Abstract
In the course of Bacillus anthracis infection, B. anthracis lethal factor (LF) and edema factor bind to a protective antigen (PA) associated with cellular receptors ANTXR1 (TEM8) or ANTXR2 (CMG2), followed by internalization of the complex via receptor-mediated endocytosis. A new group of potential antianthrax drugs, β-cyclodextrins, has recently been described. A member of this group, per-6-(3-aminopropylthio)-β-cyclodextrin (AmPrβCD), was shown to inhibit the toxicity of LF in vitro and in vivo. In order to determine which steps in lethal factor trafficking are inhibited by AmPrβCD, we developed two targeted fluorescent tracers based on LFn, a catalytically inactive fragment of LF: (i) LFn site specifically labeled with the fluorescent dye AlexaFluor-594 (LFn-Al), and (ii) LFn-decorated liposomes loaded with the fluorescent dye 8-hydroxypyrene-1,3,6-trisulfonic acid (LFn-Lip). Both tracers retained high affinity to PA/ANTXR complexes and were readily internalized via receptor-mediated endocytosis. Using fluorescent microscopy, we found that AmPrβCD inhibits receptor-mediated cell uptake but not the binding of LFn-Al to PA/ANTXR complexes, suggesting that AmPrβCD works outside the cell. Moreover, AmPrβCD and LFn-Al synergistically protect RAW 264.7 cells from PA-mediated LF toxicity, confirming that AmPrβCD did not affect the binding of LFn-Al to receptor-associated PA. In contrast, AmPrβCD did not inhibit PA-mediated internalization of LFn-Lip, suggesting that multiplexing of LFn on the liposomal surface overcomes the inhibiting effects of AmPrβCD. Notably, internalized LFn-Al and LFn-Lip protected cells that overexpressed anthrax receptor TEM8 from PA-induced, LF-independent toxicity, suggesting an independent mechanism for PA inhibition inside the cell. These data suggest the potential for the use of β-cyclodextrins in combination with LFn-Lip loaded with antianthrax drugs against intracellular targets.
Bacillus anthracis, the gram-positive bacterium that causes anthrax, produces two bipartite AB-type toxins comprising protective antigen (PA) and either lethal factor (LF) or edema factor (EF). According to a current mechanistic model of the toxicity of B. anthracis (39), the 83-kDa PA can bind to two different receptors on the target cells: tumor endothelial marker 8 (ANTXR1 or TEM8), which is expressed on epithelial cells of the skin, lung, and intestine (11, 12), and capillary morphogenesis protein 2 (ANTXR2 or CMG2), which is widely expressed in different tissues (38). Upon binding to cellular receptors, PA is cleaved by a furin-like protease(s), and the resulting 63-kDa PA fragment forms a bagel-like heptameric prepore that can bind to up to three molecules of LF, forming lethal toxin (LeTx), or EF, resulting in edema toxin. The complex is then internalized via receptor-mediated endocytosis and is trafficked to the endosomal compartment, where, under mild acidic conditions, PA undergoes a conformational transition from a prepore to a pore, leading to the formation of a 14-stranded, membrane-spanning β-barrel through which LF and EF are released into the cytosol (for a review, see reference 16).
Since targets on the cell surface are more accessible to drugs than intracellular targets, several strategies for inhibition of PA receptor binding and proteolytic cleavage, PA oligomerization, and binding of LF and/or EF to the PA prepore have been proposed (34, 36, 40). Recently, it has been reported that a β-cyclodextrin derivative, per-6-(3-aminopropylthio)-β-cyclodextrin (AmPrβCD), inhibits the conductance of the PA pore reconstituted in a bilayer lipid membrane and inhibits LF toxicity in vitro and in vivo, suggesting that AmPrβCD might inhibit translocation inside the cell (24, 25). However, AmPrβCD activity might be due to the inhibition of earlier steps that take place on the cell surface, such as the binding of LF to the PA prepore or internalization of the ligand-receptor complex.
To establish whether the PA function is inhibited inside or outside the cell, we developed two targeted fluorescent tracers based on LFn, a catalytically inactive fragment of LF capable of PA-dependent binding and internalization into cells (2). LFn expressed with a C-terminal Cys tag for site-specific modification (4) was labeled either with the fluorescent dye AlexaFluor-594, which yielded the LFn-Al tracer, or with polyethylene glycol-modified phospholipid to decorate liposomes loaded with the membrane-impermeant fluorescent dye 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS), which yielded the LFn-Lip tracer. We found that both fluorescent tracers retained high affinities to PA/receptor complexes and underwent TEM8- and CMG2-mediated endocytosis, providing new opportunities for the “tagging” of cells expressing these receptors. Although LFn was previously used for the intracellular delivery of fused protein fragments (31, 32), we were surprised to find that LFn also allows the intracellular delivery of significantly larger liposomes.
We report here that AmPrβCD inhibits receptor-mediated cell uptake of PA-bound LFn-Al by both receptors, suggesting that AmPrβCD acts via an extracellular mechanism(s). However, AmPrβCD does not inhibit receptor-mediated endocytosis of LFn-Lip, suggesting that multiplexing of LFn on the liposome surface overcomes the effects of AmPrβCD on PA/receptor complexes. Since internalized LFn-Lip protects cells that overexpress the B. anthracis receptor TEM8 from PA-induced, LF-independent toxicity, we hypothesize that LFn-Lip inhibits PA intracellularly. These data suggest the potential for the use of β-cyclodextrins in combination with LFn-Lip loaded with antianthrax drugs against intracellular targets.
MATERIALS AND METHODS
Materials.
PA and LF (List Biological, Campbell, CA) were reconstituted in 5 mM HEPES (pH 7.5)-50 mM NaCl-0.1% bovine serum albumin to final concentrations of 1 mg/ml and stored at −70°C in small aliquots. AmPrβCD was custom synthesized by Pinacle Pharmaceuticals (Charlottesville, VA). AlexaFluor-594 maleimide was from Molecular Probes (Eugene, OR). Poly(ethyleneglycol)-α-distearoyl phosphatidylethanolamine-ω-maleimide (DSPE-mPEG-maleimide; 3,400 kDa) was from Nectar Therapeutics. The fluorescent dye HPTS, trisodium salt, was from Sigma. The APO screening kit and caspase-2 fluorogenic substrate were from CalBiochem. The Cell Probe HT Caspase3/7 whole-cell assay kit was from Beckman Coulter.
Synthesis of LFn-based fluorescent tracers.
The construction of LFn fused to a C-terminal Cys tag was described previously (4). An additional His tag was introduced by cloning the Cys-tagged LFn open reading frame into the NcoI and SalI sites of the pET/28b(+) vector (Novagen). The resulting protein, LFn-Cys, was expressed in Escherichia coli BL21(DE3) and was purified as described previously (4), with an additional metal affinity chromatography step on His resin (Novagen) done according to the manufacturer's instructions. The yield of LFn-Cys was ∼20 mg/liter of more than 98% pure protein, as judged by reverse-phase high-pressure liquid chromatography (RP-HPLC) analysis, as described previously (5). For site-specific conjugation of AlexaFluor-594, LFn-Cys (25 nmol) was mixed with AlexaFluor-594 maleimide to a final protein-to-dye molar ratio of 1:2 in a volume of 0.1 ml of 50 mM NaPi (pH 7.2). The reaction mixture was incubated for 30 min and was purified by desalting (PD-10 column; GE Healthcare). The extent of AlexaFluor-594 modification was analyzed by RP-HPLC on a C4 column and was calculated as the ratio of integral peak intensities at 216 nm (for protein) and 598 nm (for AlexaFluor-594). Preparation of HPTS-loaded liposomes and modification of LFn-Cys with DSPE-mPEG-maleimide were performed as described previously (4, 6). Briefly, a twofold molar excess of mPEG-DSPE maleimide was added to LFn, and the mixture was incubated for 10 min at room temperature and then mixed with an equal volume of preformed HPTS-loaded liposomes. After a 16-h incubation at 37°C, LFn-Lip was purified by gel filtration on Sepharose 4B and was characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and RP-HPLC. An average liposome preparation contained 1 to 2 μM LFn, which corresponded to 50 to 100 LFn molecules per liposome. LFn-Lip was stored at 4°C for several weeks without any loss of LFn functional activity.
Cells.
CHO-K1 ovary hamster cells (CCL-61) and RAW 264.7 mouse monocytes (TIB-71) were from ATCC (Manassas, VA). TEM8-overexpressing cells were made by stable transfection of CHO-K1 cells with plasmid pEF6/V5-His-TOPO/TEM8 (kindly provided by B. Terman, Albert Einstein School of Medicine, Bronx, NY), which encodes the full-length human TEM8 splice variant 1 fused to c-myc, V5, and six-His-tagged epitopes (22). Lipofectamine 2000 reagent (Gibco Life Technologies) was used for transfection. Selected blasticidine-resistant clones were screened for TEM8 expression by Western blotting with a c-myc-specific monoclonal antibody (Invitrogen). A clone with the highest level of TEM8 expression was designated CHO-TEM8 and was used for this work. CHO-K1 and CHO-TEM8 cells were grown in F-12 medium. RAW 264.7 cells were grown in Dulbecco minimal essential medium. Both media included 10% fetal bovine serum (HyClone), 2 mM l-glutamine, and antibiotics. The cells were maintained at 37°C in 5% CO2.
Microscopy.
The cells were incubated in complete culture medium containing 8 nM PA, with or without AmPrβCD (10 or 100 μM), for 15 min at 37°C. The cells either were supplemented with an LFn tracer, followed by incubation at 37°C for 1 h, or were shifted to 4°C for 15 min first and then supplemented with LFn tracer for a 1-h incubation at 4°C. After the incubations with the LFn tracers, the cells were washed twice with phosphate-buffered saline (PBS), fixed in 4% formaldehyde (Polysciences, Warrington, PA) for 5 min at room temperature, and mounted in mounting medium for fluorescence with 4′,6′-diamidino-2-phenylindole (DAPI) or propidium iodide (Vector Labs) for nuclear counterstaining. Digitized images were captured on a Zeiss Axiovert 2000 fluorescent microscope and a Zeiss LSM 510 confocal microscope.
Caspase activation assay.
CHO-TEM8 cells were plated in 75-cm2 flasks at 1.5 × 106 cells/flask. Twenty hours later, PA was added to a final concentration of 30 nM, either alone or in combination with 10 μM AmPrβCD. The cells were incubated for 24 h under normal culture conditions and then harvested in 2 mM EDTA-PBS, spun down, and lysed at 4°C for 5 min in a lysis buffer (250 mM sucrose, 20 mM HEPES [pH 7.4], 1% Nonidet P-40, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 25 μg/ml antitrypsin, 25 μg/ml aprotinin). The cell lysates were clarified by centrifugation (10,000 × g for 2 min) and supplemented with 2 mM dithiothreitol to preserve the caspase enzymatic activity. The total protein in the cytosolic fractions was determined by a protein assay (Bio-Rad, Hercules, CA). Aliquots containing 40 μg of cytosolic protein were analyzed in a final volume of 100 μl of caspase activity buffer (0.1 M HEPES [pH 7.4], 2 mM dithiothreitol, 0.1% Nonidet P-40, 1% sucrose) containing 50 μM caspase substrate. The reaction mixtures were incubated for 1 h at 37°C, and fluorescence was measured at an excitation λ of 400 nm and an emission λ of 505 nm.
RESULTS
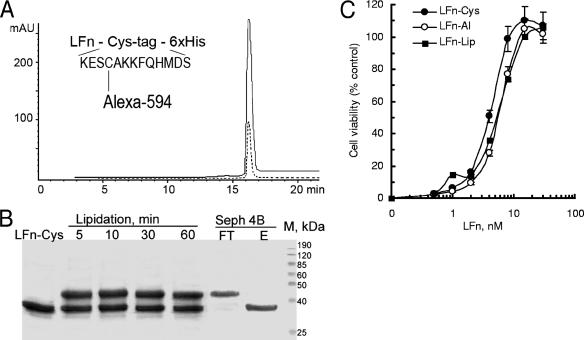
To develop LFn-based fluorescent tracers, we fused LFn to a 15-amino-acid cysteine-containing Cys tag for site-specific modification (4) and to a His tag for purification (Fig. 1A, inset). Given the crucial importance of the N-terminal portion of LFn for binding to PA (45), we chose the C terminus for tag fusion. Wild-type LFn does not contain cysteine residues, and the only reactive thiol group in the LFn-Cys fusion protein is provided by a cysteine at position 4 of the C-terminal Cys tag. To obtain LFn-Al, LFn-Cys was modified with a thiol-reactive fluorescent dye, AlexaFluor-594 maleimide, and was purified by gel filtration. RP-HPLC analysis confirmed a 1:1 ratio of protein to dye in the conjugate LFn-Al (Fig. 1A). To obtain LFn-Lip, LFn-Cys was lipidated with a thiol-reactive polyethylene glycol-modified phospholipid, mPEG-DSPE maleimide, yielding ∼50% of the modified protein within minutes (Fig. 1B). For insertion of lipidated LFn-Cys into the liposomal membrane, the lipidation reaction mixture was added to preformed liposomes loaded with HPTS, a membrane-impenetrable fluorescent dye; and the mixture was incubated for 16 h at 37°C, followed by purification of the resulting LFn-Lip from free LFn-Cys by gel filtration on Sepharose 4B (Fig. 1B).
FIG. 1.
Site-specific modification does not affect the ability of LFn to bind to cell-associated PA. (A) Purified LFn-Al was analyzed by RP-HPLC with detection at 280 nm for protein (solid line) and 598 nm for AlexaFluor-594 (dotted line). (Inset) LFn-Al with a single cysteine available for SH-directed modification. mAU, milli-absorption units. (B) LFn-Lip made by LFn-Cys lipidation, followed by insertion into preformed liposomes. The samples were analyzed by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 17.5% gel, followed by staining with SafeBlue (Bio-Rad). Purification was on a Sepharose 4B column. FT, liposome-containing flowthrough fraction; E, elution fraction containing free LFn-Cys. (C) Competition of LFn-based tracers with LF to PA associated with RAW 264.7 cells. Cells were plated on 96-well plates at 15 × 103 cells/well 20 h before the experiment. Various amounts of an LFn tracer were mixed with LF and PA in complete culture medium; and the mixture, which contained PA at a final concentration of 2 nM and LF at a final concentration of 0.2 nM, was added to cells in triplicate wells. After 3 h of incubation at 37°C in 5% CO2, viable cell numbers were determined by use of a CellTiter 96 AQueous One Solution cell proliferation assay kit (Promega).
The specificity of binding to PA was established for both LFn-based tracers by assaying their competition with full-length LF for binding to cellular PA/ANTXR complexes (the assay has been described previously [4]). In this assay, various amounts of either tracer or unmodified LFn-Cys are added to RAW 264.7 cells exposed to LeTx formed by the combination of LF and PA. LeTx is highly toxic for RAW 264.7 cells, and therefore, the ability of nontoxic LFn to compete with full-length LF for binding to cell-associated PA is readily detected by the number of viable RAW 264.7 cells after a 3-h exposure to LeTx. We found that under selected conditions, unmodified LFn-Cys rescued RAW 264.7 cells with a 50% inhibitory concentration (IC50) of 3.5 ± 0.4 nM, which is close to the activity of LFn (IC50, ∼3 nM) reported earlier (4). Notably, both LFn-Al and LFn-Lip tracers displayed similar activities in this assay, with an IC50 of 4.3 ± 0.3 nM (Fig. 1C), indicating that site-specific C-terminal attachment of either a highly charged fluorescent dye or a bulky liposome did not dramatically affect the binding of LFn-Cys to cell-associated PA.
To monitor PA-mediated LFn-Al internalization, we initially tested two ANTXR-positive cell lines: RAW 264.7 mouse monocytes expressing CMG-2 (8) and CHO-K1 hamster ovary fibroblasts expressing an unidentified ANTXR (12, 30). Receptor-mediated endocytosis was observed by fluorescent microscopy of cells incubated with LFn-Al in the presence of PA, while cells incubated with LFn-Al alone served as a control for nonspecific uptake. We found that punctate AlexaFluor-594 fluorescence was readily detectable in RAW 264.7 cells in the presence of PA but not in the absence of PA, indicating PA/CMG2-mediated endocytosis of the tracer (Fig. 2A). However, CHO-K1 cells did not accumulate detectable amounts of AlexaFluor-594 (data not shown), suggesting that these cells do not express sufficient levels of PA receptors. Indeed, according to a recent report (21), wild-type CHO cells can internalize only ∼800 LF molecules per cell. Therefore, for this study we developed CHO-K1 cells overexpressing TEM8. A selected clone with the highest level of expression of TEM8, named CHO-TEM8, demonstrated PA-dependent uptake of LFn-Al (Fig. 2A) and was used for further studies. It should be noted that a prolonged incubation (1 h) with LFn-Al at 37°C was necessary to obtain detectable levels of the fluorescent probe in both cell types, while no cell-associated fluorescence was detected after a shorter (15-min) incubation (data not shown).
FIG. 2.
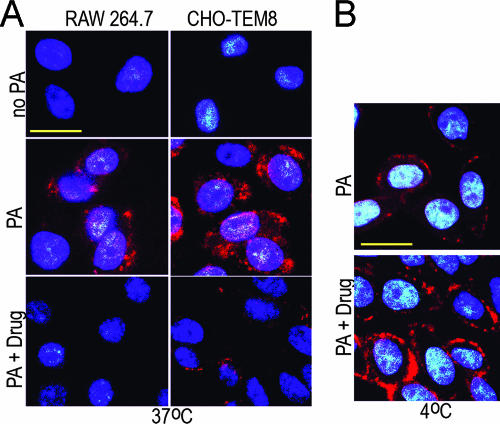
AmPrβCD inhibits internalization rather than binding of LFn-Al to PA/ANTXR complex. Cells were plated on glass coverslips at 105 cells/coverslip and 20 h later were shifted to fresh culture medium (no PA), medium supplemented with 8 nM PA alone (PA), or PA in combination with 100 μM AmPrβCD (PA + Drug) and preincubated for 15 min at 37°C. LFn-Al was added to a final concentration of 2 nM, and cells were incubated for 1 h at 37°C (A) or 4°C (B). Blue, DAPI nuclear counterstaining; red, AlexaFluor-594. Scale bars, 20 μm.
To establish the effects of AmPrβCD on the uptake of LFn-Al, RAW 264.7 and CHO-TEM8 cells were preincubated with PA alone or PA and AmPrβCD at 37°C and were then incubated with LFn-Al for 1 h at 37°C. A dramatic decrease in the level of intracellular fluorescence was observed in both cell types in the presence of 10 μM and 100 μM AmPrβCD (Fig. 2A for 100 μM AmPrβCD), indicating that either binding or uptake was inhibited by the drug. Given the relatively low affinity of LFn to PA even at room temperature (Kd, ∼1 nM) (19), the lack of membrane-bound LFn-Al at 37°C in the presence of the drug might be temperature dependent rather than drug dependent. We therefore tested how AmPrβCD affects the binding of LFn-Al at 4°C, at which the LFn/PA complexes are three- to sevenfold more stable than they are at room temperature (19). CHO-TEM8 cells were preincubated at 37°C with PA alone or PA with 10 μM AmPrβCD, shifted to 4°C for 15 min, and then supplemented with LFn-Al and incubated for 1 h at 4°C. In both systems, membrane binding of LFn-Al was readily detectable as a distinct band around significantly smaller DAPI-stained nuclei (Fig. 2B). Judging by the fluorescence intensity, not only did AmPrβCD fail to inhibit but, in fact, it stimulated LFn-Al binding to PA/TEM8 (Fig. 2B).
In similar experiments performed with RAW 264.7 cells, binding of LFn-Al to RAW 264.7 cells at 4°C was not detectable (data not shown). Although this lack of binding was, most likely, due to the low level of the endogenous CMG2, we could not rule out a possibility that AmPrβCD might directly inhibit binding of this tracer to PA/CMG2. We therefore used the protection assay described above (Fig. 1C) to compare the effects of AmPrβCD alone or in combination with LFn-Al on the CMG2-mediated toxicity of LeTx. We reasoned that if AmPrβCD does not affect PA-mediated LFn-Al binding, a combination of these two compounds might be more effective than each one alone. In our standard LeTx protection assay (0.2 nM LF, 2 nM PA, 3-h exposure), AmPrβCD alone protected RAW 264.7 cells at micromolar concentrations, with an IC50 of ∼7 μM (Fig. 3A), while LFn-Al was active in this assay, with an IC50 of 4 nM (Fig. 1C). To evaluate the relationship between the effects of LFn-Al and AmPrβCD, if any, we used an approach pioneered by Berenbaum to analyze whether the effects of drugs given simultaneously are additive, synergistic, or antagonistic (9). AmPrβCD was mixed with LFn-Al at the corresponding working concentrations, resulting in a molar ratio of 2,000:1; serially diluted in complete culture medium; and added to cells exposed to LeTx. We found that the combination of two compounds was significantly more effective in this assay than each compound alone, with IC50 values of ∼1.2 μM for AmPrβCD and ∼0.6 nM for LFn-Al. Fractional IC50 values (the ratio of the IC50 for a drug in a combination to the IC50 for a drug alone) were determined to be 0.17 for each compound (Fig. 3A; compare Fig. 3A to Fig. 1C). Since the sum of fractional IC50 values was significantly less than 2, our results, according to Berenbaum (9), indicated synergism between LFn-A and AmPrβCD, which excludes the possibility that AmPrβCD inhibited the binding of LFn-Al to PA/CMG2.
FIG. 3.
AmPrβCD-mediated cell rescue is synergistic when AmPrβCD is used in combination with LFn and PA specific. (A) Competition with full-length LF was done as described in the legend to Fig. 1C. AmPrβCD (Am-CD) alone or in combination with LFn-Al at a molar ratio of 1,000:1 was mixed with LF and PA, serially diluted, and added to the cells. (B) VEGFR-2 expressing 293/KDR cells were plated on 96-well plates at 1,000 cells/well. Twenty hours later, VEGF or AmPrβCD was serially diluted in complete culture medium containing SLT-VEGF and added to cells in triplicate wells to a final SLT-VEGF concentration of 1 nM. After 96 h of incubation at 37°C in 5% CO2, viable cell numbers were determined by use of a CellTiter 96 kit.
Since our data indicated that AmPrβCD acted outside of the cell, it was conceivable that, due to its high positive charge, AmPrβCD could bind to the cell surface and nonspecifically affect the endocytosis of many different cellular receptors. We therefore tested whether AmPrβCD could inhibit the endocytosis of a B. anthracis-unrelated ligand/receptor complex. For these experiments we used SLT-VEGF, a chimeric toxin comprising full-length Shiga-like toxin (SLT) subunit A fused to vascular endothelial growth factor (VEGF), as described elsewhere (3). SLT-VEGF is internalized via VEGF receptor-mediated endocytosis and is highly toxic for 293/KDR cells expressing VEGF receptor VEGFR-2. AmPrβCD was serially diluted in complete culture medium containing 1 nM SLT-VEGF and was added to 293/KDR cells at the concentration range used for the protection of RAW 264.7 cells from LeTx (Fig. 3A). As a positive control for cell rescue we used VEGF, which protects 293/KDR cells from the toxicity of SLT-VEGF by competing for VEGFR-2 binding (4, 5, 7). We found that AmPrβCD did not protect 293/KDR cells from SLT-VEGF-induced toxicity (Fig. 3B), indicating that its effects were limited to PA/ANTXR complexes.
To establish whether AmPrβCD inhibition of PA-mediated LF uptake depends on the presence of LF in the complex, we used a novel PA cytotoxicity assay. While characterizing CHO-TEM8 cells, we found that PA alone was cytotoxic to these cells in a dose-dependent manner (Fig. 4A). Parental CHO-K1 cells were not sensitive to PA in the same concentration range (Fig. 4A), indicating a causative role of high-level TEM8 expression for PA-induced toxicity. A similar PA-induced/LF-independent toxicity has recently been reported for RAW 264.7 cells engineered to overexpress TEM8 (37). We found that PA-induced toxicity was completely blocked by 5 μM AmPrβCD (Fig. 4A). Using fluorogenic assays with panspecific and specific caspase substrates, we also found that AmPrβCD inhibited PA-induced apoptosis in CHO-TEM8 cells by blocking the overall caspase activity and, more specifically, initiator caspase-2 and executioner caspase-3 and caspase-7 (Fig. 4B).
FIG. 4.
Rescue of CHO-TEM8 cells from PA-induced death. (A) CHO-K1 and CHO-TEM8 cells were plated on 96-well plates at 2,000 cells/well and 20 h later were exposed to either PA alone or PA in the presence of 5 μM AmPrβCD (Am-CD). After 48 h of incubation under normal culture conditions, viable cell numbers were determined by use of CellTiter 96 kit. (B) Nearly confluent CHO-TEM8 cells were exposed to 30 nM PA alone or in the presence of 5 μM AmPrβCD, incubated for 24 h under normal culture conditions, and then analyzed for caspase activation, as described in Materials and Methods. (C) CHO-TEM8 cells were plated on 96-well plates at 2,000 cells/well. Twenty hours later, LFnLFn-Lip or liposomal controls (untargeted HPTS-loaded liposomes adjusted to the same HPTS concentration) were serially diluted in complete culture medium containing 12 nM PA and added to cells in triplicate wells. After 48 h of incubation at 37°C in 5% CO2, viable cell numbers were determined by use of a CellTiter 96 kit. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
The PA-induced toxicity observed for TEM8 overexpressing RAW 264.7 cells was tentatively attributed to the formation of a large number of pores by internalized PA in endosomal compartments (37). If this suggested mechanism is correct, LF and its derivatives should also rescue CHO-TEM8 cells from PA-induced toxicity by blocking the PA membrane channel, as was recently described for PA channels reconstituted in artificial lipid bilayer membranes (35). Indeed, we found that LFn and both LFn-targeted fluorescent tracers successfully rescued CHO-TEM8 cells in a similar dose-dependent manner (Fig. 4C for LFn-Lip). As expected, untargeted HPTS-loaded liposomes did not prevent cell death in this assay (Fig. 4C).
Judging by the ability to protect RAW 264.7 cells from LeTx (Fig. 1C) and CHO-TEM8 cells from PA-induced toxicity (Fig. 4C), the LFn-Lip tracer was capable of specific binding to the PA/ANTXR complex. Since LFn has been used for the intracellular delivery of fused protein fragments (31, 32), we could not exclude the possibility of the intracellular delivery of LFn-targeted liposomes. Indeed, confocal microscopy of CHO-TEM8 cells incubated with LFn-Lip and PA revealed the intracellular localization of fluorescent signals (see Fig. 5A for the reconstructed image; see Fig. S1 in the supplemental material for individual z sections). Similar results were obtained with RAW 264.7 cells, but with a smaller number of signals and weaker punctuate signals (data not shown). For both types of cells, intracellular fluorescent signals were negligible in the absence of PA or when untargeted HPTS-loaded liposomes were used (data not shown). Surprisingly, PA-dependent uptake of LFn-Lip was readily detected in both cell lines, regardless of the presence of AmPrβCD, clearly indicating that AmPrβCD did not inhibit the uptake of LFn-Lip (Fig. 5B). This unexpected result suggested that multiplexing of LFn might overcome AmPrβCD-induced inhibition of PA-mediated endocytosis.
FIG. 5.
LFn-Lip is internalized by ANTXR-expressing cells, regardless of the presence of AmPrβCD. Cells for microscopy were plated and preincubated with 8 nM PA with or without 100 μM AmPrβCD, as described in the legend to Fig. 2. (A) LFn-Lip was added to CHO-TEM8 cells to a final concentration of 2 nM LFn for 1 h at 37°C, and then cells that had been washed with PBS were fixed and observed. Green, HPTS fluorescence. (B) Incubation with 2 nM LFn-Lip with or without 8 nM PA and 100 μM AmPrβCD for 1 h at 37°C. Green, HPTS; blue, DAPI nuclear counterstaining. Scale bars, 20 μm.
DISCUSSION
Blocking of the heptameric PA pore with heptameric β-cyclodextrins appears to be a rational starting point for the development of effective antianthrax treatments. Recent data from Karginov et al. (25) indicate that β-cyclodextrins derivatized on a primary face with amino groups inhibited the toxicity of LeTx in vitro and provided protection against B. anthracis infection in highly susceptible Fischer F344 rats. Comparison of the in vitro activities of several amino-substituted β-cyclodextrins demonstrated structure-activity relationships within this class of compounds (24), supporting the hypothesis of specific interactions between β-cyclodextrins and PA. On the basis of the ability of AmPrβCD to inhibit ion currents through the PA pore reconstituted into a lipid bilayer, it was suggested that this compound might inhibit the translocation of LF or EF inside the cell through the PA pore formed in endosomes under mildly acidic conditions (24, 25). However, we report here that AmPrβCD selectively inhibits receptor-mediated endocytosis of the PA/LFn-Al complex by either of two B. anthracis receptors, TEM8 or CMG2. Furthermore, using a new assay for TEM8-mediated LF-independent PA cytotoxicity, we found that the action of AmPrβCD does not even require the binding of LF to the PA/TEM8 complex. Taken together, these data indicate that AmPrβCD inhibits the toxicity of LF by acting in a PA-specific manner on the cell surface rather than inside the cell. On the other hand, judging by the inhibition of PA-induced/LF-independent toxicity by LFn-Al and LFn-Lip, PA can also be inhibited inside the cells.
Given the known activities of cyclodextrins, AmPrβCD might inhibit the receptor-mediated endocytosis of PA or LeTx through two different mechanisms. In a “protein-centered” model, the binding of AmPrβCD to a protein component(s) of the complex could stabilize protein conformations that are poorly suitable for endocytosis (13, 41, 43; for a review, see reference 18). Alternatively, in a “membrane-centered” model, reversible binding of cyclodextrin to the PA/receptor complex might lead to the depletion of cholesterol from the underlying lipid raft, rendering the whole membrane region poorly suitable for endocytosis. Indeed, at millimolar concentrations, β-cyclodextrin derivatives are known to affect many membrane processes, including receptor-mediated endocytosis of viral and bacterial proteins, by sequestering cholesterol from lipid rafts (10, 14, 15, 17, 20, 26-29, 33, 44; for a review, see reference 18). In fact, Abrami et al. convincingly demonstrated that β-methyl cyclodextrin inhibits the lipid raft-mediated endocytosis of LF/PA (1). Judging by the relatively high IC50 of ∼7 μM, the binding of AmPrβCD is, most likely, reversible; and therefore, it might continuously deplete cholesterol from the lipid raft in the area of the PA/receptor complex. This putative mechanism, however, still requires a PA/AmPrβCD interaction, since the lipid raft-mediated internalization of VEGFR-2 (23) was not affected in the presence of AmPrβCD. Further experiments are in progress to discriminate between the “protein-centered” and “membrane-centered” mechanisms of AmPrβCD action and to accommodate the recent discovery that low-density lipoprotein receptor-related protein 6 (LRP6) acts as an accessory protein in toxin internalization (42).
Regardless of the mechanism, it is particularly interesting that cells take up liposomes targeted by LFn via a PA-mediated mechanism, despite the presence of AmPrβCD. It suggests that properly designed LFn-Lip loaded with specific inhibitors of LF or EF might be used together with cyclodextrins to target the same cells. Indeed, cyclodextrin would inhibit the PA-mediated internalization of LF and EF but not LFn-Lip, while internalized LFn-Lip might release drugs that inhibit LF and EF inside the same cell. It should be noted, however, that it would require a clear demonstration that the entrapped drugs are released into the cytosol, which might depend on the selective structure of the drug and the compositions of liposomes. We hypothesize that such a combination of a targeted drug and the targeted delivery of a different drug might provide significant therapeutic benefits for the treatment for B. anthracis infection.
Supplementary Material
Acknowledgments
We thank S. H. Leppla for critical review of the manuscript.
This work was supported by National Institutes of Health grant 1 R43AI054060-01.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Abrami, L., S. Liu, P. Cosson, Leppla, and S. H. F. G. van der Goot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora, N., and S. H. Leppla. 1993. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J. Biol. Chem. 268:3334-3341. [PubMed] [Google Scholar]
- 3.Backer, M. V., and J. M. Backer. 2001. Targeting endothelial cells overexpressing VEGFR-2: selective toxicity of Shiga-like toxin-VEGF fusion proteins. Bioconjugate Chem. 12:1066-1073. [DOI] [PubMed] [Google Scholar]
- 4.Backer, M. V., V. Patel, B. T. Jehning, and J. M. Backer. 2006. Self-assembled “dock & lock” system for linking payloads to targeting proteins. Bioconjugate Chem. 17:912-919. [DOI] [PubMed] [Google Scholar]
- 5.Backer, M. V., J. Elliot, T. I. Gaynutdinov, and J. M. Backer. 2004. Assembly of targeting complexes driven by a single-chain antibody. J. Immunol. Methods 289:35-43. [DOI] [PubMed] [Google Scholar]
- 6.Backer, M. V., T. Gaynutdinov, V. Patel, B. Jehning, E. Myshkin, and J. M. Backer. 2004. Adapter protein for site-specific conjugation of payloads for targeted drug delivery. Bioconjugate Chem. 15:1021-1029. [DOI] [PubMed] [Google Scholar]
- 7.Backer, M. V., T. I. Gaynutdinov, V. Patel, A. K. Bandyopadhyaya, B. T. S. Thirumamagal, W. Tjarks, R. Barth, K. P. Claffey, and J. M. Backer. 2005. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol. Cancer Ther. 4:1423-1429. [DOI] [PubMed] [Google Scholar]
- 8.Banks, D. J., M. Barnajian, F. J. Maldonado-Arocho, A. M. Sanchez, and K. A. Bradley. 2005. Anthrax toxin receptor 2 mediates Bacillus anthracis killing of macrophages following spore challenge. Cell. Microbiol. 7:1173-1185. [DOI] [PubMed] [Google Scholar]
- 9.Berenbaum, M. C.. 1978. A method for testing synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 10.Bom, A., M. Bradley, K. Cameron, J. K. Clark, J. Van Egmond, H. Feilden, E. J. MacLean, A. W. Muir, R. Palin, D. C. Rees, and M. Q. Zhang. 2002. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew. Chem. Int. Ed. Engl. 41:266-270. [DOI] [PubMed] [Google Scholar]
- 11.Bonuccelli, G., F. Sotgia, P. G. Frank, T. M. Williams, C. J. de Almeida, H. B. Tanowitz, P. E. Scherer, K. A. Hotchkiss, B. I. Terman, B. Rollman, A. Alileche, J. Brojatsch, and M. P. Lisanti. 2005. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis' three sites of entry: implications for the pathogenesis of anthrax infection. Am. J. Physiol. Cell Physiol. 288:C1402-C1410. [DOI] [PubMed] [Google Scholar]
- 12.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. [DOI] [PubMed] [Google Scholar]
- 13.Brewster, M. E., J. W. Simpkins, M. S. Hora, W. C. Stern, and N. Bodor. 1989. The potential use of cyclodextrins in parenteral formulations. J. Parenter. Sci. Technol. 43:231-240. [PubMed] [Google Scholar]
- 14.Chark, D., A. Nutikka, N. Trusevych, J. Kuzmina, and C. Lingwood. 2004. Differential carbohydrate epitope recognition of globotriaosyl ceramide by verotoxins and a monoclonal antibody. Eur. J. Biochem. 271:405-417. [DOI] [PubMed] [Google Scholar]
- 15.Choi, K. S., H. Aizaki, and M. M. Lai. 2005. Murine coronavirus requires lipid rafts for virus entry and cell-cell fusion but not for virus release. J. Virol. 79:9862-9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 17.Danthi, P., and M. Chow. 2004. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J. Virol. 78:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis, M. E., and M. E. Brewster. 2004. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3:1023-1035. [DOI] [PubMed] [Google Scholar]
- 19.Elliott, J. L., J. Mogridge, and R. J. Collier. 2000. A quantitative study of the interactions of Bacillus anthracis edema factor and lethal factor with activated protective antigen. Biochemistry 39:6706-6713. [DOI] [PubMed] [Google Scholar]
- 20.Graham, D., E. Chertova, J. Hilburn, L. Arthur, and J. E. K. Hildreth. 2003. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with β-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J. Virol. 77:8237-8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobson, J. P., S. Liu, B. Rono, S. H. Leppla, and T. H. Bugge. 2006. Imaging specific cell-surface proteolytic activity in single living cells. Nat. Methods 3:259-261. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss, K. A., C. M. Basile, S. C. Spring, G. Bonuccelli, M. P. Lisanti, and B. I. Terman. 2005. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp. Cell Res. 305:133-144. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda, S., M. Ushio-Fukai, L. Zuo, T. Tojo, S. Dikalov, N. A. Patrushev, and R. W. Alexander. 2005. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circulation Res. 96:467-475. [DOI] [PubMed] [Google Scholar]
- 24.Karginov, V. A., A. Yohannes, T. M. Robinson, N. E. Fahmi, K. Alibek, and S. M. Hecht. 2006. β-Cyclodextrin derivatives that inhibit anthrax lethal toxin. Biorg. Med. Chem. 14:33-40. [DOI] [PubMed] [Google Scholar]
- 25.Karginov, V. A., E. M. Nestorovich, M. Moayeri, S. H. Leppla, and S. M. Bezrukov. 2005. Blocking anthrax lethal toxin at the protective antigen channel by using structure-inspired drug design. Proc. Natl. Acad. Sci. USA 102:15075-15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowalski, M. P., and G. B. Pier. 2004. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J. Immunol. 172:418-425. [DOI] [PubMed] [Google Scholar]
- 27.Labrecque, L., I. Royal, D. S. Surprenant, C. Patterson, D. Gingras, and R. Beliveau. 2003. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol. Biol. Cell 14:334-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lafont, F., G. Tran Van Nhieu, K. Hanada, P. Sansonetti, and F. G. van der Goot. 2002. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 21:4449-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao, Z., D. Graham, and J. E. K. Hildreth. 2003. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res. Hum. Retrovir. 19:675-687. [DOI] [PubMed] [Google Scholar]
- 30.Liu, S., and S. H. Leppla. 2003. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J. Biol. Chem. 278:5227-5234. [DOI] [PubMed] [Google Scholar]
- 31.McEvers, K., M. Elrefaei, P. Norris, S. Deeks, J. Martin, Y. Lu, and H. Cao. 2005. Modified anthrax fusion proteins deliver HIV antigens through MHC class I and II pathways. Vaccine 23:4128-4135. [DOI] [PubMed] [Google Scholar]
- 32.Milne, J. C., S. R. Blanke, P. C. Hanna, and R. J. Collier. 1995. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol. Microbiol. 15:661-666. [DOI] [PubMed] [Google Scholar]
- 33.Miyata, S., J. Minami, E. Tamai, O. Matsushita, S. Shimamoto, and A. Okabe. 2002. Clostridium perfringens epsilon-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J. Biol. Chem. 277:39463-39468. [DOI] [PubMed] [Google Scholar]
- 34.Mourez, M., R. S. Kane, J. Mogridge, S. Metallo, P. Deschatelets, B. R. Sellman, G. M. Whitesides, and R. J. Collier. 2001. Designing a polyvalent inhibitor of anthrax toxin. Nat. Biotechnol. 19:958-961. [DOI] [PubMed] [Google Scholar]
- 35.Neumeyer, T., F. Tonello, F. Dal Molin, B. Schiffler, F. Orlik, and R. Benz. 2006. Anthrax lethal factor (LF) mediated block of the anthrax protective antigen (PA) ion channel: effect of ionic strength and voltage. Biochemistry 45:3060-3068. [DOI] [PubMed] [Google Scholar]
- 36.Rainey, G. J., and J. A. Young. 2004. Antitoxins: novel strategies to target agents of bioterrorism. Nat. Rev. Microbiol. 2:721-726. [DOI] [PubMed] [Google Scholar]
- 37.Salles, I. I., D. E. Voth, S. C. Ward, K. M. Averette, R. K. Tweten, K. A. Bradley, and J. D. Ballard. 2006. Cytotoxic activity of Bacillus anthracis protective antigen observed in a macrophage cell line overexpressing ANTXR. Cell. Microbiol. 8:1272-1281. [DOI] [PubMed] [Google Scholar]
- 38.Scobie, H. M., G. J. Rainey, K. A. Bradley, and J. A. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 100:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scobie, H. M., and J. A. Young. 2005. Interactions between anthrax toxin receptors and protective antigen. Curr. Opin. Microbiol. 8:106-112. [DOI] [PubMed] [Google Scholar]
- 40.Sellman, B. R., M. Mourez, and R. J. Collier. 2001. Dominant-negative mutants of a toxin subunit: an approach to therapy of anthrax. Science 292:695-697. [DOI] [PubMed] [Google Scholar]
- 41.Shin, J. S., Z. Gao, and S. N. Abraham. 1999. Bacteria-host cell interaction mediated by cellular cholesterol/glycolipid-enriched microdomains. Biosci. Rep. 19:421-432. [DOI] [PubMed] [Google Scholar]
- 42.Wei, W., Q. Lu, G. J. Chaudry, S. H. Leppla, and S. N. Cohen. 2006. The LDL receptor-related protein LRP6 mediates internalization and lethality of anthrax toxin. Cell 124:1141-1154. [DOI] [PubMed] [Google Scholar]
- 43.Wielgosz, M. M., D. A. Rauch, K. S. Jones, Ruscetti. F. W., and L. Ratner. 2005. Cholesterol dependence of HTLV-I infection. AIDS Res. Hum. Retrovir. 21:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf, A. A., Y. Fujinaga, and W. I. Lencer. 2002. Uncoupling of the cholera toxin-G(M1) ganglioside receptor complex from endocytosis, retrograde Golgi trafficking, and downstream signal transduction by depletion of membrane cholesterol. J. Biol. Chem. 277:16249-16256. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, S., A. Finkelstein, and R. J. Collier. 2004. Evidence that translocation of anthrax toxin's lethal factor is initiated by entry of its N terminus into the protective antigen channel. Proc. Natl. Acad. Sci. USA 101:16756-16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.