Abstract
RWJ-416457, an investigational pyrrolopyrazolyl-substituted oxazolidinone, inhibited the growth of linezolid-susceptible staphylococci, enterococci, and streptococci at concentrations of ≤4 μg/ml, generally exhibiting two- to fourfold-greater potency than that of linezolid. Time-kill studies demonstrated bacteriostatic effects for both RWJ-416457 and linezolid.
Gram-positive bacteria are a frequent cause of infection among patients in the hospital and community (4). The emergence of vancomycin-resistant Staphylococcus aureus and the increasing incidence of community-acquired methicillin-resistant S. aureus (MRSA) (6), penicillin- and macrolide-resistant Streptococcus pneumoniae (2, 8), and vancomycin-resistant enterococci are indications of the increasing resistance of gram-positive bacteria to available antimicrobial agents and provide an impetus to discover novel or more efficacious antimicrobial agents (2-4).
Oxazolidinones are one of the newest classes of antibacterial agents, with linezolid as the only member of this class to gain regulatory approval thus far (14). Linezolid is currently approved to treat skin and respiratory infections caused by gram-positive pathogens, including multidrug-resistant staphylococci, enterococci, and streptococci (14). It is currently the only drug that can be used both orally and parenterally to treat infections caused by MRSA and vancomycin-resistant enterococci. In the quest for a new oxazolidinone with enhanced characteristics compared with linezolid, RWJ-416457 (Fig. 1), an investigational, orally active, oxazolidinone, was identified (7a, 11a).
FIG. 1.
Structure of RWJ-416457.
This study describes the in vitro activity of RWJ-416457 against clinically important bacteria using linezolid and other relevant agents as comparators. Targeted organisms included susceptible and multidrug-resistant staphylococci, enterococci, and pneumococci, Haemophilus influenzae, and Moraxella catarrhalis from the Johnson & Johnson Pharmaceutical Research & Development culture collection and the atypical intracellular respiratory tract pathogens Chlamydia pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae from Focus Bio-Inova.
MICs were determined by the CLSI (formerly NCCLS) broth microdilution procedure for susceptibility testing for bacteria that grow aerobically (11). C. pneumoniae susceptibility testing was performed in HEp-2 cells by the method of Hammerschlag et al. (7). Frozen aliquots of C. pneumoniae were diluted in antibiotic-free Chlamydia overlay medium (COM) and inoculated into a 72-h monolayer of HEp-2 cells. After preincubation, COM plus antibiotic were serially diluted in twofold steps. Following a 72-h incubation at 35°C, the monolayer was fixed and stained. The MIC end point was determined as the lowest concentration that showed significant or complete inhibition of inclusion-forming units. M. pneumoniae susceptibility was determined by the microbroth method of Waites et al. (13). Serial 10-fold dilutions of each isolate were prepared in SP4 glucose broth (Remel) to determine the number of color changing units (CCU). Microtiter plates were prepared with antibiotics serially diluted in antibiotic-free SP4 glucose broth, inoculated (104 CCU/ml), and incubated at 35°C until color changed in control wells. The MIC end point was the lowest concentration at which growth was inhibited on the basis of color change. L. pneumophila susceptibility was performed by broth microdilution. Briefly, the inoculum was suspended in buffered yeast extract broth and transferred to microtiter wells containing serial dilutions of antibiotic and incubated aerobically at 34°C to 36°C for 48 h. The MIC end point was determined as the lowest concentration that inhibited visible growth.
Time-kill experiments with RWJ-416457 and linezolid were performed at four times the MIC by CLSI methodology (10) against S. pneumoniae ATCC 6305 and OC 4446, Enterococcus faecalis ATCC 29212 and ATCC 51299, and S. aureus ATCC 29213 and MRSA OC 2878. The limit of detection was 100 CFU/ml.
Oxazolidinones have a unique mechanism of action, inhibiting bacterial protein synthesis by binding at the peptidyl transferase center of the 50S ribosomal subunit; studies suggest this binding interferes with ribosomal assembly and formation of the initial peptide bond (1, 5, 9, 12). In a bacterial cell-free transcription-dependent translation assay system, RWJ-416457 inhibited protein synthesis at a concentration twofold lower than linezolid, with 50% inhibitory concentrations of 1.4 and 2.9 μM, respectively (D. Montenegro and A. M. Queenan, unpublished data).
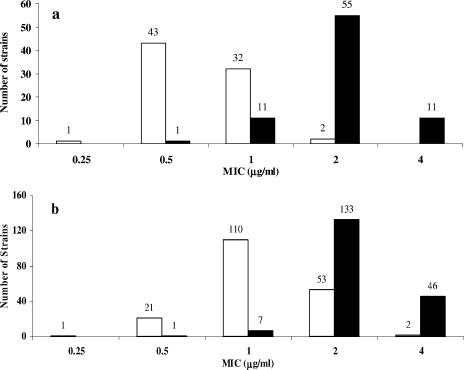
The in vitro antibacterial activities of RWJ-416457 and linezolid against S. aureus and coagulase-negative staphylococci (CoNS) are shown in Table 1. RWJ-416457 had MIC90 values of ≤2 μg/ml against staphylococci (including methicillin-susceptible Staphylococcus aureus [MSSA], MRSA, vancomycin-intermediate S. aureus, and CoNS) (Table 1), consistently twofold lower than those of linezolid. Against a single vancomycin-resistant S. aureus isolate, RWJ-416457 was equipotent to linezolid, with an MIC of 1 μg/ml (B. Bozdogan and P. C. Appelbaum, personal communication). Against vancomycin-susceptible and -resistant E. faecalis and Enterococcus faecium, RWJ-416457 had MIC90 values of 1 μg/ml, two- and fourfold lower than those of linezolid, respectively (Table 1). As illustrated by the MIC distributions of RWJ-416457 and linezolid against S. aureus (MSSA and MRSA), E. faecium, and E. faecalis (Fig. 2), individual MICs of RWJ-416457 were generally two- to fourfold lower than those of linezolid against these pathogens.
TABLE 1.
In vitro activities of RWJ-416457 and comparators against gram-positive bacteria and respiratory tract pathogens
| Organism | No. of isolates | Compound | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|---|---|---|
| S. aureus | |||||
| All | 191 | RWJ-416457 | 0.25-16 | 1 | 2 |
| 191 | Linezolid | 0.5->32 | 4 | 4 | |
| 181 | Vancomycin | 0.5-8 | 1 | 4 | |
| Methicillin-susceptible | 72 | RWJ-416457 | 0.5-4 | 1 | 2 |
| Linezolid | 2-4 | 2 | 4 | ||
| Vancomycin | 1-2 | 1 | 2 | ||
| Methicillin-resistant (hospital-associated MRSA, linezolid-susceptible)a | 102 | RWJ-416457 | 0.25-4 | 1 | 2 |
| Linezolid | 0.5-4 | 4 | 4 | ||
| Vancomycin | 0.5-8 | 1 | 4 | ||
| Oxacillin | 4->32 | >32 | >32 | ||
| Vancomycin-intermediate (MRSA)a | 13 | RWJ-416457 | 0.25-2 | 0.5 | 2 |
| Linezolid | 0.5-4 | 1 | 4 | ||
| Vancomycin | 2-8 | 4 | 8 | ||
| Oxacillin | 4->32 | >32 | >32 | ||
| Linezolid-resistant (MRSA)a | 4 | RWJ-416457 | 4-16 | NAb | NA |
| Linezolid | 8->32 | NA | NA | ||
| Vancomycin | 1-2 | NA | NA | ||
| Oxacillin | >32 | NA | NA | ||
| Community-acquired MRSA | 13 | RWJ-416457 | 1-2 | 1 | 2 |
| Linezolid | 2-4 | 4 | 4 | ||
| Vancomycin | 1 | 1 | 1 | ||
| Oxacillin | 16-32 | 32 | 32 | ||
| Coagulase-negative staphylococci | |||||
| All | 67 | RWJ-416457 | 0.25-1 | 0.5 | 1 |
| Linezolid | 0.5-2 | 2 | 2 | ||
| Vancomycin | 0.5-4 | 2 | 4 | ||
| Oxacillin | 0.12->16 | >16 | >16 | ||
| Methicillin-susceptible | 14 | RWJ-416457 | 0.25-1 | 0.5 | 0.5 |
| Linezolid | 1-2 | 1 | 2 | ||
| Vancomycin | 1-4 | 2 | 4 | ||
| Oxacillin | 0.12-0.25 | 0.12 | 0.25 | ||
| Methicillin-resistant | 53 | RWJ-416457 | 0.25-1 | 0.5 | 1 |
| Linezolid | 0.5-2 | 2 | 2 | ||
| Vancomycin | 0.5-4 | 2 | 4 | ||
| Oxacillin | 0.5->16 | >16 | >16 | ||
| E. faecalis | |||||
| Linezolid-susceptible | 42 | RWJ-416457 | 0.5-2 | 0.5 | 1 |
| Linezolid | 1-4 | 2 | 2 | ||
| Vancomycin | 1->16 | 4 | >16 | ||
| Linezolid-resistant | 1 | RWJ-416457 | 32 | NA | NA |
| Linezolid | 64 | NA | NA | ||
| Vancomycin | >128 | NA | NA | ||
| E. faecium | |||||
| Linezolid-susceptible | 36 | RWJ-416457 | 0.25-1 | 1 | 1 |
| Linezolid | 0.5-4 | 2 | 4 | ||
| Vancomycin | 1->16 | >16 | >16 | ||
| Linezolid-resistant | 2 | RWJ-416457 | 16 | NA | NA |
| Linezolid | 32-64 | NA | NA | ||
| Vancomycin | >128 | NA | NA | ||
| Enterococcus gallinarum (linezolid-resistant) | 1 | RWJ-416457 | 16 | NA | NA |
| Linezolid | 64 | NA | NA | ||
| Vancomycin | >128 | NA | NA | ||
| Streptococcus pyogenes (37 macrolide-resistant isolates) | 39 | RWJ-416457 | 0.25-0.5 | 0.5 | 0.5 |
| Linezolid | 1-2 | 1 | 2 | ||
| Erythromycin | 0.03->16 | 8 | >16 | ||
| S. pneumoniae | 108 | RWJ-416457 | 0.06-1 | 0.5 | 0.5 |
| All | Linezolid | 0.25-2 | 1 | 1 | |
| Penicillin-resistant | 31 | RWJ-416457 | 0.25-1 | 0.5 | 0.5 |
| Linezolid | 0.5-2 | 1 | 1 | ||
| Penicillin | 2-4 | 2 | 4 | ||
| Macrolide-resistant | 51 | RWJ-416457 | 0.25-1 | 0.5 | 0.5 |
| Linezolid | 0.5-2 | 1 | 1 | ||
| Erythromycin | 1->16 | 4 | >16 | ||
| Fluoroquinolone-resistant | 20 | RWJ-416457 | 0.06-1 | 0.5 | 0.5 |
| Linezolid | 0.5-2 | 0.5 | 1 | ||
| Levofloxacin | 8->32 | 16 | >16 | ||
| H. influenzae | 4 | RWJ-416457 | 8->16 | NA | NA |
| Linezolid | 4-16 | NA | NA | ||
| Erythromycin | 2-8 | NA | NA | ||
| M. catarrhalis | 12 | RWJ-416457 | 2-4 | 2 | 4 |
| Linezolid | 4-8 | 8 | 8 | ||
| Erythromycin | 0.12-0.25 | 0.25 | 0.25 | ||
| L. pneumophila | 17 | RWJ-416457 | 2-64 | 4 | 8 |
| Linezolid | 1-64 | 4 | 16 | ||
| Levofloxacin | 0.03-0.5 | 0.12 | 0.5 | ||
| M. pneumoniae | 17 | RWJ-416457 | 16-32 | 16 | 32 |
| Linezolid | 64->64 | >64 | >64 | ||
| Levofloxacin | 0.5-1 | 0.5 | 1 | ||
| C. pneumoniae | 3 | RWJ-416457 | 16-64 | NA | NA |
| Linezolid | 64->64 | NA | NA | ||
| Levofloxacin | 0.5 | NA | NA |
Isolates received through the Network on Antimicrobial Resistance in S. aureus (NARSA) program.
NA, not applicable.
FIG. 2.
(a) MIC distribution of RWJ-416457 and linezolid against enterococci (36 E. faecium isolates and 42 E. faecalis isolates). (b) MIC distribution of RWJ-416457 and linezolid against S. aureus (72 MSSA isolates, 102 hospital-associated MRSA isolates, and 13 community-acquired MRSA isolates). The white bars depict RWJ-416457 values, and the black bars depict linezolid values. The numbers of isolates are indicated above the bars.
Against linezolid-resistant staphylococci and enterococci, RWJ-416457 MICs ranged from 4 to 32 μg/ml, values that were again two- to fourfold lower than those of linezolid. Because concentrations of 16 and 32 μg/ml are not likely to be achieved clinically on the basis of the anticipated drug levels and pharmacodynamic profile (6a), this compound is not expected to have utility against most linezolid-resistant isolates.
When tested against respiratory pathogens, the MIC90 of RWJ-416457 for penicillin-, macrolide-, and fluoroquinolone-resistant S. pneumoniae was 0.5 μg/ml, twofold lower than that of linezolid. RWJ-416457 MICs were 2 to >16 μg/ml against the gram-negative pathogens H. influenzae and M. catarrhalis. RWJ-416457 and linezolid had MIC90 values of 8 and 16 μg/ml, respectively, against L. pneumophila and MICs of ≥16 and ≥64 μg/ml, respectively, against M. pneumoniae and C. pneumoniae. Thus, although RWJ-416457 generally retained at least a twofold advantage in potency over linezolid against the atypical respiratory tract pathogens, MICs of both agents frequently exceeded 8 μg/ml.
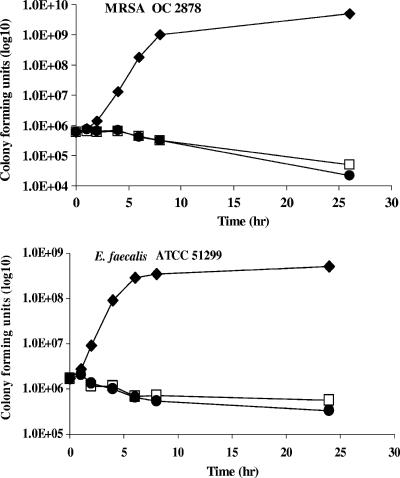
Time-kill experiments demonstrated that both RWJ-416457 and linezolid were bacteriostatic against the six S. pneumoniae, S. aureus, and E. faecalis strains tested at four times the MIC levels (Fig. 3 for representative time-kill curves). These observations are consistent with the protein synthesis inhibitory mechanism of action for both agents.
FIG. 3.
Time-kill analysis of isolates treated with RWJ-416457 or linezolid at four times the MIC. Isolates were grown in control medium (♦), medium with RWJ-416457 (□), and medium with linezolid (•). Against MRSA OC 2878, the RWJ-416457 MIC was 1 μg/ml and the linezolid MIC was 2 μg/ml; against E. faecalis ATCC 51922, the RWJ-416457 MIC was 1 μg/ml and the linezolid MIC was 4 μg/ml.
In conclusion, the phase 1 investigational oxazolidinone RWJ-416457 demonstrated inhibitory activity against many bacteria resistant to vancomycin, macrolides, and fluoroquinolones. RWJ-416457 generally had two- to fourfold-lower MICs than those of linezolid against most pathogens tested. Its in vitro microbiological activity warrants further investigation to determine an appropriate dosing regimen, in addition to its safety and efficacy in human trials.
Acknowledgments
We thank Deborah Montenegro and Anne Marie Queenan for conducting protein synthesis assays.
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Barbachyn, M. R., and C. W. Ford. 2003. Oxazolidinone structure-activity relationships leading to linezolid. Angew. Chemie 42:2010-2023. [DOI] [PubMed] [Google Scholar]
- 2.Bush, K. 2004. Antibacterial drug discovery in the 21st century. Clin. Microbiol. Infect. 10:(Suppl. 4):10-17. [DOI] [PubMed] [Google Scholar]
- 3.Bush, K. 2004. Why it is important to continue antibacterial drug discovery. ASM News 70:282-286. [Google Scholar]
- 4.Clark, N. M., E. Hershberger, M. J. Zervosc, and J. P. Lynch III. 2003. Antimicrobial resistance among gram-positive organisms in the intensive care unit. Curr. Opin. Crit. Care 9:403-412. [DOI] [PubMed] [Google Scholar]
- 5.Colca, J. R., W. G. McDonald, D. J. Waldon, L. M. Thomasco, R. C. Gadwood, E. T. Lund, G. S. Cavey, W. R. Mathews, L. D. Adams, E. T. Cecil, J. D. Pearson, J. H. Bock, J. E. Mott, D. L. Shinabarger, L. Xiong, and A. S. Mankin. 2003. Cross-linking in the living cell locates the site of action of oxazolidinone antibiotics. J. Biol. Chem. 278:21972-21979. [DOI] [PubMed] [Google Scholar]
- 6.Diederen, B. M. W., and J. Kluytmans. 2006. The emergence of infections with community-associated methicillin resistant Staphylococcus aureus. J. Infect. 52:157-168. [DOI] [PubMed] [Google Scholar]
- 6a.Drusano, G. L., W. Liu, K. Bush, M. R. Deziel, M. Drusano, and A. Louie. 2005. Pharmacodynamics (PD) of RWJ-416457, a new oxazolidinone antibiotic, in a neutropenic mouse thigh model of Staphylococcus aureus (SA) infection, abstr. F-1248, p. 196. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 7.Hammerschlag, M. R., K. K. Qumei, and P. M. Roblin. 1992. In vitro activities of azithromycin, clarithromycin, l-ofloxacin, and other antibiotics against Chlamydia pneumoniae. Antimicrob. Agents Chemother. 36:1573-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Hilliard, J. J., J. Melton, J. Fernandez, S. Paget, M. Macielag, R. Goldschmidt, and K. Bush. 2005. In vivo activity of the novel oxazolidinone RWJ-416457, abstr. F-1247, p. 195-196. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 8.Klugman, K. P., and J. R. Lonks. 2005. Hidden epidemic of macrolide-resistant pneumococci. Emerg. Infect. Dis. 11:802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livermore, D. M. 2003. Linezolid in vitro: mechanism and antibacterial spectrum. J. Antimicrob. Chemother. 51:(Suppl. S2)ii9-ii16. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 11.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically. Approved standard, 6th ed. M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 11a.Paget, S. D., C. M. Boggs, B. D. Foleno, R. M. Goldschmidt, M. Weidner-Wells, E. Wira, K. Bush, and M. Macielag. 2005. Synthesis and in vitro antibacterial activity of pyrrolopyrazolyl-substituted oxazolidinones, abstr. F-1242, p. 194. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 12.Shinabarger, D. L., K. R. Marotti, R. W. Murray, A. H. Lin, E. P. Melchior, S. M. Swaney, D. S. Dunyak, W. F. Demyan, and J. M. Buysse. 1997. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waites, K. B., L. B. Duffy, T. Schmid, D. Crabb, M. S. Pate, and G. H. Cassell. 1991. In vitro susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum to sparfloxacin and PD 127391. Antimicrob. Agents Chemother 35:1181-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox, M. H. 2005. Update on linezolid: the first oxazolidinone antibiotic. Expert Opin. Pharmacother. 6:2315-2326. [DOI] [PubMed] [Google Scholar]