Percentage cytotoxicity was calculated as the quotient of released LDH/total LDH (i.e., intracellular LDH of the corresponding adherent cells plus released LDH).
Proliferation.
The quantification of cell proliferation was based on the measurement of bromodeoxyuridine (BrdU) incorporation during DNA synthesis in proliferating cells. Twenty-four hours after seeding, the cells were treated with the antibiotics and nurtured in their presence for 48 h. The primary human osteoblasts were labeled with BrdU for 48 h, and the cells lines were labeled with BrdU for 18 h. If not explicitly stated otherwise, primary human osteoblasts were incubated in differentiating medium, and the cells lines were incubated in standard medium containing 10% FCS. The incorporated BrdU was quantified using the Cell Proliferation ELISA following the manufacturers' instructions.
Metabolic activity.
The overall metabolic activity in the cell populations was determined via reduction of MTT by NAD-dependent dehydrogenase activity to form a colored reaction product. Twenty-four hours after the seeding medium was changed, the cells were treated with the antibiotics and nurtured in their presence for 48 h. Afterwards they were incubated for 2 to 3 h with 20 μl MTT solution (1% MTT in PBS) at 37°C. The supernatant was removed, and the formazan was solubilized with 2-propanol and measured photometrically at 450 nm. Equally treated wells without cells served as blanks.
To investigate a possible direct reaction with MTT, every antibiotic was incubated with MTT but without cells in PBS, and the extinction of the supernatant was measured.
Lactate production.
The measurement of lactate was performed with Lactate Reagent, which is based on the conversion of lactic acid to pyruvate and hydrogen peroxide. The increase in absorbance at 540 nm is directly proportional to the lactate concentration in the sample and was measured with an ELISA reader. Because of the high lactate content of FCS, all cultures were cultivated under serum-free conditions with Ultroser G. The medium was changed 24 h after seeding, and the cells were treated with the antibiotics and nurtured in their presence for 48 h. Because of the extreme sensitivity of the lactate measurement, the optimal quantity of the cell culture supernatant sample was determined in every experiment. Usually 2 to 10 μl of supernatant was sufficient for a precise determination with 200 μl lactate reagent on 96-well plates. The concentration of lactate was calculated in relation to a lactate standard in known concentration.
To analyze the lactate production in time kinetic experiments, the medium was removed and replaced with 5 ml medium supplemented with 2% Ultroser G containing the substance to be tested at time point zero, and the cultures were incubated at 37°C during the experiment. Every hour, including time point zero, 20 μl cell culture supernatant was collected for determination of lactate concentration.
No direct reaction of the lactate reagent with any antibiotic was observed.
Data analysis.
The untreated control of every experiment was defined as 100% proliferation, metabolic activity, or lactate production, and the values of the antibiotic-treated cultures were calculated in relation to the control. Standard deviations displayed in dose-response curves were calculated on the basis of six replicates in the same experiment. Displayed graphs are representative for the corresponding experiment. Experiments with PHO were repeated with cells derived from different subjects. All experiments were repeated at least four times to determine inhibition of proliferation of primary human osteoblasts and cell lines, four times to determine metabolic activity and extracellular lactate production of the cell lines, four times to determine cytotoxicity, and at least six times to determine metabolic activity and lactate production of PHO. Twenty percent inhibitory concentrations (IC20s) and IC50s were determined graphically for every experiment, and mean values were calculated for the tabulation.
RESULTS
Possible detrimental influence of solvents in the concentrations used was excluded prior to the experiments.
Direct cytotoxicity of antibiotics for primary human osteoblasts.
Direct cytotoxicity measured via LDH release could not be observed 24 h after treatment of PHO with inhibitors of the bacterial cell wall synthesis, aminoglycosides, tetracycline, rifampin, and lincomycin, in any concentration tested. The fluoroquinolones ciprofloxacin and moxifloxacin caused 10 to 20% cytotoxicity only in concentrations of 400 μg/ml. Clindamycin and erythromycin caused a cytotoxicity of 10 to 25% and 40%, respectively, in concentrations of 400 μg/ml, but in contrast to azithromycin and roxithromycin, no LDH release could be detected with lower concentrations. Azithromycin and roxithromycin both caused 20 to 30% cytotoxicity at 100 μg/ml, 30 to 45% at 200 μg/ml, and 40 to 60% at 400 μg/ml (data not shown). Light microscopic examination during incubation revealed that very high concentrations of clindamycin, erythromycin, and, more distinctly, azithromycin and roxithromycin caused a rounding of PHO, which resulted in a detachment from the surface within a few hours.
Effect of antibiotics on proliferation, metabolic activity, and lactate production of primary human osteoblasts, MG63 cells, and HeLa cells.
The aminoglycosides and the inhibitors of the bacterial cell wall synthesis, with exception of cefazolin, displayed no influence on proliferation, metabolic activity, and lactate production. Cefazolin decreased the proliferation of PHO slightly in the highest concentration of 400 μg/ml, yet the proliferation of MG63 and HeLa cells was inhibited in lower concentrations (Table 1). It decreased the metabolic activity and lactate production of PHO only slightly, whereas the metabolic activity and lactate production of the cell lines was almost unchanged.
TABLE 1.
Mean IC20s and IC50s for all experiments for proliferation and metabolic activity for 48 h of incubation of PHO, MG63 cells, and HeLa cells with different antibiotics
| Antibiotic | Mean IC (proliferation, metabolic activity, in μg/ml) and cell typea
|
|||||
|---|---|---|---|---|---|---|
| IC20 PHO | IC50 PHO | IC20 MG63 | IC50 MG63 | IC20 HeLa | IC50 HeLa | |
| Penicillin G | No effect | No effect | No effect | No effect | No effect | No effect |
| Flucloxacillin | No effect | No effect | No effect | No effect | No effect | No effect |
| Amoxicillin | No effect | No effect | No effect | No effect | No effect | No effect |
| Cefazolin | 380, >400 | >400, >400 | 230, 400 | >400, >400 | 270, >400 | >400, >400 |
| Vancomycin | No effect | No effect | No effect | No effect | No effect | No effect |
| Fosfomycin | No effect | No effect | No effect | No effect | No effect | No effect |
| Gentamicin | No effect | No effect | No effect | No effect | No effect | No effect |
| Streptomycin | No effect | No effect | No effect | No effect | No effect | No effect |
| Tobramycin | No effect | No effect | No effect | No effect | No effect | No effect |
| Ciprofloxacin | 70, 260 | 170, >400 | 80, 60 | 160, 150 | 100, 70 | 290, 120 |
| Moxifloxacin | 80, 190 | 160, >400 | 110, 30 | 230, 170 | 90, 40 | 320, 110 |
| Tetracycline | 60, ÷b | 180, ÷ | 60, ÷ | 180, ÷ | 200, ÷ | >400, ÷ |
| Rifampin | 30, ÷ | 130, ÷ | 120, ÷ | 240, ÷ | 180, ÷ | 270, ÷ |
| Clindamycin | 40, 340 | 150, >400 | 160, 200 | 250, >400 | 230, 80 | >400, 200 |
| Lincomycin | No effect | No effect | No effect | No effect | No effect | No effect |
| Erythromycin | 30, 210 | 180, >400 | 130, 180 | 300, 310 | 210, 110 | >400, 170 |
| Roxithromycin | 20, 110 | 70, 210 | 50, 50 | 180, 110 | 100, 30 | 160, 90 |
| Azithromycin | 20, 80 | 25, 160 | 30, 70 | 190, 180 | 110, 50 | 240, 160 |
| Chloramphenicol | 60, 260 | 230, >400 | 200, 110 | >400, 400 | 340, 100 | >400, 340 |
| Linezolid | 240, >400 | >400, >400 | No effect, 250 | No effect, >400 | No effect, 300 | No effect, >400 |
ICs over 400 μg/ml could not be determined, because this was the highest tested concentration.
÷, ICs could not be determined because of direct reaction of MTT with antibiotic.
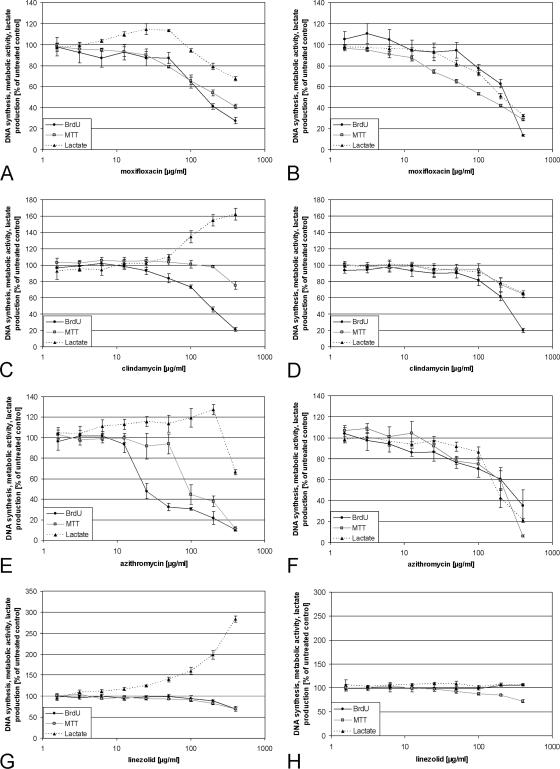
The fluoroquinolones ciprofloxacin and moxifloxacin inhibited the proliferation of PHO, starting with concentrations of 10 μg/ml (Fig. 1A; Table 1). Both decreased concentration-dependent metabolic activity and proliferation in a similar fashion. They increased the lactate production about 10 to 20% above control values in concentrations of 25 to 50 μg/ml, but higher concentrations caused a decrease, about 30% below control values. Although the fluoroquinolones inhibited the proliferation of the cells lines MG63 and HeLa in higher concentrations than PHO, the IC50s for the inhibition of the metabolic activity were significantly below the IC50s of PHO (Fig. 1B; Table 1). The lactate production of MG63 and HeLa was only very slightly or was not influenced by the fluoroquinolones. Because of the great similarity in the effect of antibiotics on both cell lines, only representative graphs of MG63 cells are displayed in the results.
FIG. 1.
Effect of moxifloxacin, clindamycin, azithromycin, and linezolid on PHO and MG63 cells. PHO (A, C, E, and G) and MG63 cells (B, D, F, and H) were treated with moxifloxacin, clindamycin, azithromycin, or linezolid in concentrations of 1.5 to 400 μg/ml for 48 h. Proliferation was determined via BrdU incorporation, metabolic activity via MTT assay, and glycolytic activity by means of lactate production. The untreated control was defined as 100% proliferation, metabolic activity, or lactate production, and all other values were calculated in relation to the control values. Displayed are representative graphs of the particular experiments. The error bars demonstrate the standard deviations of six independent replicates of the same experiment.
Rifampin and tetracycline inhibited the proliferation of PHO with ICs similar to those of the fluoroquinolones (Table 1). Tetracycline inhibited proliferation of MG63 cells in a fashion similar to that of PHO, but concentrations above 400 μg/ml were necessary for 50% inhibition of proliferation of HeLa cells. In the case of rifampin, both cell lines exhibited higher ICs for proliferation than PHO (Table 1). Both tetracycline and rifampin converted MTT directly to formazan in the absence of cells. Rifampin converted almost four times more MTT than did tetracycline in the same time. Microscopic examination of PHO after MTT incubation revealed a significantly stronger formazan staining of the individual cells in cultures treated with high concentrations of rifampin compared to cells in control cultures (data not shown). MTT is probably converted directly by intracellular sources of rifampin and tetracycline. Because they increased the metabolic activity, no ICs could be determined. Tetracycline increased the maximal lactate production of PHO and averaged about 30% at 190 μg/ml, but the increase measured in seven different experiments varied strongly between 0% and 60%. It increased the lactate production of MG63 and HeLa cells only in lower concentrations about 7.5% at most compared to the control, but in contrast to PHO it decreased the dose-dependent lactate production about 30 to 70% in the highest concentration. Rifampin increased the maximal lactate production of PHO and averaged about 40% in concentrations of 400 μg/ml, but as for tetracycline the values acquired from seven independent experiments varied strongly between 0% and 100%. Instead of increasing, it decreased the lactate production of MG63 and HeLa cells about 50 to 60% at 400 μg/ml (data not shown). The observed variability in the effect on primary human osteoblasts was never observed with any other antibiotics besides tetracycline and rifampin.
Clindamycin and the macrolides displayed similar effects on PHO and cell lines, whereas lincomycin had no effect at all. Azithromycin and roxithromycin inhibited the proliferation and metabolic activity of PHO much more than erythromycin and clindamycin with the lowest ICs of all antibiotics tested, whereas the ICs of the metabolic activity were two to six times higher than the ICs for proliferation (Table 1; Fig. 1C and E). This is in contrast to the cell lines. Although the ICs of proliferation were higher than the ICs of PHO, the ICs of the metabolic activity were always lower than the corresponding ICs of PHO (Table 1; Fig. 1D and F). Clindamycin as well as the macrolides decreased the lactate production of the MG63 and HeLa cells, comparable to the inhibition of proliferation. In contrast, the concentration-dependent lactate production of PHO was dramatically increased by clindamycin and erythromycin. Concentrations of 25 μg/ml clindamycin and erythromycin already caused an increase in lactate production, with a maximal increase of about 60 to 70% compared to the control at 400 μg/ml (Fig. 1C). The lactate production was increased by azithromycin about 20 to 30% at most but dropped at 400 μg/ml to about 30 to 40% below control levels (Fig. 1E). Increasing concentrations of roxithromycin caused at first a decrease in lactate production of about 20%, but concentrations of 100 μg/ml and more increased the lactate production again to control levels. The singular course of the graph was exactly reproducible in every experiment (graphs not shown). To demonstrate that clindamycin is actively transported into the cells via the nucleoside transporters, PHO were incubated with 400 μg/ml clindamycin in the presence of 20 mM adenosine. Adenosine blocked almost completely the increase of lactate production as well as the LDH release induced by 400 μg/ml clindamycin during 10 h of incubation (data not shown).
Chloramphenicol decreased the proliferation of PHO concentration dependently, whereas the metabolic activity was much less influenced (Table 1). In contrast, the proliferation of MG63 and HeLa cells was much less inhibited by chloramphenicol than was the metabolic activity. Linezolid inhibited both proliferation and metabolic activity of PHO about 30% at most only in very high concentrations (Table 1; Fig. 1G). No inhibitory influence on proliferation of MG63 and HeLa cells could be found with any concentration during 48 h of incubation, but linezolid decreased the concentration-dependent metabolic activity in higher concentrations (Table 1; Fig. 1H).
The increase of lactate production of PHO induced by chloramphenicol and linezolid was remarkable. Even in the presence of 1.5 μg/ml chloramphenicol, an increase of 12 to 31% lactate production of PHO could be measured, with a maximal increase of 150% on average at 400 μg/ml compared to control values. An increase of lactate production of PHO caused by linezolid was already detectable at 6 μg/ml, with an increase of 13% on average. Concentrations of 400 μg/ml caused an increase of 120% on average, but almost 200% could be reached in some experiments. Chloramphenicol and linezolid both increased the lactate production of MG63 and HeLa cells by only about 25% on average (data not shown).
Usually all osteoblasts were cultivated with DMEM containing the serum replacement Ultroser G. To demonstrate that the effect of at least clindamycin, erythromycin, chloramphenicol, and gentamicin on PHO was not dependent on the absence of FCS, some experiments were performed in medium containing 10% FCS. Gentamicin did not inhibit the proliferation of PHO during 48 h of incubation, regardless of supplementing the medium with FCS or Ultroser G (data not shown). Clindamycin as well as erythromycin and chloramphenicol inhibited the proliferation of PHO cultivated in medium containing FCS with the same IC50s that were determined for PHO cultivated in Ultroser G.
The dose-response curve characteristics and IC50s were always reproducible with PHO gained from different individuals (data not shown).
Effect of repeated treatment with low concentrations of clindamycin and erythromycin on PHO.
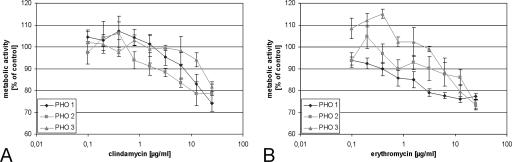
To investigate if low concentrations can influence the metabolic activity of PHO after repeated treatment instead of a single application, PHO were seeded in high density on 96-well plates in medium supplemented with 10% FCS. After 24 h the antibiotics were added at concentrations of 0.01 μg/ml up to 25 μg/ml. The medium was changed daily, and fresh clindamycin or erythromycin was added at the corresponding concentration. The antibiotic-free medium of control cultures was changed daily as well. After 6 days, the metabolic activity of the cultures was determined via MTT assay. Figure 2 shows dose-response curves for three different experiments with osteoblasts gained from different individuals. IC50s could not be determined in these experiments, but the IC20s of metabolic activity were 20 μg/ml for clindamycin and 11 μg/ml for erythromycin, significantly below the IC20s determined after 48 h of incubation (Table 1).
FIG. 2.
Influence of clindamycin and erythromycin on metabolic activity of confluent PHO when cell culture medium containing the antibiotics was changed daily. The cultures were incubated with clindamycin (A) or erythromycin (B) in concentrations of 0.01 to 25 μg/ml. The medium was changed daily, and fresh antibiotics at the same concentration as before were added. After 6 days the metabolic activity of the cultures was determined via MTT. The untreated control was defined as 100% metabolic activity, and all other values were calculated in relation to the control values. Displayed are dose-response curves of three independent experiments with osteoblasts gained from different individuals (PHO 1 to 3). The error bars demonstrate the standard deviations of six independent replicates of the same experiment.
Inhibition of glycolysis and respiratory chain of PHO and MG63 cells.
The increased lactate production can be an indicator of impaired mitochondrial energetics. To prove this hypothesis, PHO and MG63 cells were treated with known inhibitors of glycolysis and the respiratory chain.
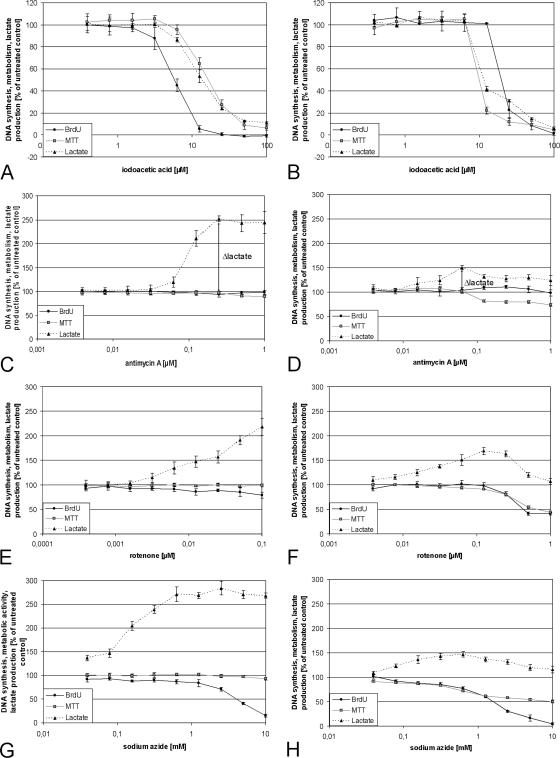
Inhibition of glycolysis with iodoacetic acid caused a concentration-dependent decrease in proliferation, metabolic activity, and lactate production of PHO as well as of MG63 cells (Fig. 3A and B). In cultures of PHO as well as in cultures of MG63 cells, the lactate production dropped almost simultaneously with the overall metabolic activity measured via MTT. The proliferation of PHO was inhibited in lower concentrations than lactate production and metabolic activity, whereas in contrast the lactate production and metabolic activity of MG63 cells decreased before the proliferation was inhibited.
FIG. 3.
Inhibition of glycolysis and respiratory chain. PHO (A, C, E, and G) and MG63 cells (B, D, F, and H) were treated with iodoacetic acid (inhibition of glycolysis), antimycin A (inhibition of respiratory chain complex III), rotenone (inhibition of respiratory chain complex I), or sodium azide (inhibition of respiratory chain complex IV) in different concentrations for 48 h. Proliferation was determined via BrdU incorporation, metabolic activity via MTT assay, and glycolytic activity by means of lactate production. The untreated control was defined as 100% proliferation, metabolic activity, or lactate production, and all other values were calculated in relation to the control values. Displayed are representative graphs of the particular experiments. The error bars demonstrate the standard deviations of six independent replicates of the same experiment. Δlactate indicates the difference between “basal” lactate (spontaneously produced) and respiratory chain inhibitor-induced maximal lactate production.
Antimycin A did not affect proliferation and metabolic activity of PHO in concentrations of less than 1 μM, but the lactate production was increased about 150% at most in concentrations over 0.25 μM (Fig. 3C), whereas the lactate production of MG63 cells was increased about 50% at most compared to the control at 50 nM (Fig. 3D). Antimycin A did not influence the proliferation of MG63 cells, but the metabolic activity decreased to 70% of control. As long as the lactate production increased with rising concentrations of antimycin A, the metabolic activity of MG63 cells was not influenced but decreased in parallel to the lactate production after reaching their maximum. Similar results were obtained with rotenone, but the induced increase in lactate production began at lower concentrations (Fig. 3E and F).
Sodium azide increased the lactate production of PHO to more than 150% compared to the control without influencing the metabolic activity, like antimycin A and rotenone, but in contrast it strongly decreased the proliferation about 80 to 90% in concentrations of more than 1 mM (Fig. 3G). It increased the lactate production of MG63 cells about 20 to 50% but decreased again with higher concentrations of sodium azide. The proliferation of MG63 cells was inhibited comparable to that of PHO, but the metabolic activity decreased slightly in lower concentrations and decreased beginning with the maximum of lactate production parallel to it (Fig. 3H).
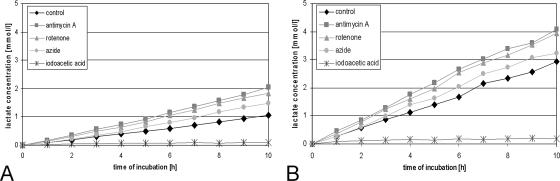
Measurement of lactate production by time kinetics over 10 h revealed that MG63 cells produced three times more absolute lactate than PHO under noninfluenced conditions when seeded in the same density 24 h before the experiment (Fig. 4). Increase of lactate production caused by inhibition of respiration with antimycin A (1 μM), rotenone (1 μM), or azide (1 mM) was already visible after 1 h of incubation in both cell systems. Antimycin A caused a doubling of lactate production of PHO after 10 h of incubation (7 μmol to 14 μmol), whereas the lactate production of MG63 cells was increased only about 25% compared to the untreated control (21 μmol to 28 μmol). Iodoacetic acid (100 μM) blocked lactate production of PHO and MG63 cells almost completely after 1 h. It is noteworthy that complete inhibition of the respiratory chain with antimycin A and rotenone caused in both cultures an absolute increase of about 7 μmol lactate after 10 h. This corresponds to 7 μmol ATP that has been produced aerobically in this experiment when the respiratory chain was not inhibited.
FIG. 4.
Absolute lactate production within 10 h of incubation with different inhibitors. PHO (A) and MG63 cells (B) were incubated with the inhibitors of the respiratory chain antimycin A (1 μM), rotenone (1 μM), and sodium azid (1 mM) as well as the inhibitor of glycolysis, iodoacetic acid (100 μM). Every hour, aliquots of the cell culture supernatant were removed, and the lactate concentration was determined in comparison to a lactate standard in a known concentration.
DISCUSSION
We demonstrated that antibiotics in high concentrations, which are reached in vivo in bone during local antibiotic therapy, can be cytotoxic or cytostatic for primary human osteoblasts (PHO) and the cell lines MG63 and HeLa. By means of measuring the lactate concentration in the cell culture supernatant as an indicator of glycolysis, we showed that a lot of antibiotics can impair mitochondria, which are believed to be direct descendants of a bacterial endosymbiont that became established at an early stage in a nucleus-containing host cell (19).
While most cells require oxygen for respiration in vivo, cultured cells often rely on glycolysis, a high proportion of which, as in transformed cells, may be anaerobic, because hemoglobin as an O2 carrier is absent (18). Reduced or ceased mitochondrial activity results in a reduction of pyruvate to lactate by NADH formed via glycolysis, which is catalyzed by cytosolic lactate hydrogenase (LDH). At the same time, glycolysis is stimulated with the aim of maintaining the ATP production constant over time, resulting in an enhancement of lactate production and secretion in the cell culture supernatant. D'Aurelio et al. (13) called the difference between “basal” lactate (spontaneously produced) and respiratory chain inhibitor-induced lactate production “Δlactate” and defined it as an index of mitochondrial function (13, 36, 42). The osteosarcoma MG63 cells consumed much more energy than PHO and hence had to produce more glycolytic ATP, because oxygen is the limiting factor in energy production under cell culture conditions (18). The MTT assay provided information about the overall metabolic activity, because it was shown that most cellular reduction of MTT is dependent on the reduced pyridine nucleotides NADH and NADPH, not on mitochondrial succinate as had been believed previously (3). PHO compensated for the complete inhibition of aerobic ATP production via glycolysis, whereas the overall metabolic activity of MG63 cells decreased when the glycolytic rate could not be enhanced further, demonstrating a connection between lactate production and metabolic activity. Maybe because of a metabolic shift the lactate production of MG63 cells declined after reaching a maximum and lactate was consumed instead of produced (15).
Some of our observed cytotoxic and cytostatic effects of clindamycin, macrolides, fluoroquinolones, linezolid, chloramphenicol, rifampin, and tetracycline on PHO can be explained by an impairment of mitochondrial energetics, whereas the inhibitors of the bacterial cell wall syntheses (with the exception of cefazolin with an unknown eukaryotic target [16]) and aminoglycosides displayed no effect on PHO because of absence of a specific target or because they could not enter the cells in the absence of a specific receptor (34, 43). In contrast, chloramphenicol and linezolid increased the lactate production comparable to the specific inhibitors of the respiratory chain presumably by inhibition of mitochondrial protein synthesis, which was actually shown in vitro for chloramphenicol (30, 39). Reports about lactate acidosis (2), neuropathy (12), thrombocytopenia, and pancytopenia (21) suggest an impairment of mitochondrial function by linezolid in vivo as well (24). Our experiments indicate that clindamycin, macrolides, fluoroquinolones, tetracycline, and rifampin also can impair mitochondria via different mechanisms of action. Macrolides and clindamycin like linezolid and chloramphenicol inhibit bacterial protein synthesis by binding the bacterial 50S ribosomal subunit and might inhibit mitochondrial protein synthesis as well, although this has not been shown directly so far (39). The impairment of mitochondrial energetics could also be caused by direct interaction with mitochondrial membranes, because at least macrolides are known to interact directly with phospholipids (35, 47) and macrolides as well as clindamycin are accumulated intracellularly (22, 48). The increase in lactate production caused by the fluoroquinolones was only marginal, because it was superposed with direct toxic effects in higher concentrations. A more distinct increase in lactate production could be observed after 9 days of incubation of PHO with 25 μg/ml ciprofloxacin, which resulted in Δlactate values of 70 to 80% (N. Duewelhenke, unpublished data). Fluoroquinolones may affect mitochondria via a mitochondrial DNA gyrase, the presence and functioning of which was demonstrated (10). Lawrence et al. postulated that the cytotoxicity of ciprofloxacin could be mediated by influencing mitochondrial topoisomerase II activity, resulting in loss of mitochondrial DNA (31, 32). The detrimental effect of fluoroquinolones on mitochondria could possibly be a cytotoxic effect comparable to the antibacterial mechanism due to inhibition of the gyrase. Tetracycline is known to inhibit bacterial protein synthesis as well as eukaryotic protein synthesis and protein synthesis of isolated mitochondria (39). Variations in the expected increased lactate production between experiments with PHO from different individuals might be caused by varying diffusion into cells derived from different individuals. Rifampin, the antibacterial action of which is based on the inhibition of bacterial RNA polymerases, does not seem to interfere with eukaryotic RNA polymerases (11), but it was shown to inhibit eukaryotic protein synthesis and RNA synthesis in mitochondria (8, 9). Probably both mechanisms contribute to the observed in vitro cytotoxicity.
Metabolic inhibitors and antibiotics have always affected the proliferation of PHO in lower concentrations than the metabolic activity, but the reverse is true for MG63 and HeLa cells. Both MG63 and HeLa cells are transformed immortalized stable cell lines which mainly aim to proliferate. Even if they were short of energy, they maintained their growth rate first of all, whereas the nontransformed and highly specialized primary human osteoblasts reacted differently by reducing cell proliferation and saving energy first. The reaction of PHO, and probably of other primary cells as well, reflects much better the reaction of normal body tissue on substances influencing energetics than do cell lines. However, mitochondria are not only responsible for the oxidative phosphorylation, they have also been shown to play a central role in activating apoptotic cell death (20) and regulation of calcium homeostasis. Since it had been shown that they can accumulate calcium, some evidence was provided that mitochondria could be involved in biological mineralization (4, 33, 44, 45). Because they could impair mitochondrial function, macrolides, fluoroquinolones, clindamycin, rifampin, tetracycline, chloramphenicol, and linezolid could be cytotoxic or cytostatic for bone cells in vivo after local administration, at least in high concentrations. Some of them might impair bone metabolism even in therapeutic serum concentrations. In vitro and animal experiments demonstrated a chondrotoxicity of fluoroquinolones (7, 17) and an impairment of fracture repair in early stages (23). An inhibition of proliferation of osteoblast-like cells in vitro in low concentrations of rifampin was reported before (25a) and should be investigated further, because rifampin proved to be an effective agent for treating bacteria in biofilms, which is extremely important in the pathogenesis of bone and joint infections. Although aminoglycosides, the most frequently used antibiotics for local treatment of bone infections, are known to affect mitochondrial protein synthesis (14), we did not find any effect on PHO or the cell lines. This is in contrast to reported in vitro detrimental effects of gentamicin on osteoblasts and bone tissue (25, 37). Possible differences in the effect on osteoblasts may depend on the state of differentiation, expressed surface receptors, and time of incubation. The ICs presented here were determined for 48 h of incubation, but additionally we demonstrated that ICs are principally dependent on the experimental setup and, probably because of active uptake, some antibiotics can accumulate intracellularly and may reach cytotoxic concentrations after prolonged administration of low concentrations.
Acknowledgments
We thank Bayer AG for kindly providing moxifloxacin as a compound, GlaxoSmithKline GmbH & Co. KG for providing amoxicillin and flucloxacillin as compounds, and Pfizer GmbH for providing azithromycin and linezolid as compounds.
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Adams, K., L. Couch, G. Cierny, J. Calhoun, and J. T. Mader. 1992. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin. Orthop. Relat. Res. 278:244-252. [PubMed] [Google Scholar]
- 2.Apodaca, A. A., and R. M. Rakita. 2003. Linezolid-induced lactic acidosis. N. Engl. J. Med. 348:86-87. [DOI] [PubMed] [Google Scholar]
- 3.Berridge, M. V., and A. S. Tan. 1993. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303:474-482. [DOI] [PubMed] [Google Scholar]
- 4.Bordat, C., J. L. Guerquin-Kern, M. Lieberherr, and G. Cournot. 2004. Direct visualization of intracellular calcium in rat osteoblasts by energy-filtering transmission electron microscopy. Histochem. Cell Biol. 121:31-38. [DOI] [PubMed] [Google Scholar]
- 5.Bronner, F., and M. C. Farach-Carson. 2004. Bone formation. Springer-Verlag, Heidelberg, Germany.
- 6.Buchholz, H. W., and H. Engelbrecht. 1970. Depot effects of various antibiotics mixed with Palacos resins. Chirurg 41:511-515. [PubMed] [Google Scholar]
- 7.Burkhardt, J. E., M. A. Hill, and W. W. Carlton. 1992. Morphologic and biochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin. Toxicol. Pathol. 20:246-252. [DOI] [PubMed] [Google Scholar]
- 8.Buss, W. C., and E. Kun. 1978. Effects of rifampicin on RNA and protein synthesis in isolated rat liver mitochondria. Biochem. Pharmacol. 27:2139-2145. [DOI] [PubMed] [Google Scholar]
- 9.Buss, W. C., R. Morgan, J. Guttmann, T. Barela, and K. Stalter. 1978. Rifampicin inhibition of protein synthesis in mammalian cells. Science 200:432-434. [DOI] [PubMed] [Google Scholar]
- 10.Castora, F. J., F. F. Vissering, and M. V. Simpson. 1983. The effect of bacterial DNA gyrase inhibitors on DNA synthesis in mammalian mitochondria. Biochim. Biophys. Acta 740:417-427. [DOI] [PubMed] [Google Scholar]
- 11.Clark, R. J. 1971. Antiviral action of rifampin. N. Engl. J. Med. 284:675. [DOI] [PubMed] [Google Scholar]
- 12.Corallo, C. E., and A. E. Paull. 2002. Linezolid-induced neuropathy. Med. J. Aust. 177:332. [DOI] [PubMed] [Google Scholar]
- 13.D'Aurelio, M., P. M. Merlo, L. Catani, G. L. Sgarbi, C. Bovina, G. Formiggini, C. G. Parenti, H. Baum, S. Tura, and G. Lenaz. 2001. Decreased Pasteur effect in platelets of aged individuals. Mech. Ageing Dev. 122:823-833. [DOI] [PubMed] [Google Scholar]
- 14.Davey, P. J., J. M. Haslam, and A. W. Linnane. 1970. Biogenesis of mitochondria. 12. The effects of aminoglycoside antibiotics on the mitochondrial and cytoplasmic protein-synthesizing systems of Saccharomyces cerevisiae. Arch. Biochem. Biophys. 136:54-64. [DOI] [PubMed] [Google Scholar]
- 15.DeZengotita, V. M., W. M. Miller, J. G. Aunins, and W. Zhou. 2000. Phosphate feeding improves high-cell-concentration NS0 myeloma culture performance for monoclonal antibody production. Biotechnol. Bioeng. 69:566-576. [DOI] [PubMed] [Google Scholar]
- 16.Edin, M. L., T. Miclau, G. E. Lester, R. W. Lindsey, and L. E. Dahners. 1996. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin. Orthop. 333:245-251. [PubMed] [Google Scholar]
- 17.Egerbacher, M., G. Seiberl, B. Wolfesberger, and I. Walter. 2000. Ciprofloxacin causes cytoskeletal changes and detachment of human and rat chondrocytes in vitro. Arch. Toxicol. 73:557-563. [DOI] [PubMed] [Google Scholar]
- 18.Freshney, R. I. 1994. Culture of animal cells. Wiley-Liss, New York, N.Y.
- 19.Gray, M. W., G. Burger, and B. F. Lang. 1999. Mitochondrial evolution. Science 283:1476-1481. [DOI] [PubMed] [Google Scholar]
- 20.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 21.Green, S. L., J. C. Maddox, and E. D. Huttenbach. 2001. Linezolid and reversible myelosuppression. JAMA 285:1291. [DOI] [PubMed] [Google Scholar]
- 22.Hand, W. L., and N. L. King-Thompson. 1982. Membrane transport of clindamycin in alveolar macrophages. Antimicrob. Agents Chemother. 21:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huddleston, P. M., J. M. Steckelberg, A. D. Hanssen, M. S. Rouse, M. E. Bolander, and R. Patel. 2000. Ciprofloxacin inhibition of experimental fracture healing. J. Bone Joint Surg. Am. 82:161-173. [DOI] [PubMed] [Google Scholar]
- 24.Hutchinson, D. K. 2003. Oxazolidinone antibacterial agents: a critical review. Curr. Top. Med. Chem. 3:1021-1042. [DOI] [PubMed] [Google Scholar]
- 25.Isefuku, S., C. J. Joyner, and A. H. Simpson. 2003. Gentamicin may have an adverse effect on osteogenesis. J. Orthop. Trauma. 17:212-216. [DOI] [PubMed] [Google Scholar]
- 25a.Isefuku, S., C. J. Joyner, and A. H. Simpson. 2001. Toxic effect of rifampicin on human osteoblast-like cells. J. Orthop. Res. 19:950-954. [DOI] [PubMed] [Google Scholar]
- 26.Jaiswal, N., S. E. Haynesworth, A. I. Caplan, and S. P. Bruder. 1997. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell Biochem. 64:295-312. [PubMed] [Google Scholar]
- 27.Kanellakopoulou, K., and E. J. Giamarellos-Bourboulis. 2000. Carrier systems for the local delivery of antibiotics in bone infections. Drugs 59:1223-1232. [DOI] [PubMed] [Google Scholar]
- 28.Kartsogiannis, V., and K. W. Ng. 2004. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell Endocrinol. 228:79-102. [DOI] [PubMed] [Google Scholar]
- 29.Klemm, K. 1979. Gentamicin-PMMA-beads in treating bone and soft tissue infections. Zentralbl. Chir. 104:934-942. [PubMed] [Google Scholar]
- 30.Kroon, A. M., and B. C. Van den. 1983. Antibacterial drugs and their interference with the biogenesis of mitochondria in animal and human cells. Pharm. Weekbl. Sci. 5:81-87. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence, J. W., D. C. Claire, V. Weissig, and T. C. Rowe. 1996. Delayed cytotoxicity and cleavage of mitochondrial DNA in ciprofloxacin-treated mammalian cells. Mol. Pharmacol. 50:1178-1188. [PubMed] [Google Scholar]
- 32.Lawrence, J. W., S. Darkin-Rattray, F. Xie, A. H. Neims, and T. C. Rowe. 1993. 4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells. J. Cell Biochem. 51:165-174. [DOI] [PubMed] [Google Scholar]
- 33.Manston, J., and E. Katchburian. 1984. Demonstration of mitochondrial mineral deposits in osteoblasts after anhydrous fixation and processing. J. Microsc. 134:177-182. [DOI] [PubMed] [Google Scholar]
- 34.Moestrup, S. K., S. Cui, H. Vorum, C. Bregengard, S. E. Bjorn, K. Norris, J. Gliemann, and E. I. Christensen. 1995. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J. Clin. Investig. 96:1404-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montenez, J. P., F. Van Bambeke, J. Piret, R. Brasseur, P. M. Tulkens, and M. P. Mingeot-Leclercq. 1999. Interactions of macrolide antibiotics (erythromycin A, roxithromycin, erythromycylamine [dirithromycin], and azithromycin) with phospholipids: computer-aided conformational analysis and studies on acellular and cell culture models. Toxicol. Appl. Pharmacol. 156:129-140. [DOI] [PubMed] [Google Scholar]
- 36.Muscari, C., C. Gamberini, F. Bonafe', E. Giordano, C. Bianchi, G. Lenaz, and C. M. Caldarera. 2004. Evaluation of cellular energetics by the Pasteur effect in intact cardiomyoblasts and isolated perfused hearts. Mol. Cell Biochem. 258:91-97. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen, J. G., and B. Lund. 1988. Effects of gentamicin and monomer on bone. An in vitro study. J. Arthroplasty 3(Suppl.):S63-S68. [DOI] [PubMed] [Google Scholar]
- 38.Reyes, M., T. Lund, T. Lenvik, D. Aguiar, L. Koodie, and C. M. Verfaillie. 2001. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 98:2615-2625. [DOI] [PubMed] [Google Scholar]
- 39.Riesbeck, K., A. Bredberg, and A. Forsgren. 1990. Ciprofloxacin does not inhibit mitochondrial functions but other antibiotics do. Antimicrob. Agents Chemother. 34:167-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robey, P. G., and J. D. Termine. 1985. Human bone cells in vitro. Calcif. Tissue Int. 37:453-460. [PubMed] [Google Scholar]
- 41.Sakaguchi, Y., I. Sekiya, K. Yagishita, S. Ichinose, K. Shinomiya, and T. Muneta. 2004. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood 104:2728-2735. [DOI] [PubMed] [Google Scholar]
- 42.Salvioli, S., G. Storci, M. Pinti, D. Quaglino, L. Moretti, M. Merlo-Pich, G. Lenaz, S. Filosa, A. Fico, M. Bonafe, D. Monti, L. Troiano, M. Nasi, A. Cossarizza, and C. Franceschi. 2003. Apoptosis-resistant phenotype in HL-60-derived cell HCW-2 is related to changes in expression of stress-induced proteins that impact on redox status and mitochondrial metabolism. Cell Death Differ. 10:163-174. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz, C., J. Hilpert, C. Jacobsen, C. Boensch, E. I. Christensen, F. C. Luft, and T. E. Willnow. 2002. Megalin deficiency offers protection from renal aminoglycoside accumulation. J. Biol. Chem. 277:618-622. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro, I. M., E. E. Golub, S. Kakuta, J. Hazelgrove, J. Havery, B. Chance, and P. Frasca. 1982. Initiation of endochondral calcification is related to changes in the redox state of hypertrophic chondrocytes. Science 217:950-952. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro, I. M., and J. S. Greenspan. 1969. Are mitochondria directly involved in biological mineralisation? Calcif. Tissue Res. 3:100-102. [DOI] [PubMed] [Google Scholar]
- 46.Sottile, V., C. Halleux, F. Bassilana, H. Keller, and K. Seuwen. 2002. Stem cell characteristics of human trabecular bone-derived cells. Bone 30:699-704. [DOI] [PubMed] [Google Scholar]
- 47.Tyteca, D., A. Schanck, Y. F. Dufrene, M. Deleu, P. J. Courtoy, P. M. Tulkens, and M. P. Mingeot-Leclercq. 2003. The macrolide antibiotic azithromycin interacts with lipids and affects membrane organization and fluidity: studies on Langmuir-Blodgett monolayers, liposomes and J774 macrophages. J. Membr. Biol. 192:203-215. [DOI] [PubMed] [Google Scholar]
- 48.Villa, P., D. Sassella, M. Corada, and I. Bartosek. 1988. Toxicity, uptake, and subcellular distribution in rat hepatocytes of roxithromycin, a new semisynthetic macrolide, and erythromycin base. Antimicrob. Agents Chemother. 32:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wahlig, H., and E. Dingeldein. 1980. Antibiotics and bone cements. Experimental and clinical long-term observations. Acta Orthop. Scand. 51:49-56. [DOI] [PubMed] [Google Scholar]
- 50.Walenkamp, G. H. I. M. 2001. Two-Stage revision of infected arthroplasty, p. 143-146. In G. H. I. M. Walenkamp and D. W. Murray (ed.), Bone cements and cementing technique. Springer Verlag, Berlin, Germany.