Abstract
The present study tested in vitro susceptibility of Candida bloodstream isolates to fluconazole to determine if the ratio of the fluconazole area under the concentration-time curve (AUC) or weight-normalized daily dose (dosewn) to MIC correlated with mortality. Fluconazole susceptibility and outcome data were determined for 77 patients with a positive Candida blood culture between 2002 and 2005. The most commonly isolated Candida species were C. albicans (64%), C. glabrata (14%), C. parapsilosis (8%), C. tropicalis (6%), and C. lusitaniae (4%). Only two isolates were classified as fluconazole resistant by the CLSI M27-A2 method. Fluconazole MICs were highest against C. glabrata relative to other Candida species. Overall the crude mortality assessed at hospital discharge was 19.4% (n = 15). Mortality rates by species were as follows: C. albicans, 16.3%; C. glabrata, 36.4%; C. parapsilosis, 0%; C. tropicalis, 0%; C. lusitaniae, 33.3%. A mortality rate of 50% was noted among patients infected with nonsusceptible isolates (MIC ≥ 16 μg/ml) compared to 18% for patients infected with susceptible (MIC ≤ 8 μg/ml) isolates (P = 0.17). The fluconazole dosewn/MIC (24-h) values were significantly higher for the 62 survivors (13.3 ± 10.5 [mean ± standard deviation]) compared to the 15 nonsurvivors (7.0 ± 8.0) (P = 0.03). The fluconazole AUC/MIC (24 h) values also trended higher for survivors (775 ± 739) compared to nonsurvivors (589 ± 715) (P = 0.09). These data support the dose-dependent properties of fluconazole. Underdosing fluconazole against less-susceptible Candida isolates has the potential to increase the risk of mortality associated with candidemia.
Candida species are the fourth most common cause of nosocomial bloodstream infection and are associated with attributable morality rates of 40% (6). Fluconazole is recommended for treatment of candidemia in nonneutropenic patients due to concentration-dependent activity, proven efficacy, a favorable safety profile, and high bioavailability, which allows for intravenous or oral use (25). Most Candida species are susceptible to fluconazole; exceptions are Candida glabrata, which has reduced susceptibility to fluconazole, and Candida krusei, which has intrinsic resistance (25, 27). Fluconazole resistance in other species such as Candida albicans, Candida tropicalis, and Candida parapsilosis has been rare although strains with reduced susceptibility to fluconazole have been reported (26). Since 1997, standardized testing published by the Clinical Laboratory Standards Institute (CLSI, formerly NCCLS) has been available to assess the in vitro susceptibility of Candida species to fluconazole and has been promoted as a tool to help clinicians drive therapeutic drug selection (22, 23). Unfortunately, testing of antifungal susceptibility is generally not considered routine (24, 29). Reasons commonly cited for lack of routine susceptibility testing include delays in receiving results and conflicting data correlating fluconazole MICs to clinical outcomes (24).
Animal models of candidiasis using fluconazole have shown that the area under the concentration-time curve (AUC)-to-MIC ratio (AUC/MIC) and the maximum concentration of drug in serum (Cmax)-to-MIC ratio (Cmax/MIC) are the pharmacodynamics indices that best correlate with mycologic response (4, 16, 17). In mouse models, the 24-h AUC/MIC which corresponded to the dose required to achieve 50% of the maximum effect varied from 25 to 44. A similar pharmacodynamic relationship has been established with other triazoles (1-3). The AUC and Cmax of fluconazole in adults can be predicted with high accuracy given the patient's body weight and renal function (17). Unfortunately, previous human studies that have assessed the relationship between fluconazole susceptibility and outcomes have used nonstandardized susceptibility testing methodology or have not taken into account the wide range of Cmax and AUC values possible for patients given a fixed dose of fluconazole due to differences in body mass and renal function (8, 14, 15). A small study of 32 patients with candidemia given various doses of fluconazole demonstrated that a lower dose compared to the fluconazole MIC was associated with increased likelihood of persistent or breakthrough candidemia (8). However, this study used data from one medical center and did not assess the impact of fluconazole pharmacodynamics on mortality. The purpose of the present study was to evaluate the relationship between fluconazole AUC/MIC and weight-normalized daily dose (dosewn)/MIC ratios and mortality in hospitalized, nonneutropenic patients with candidemia.
MATERIALS AND METHODS
Subject enrolment and data collection.
Candida isolates were obtained from the bloodstreams of patients enrolled from two general surgical/medical hospitals participating in a multicenter, retrospective observational trial of candidemia as previously described (10). To be enrolled in the trial, patients had to be hospitalized between 2002 and 2005, have a blood culture positive for Candida, be treated with fluconazole, and be hospitalized for greater than 48 h prior to blood culture collection. Exclusion criteria included previous bloodstream infections by C. albicans or by a Candida species other than C. albicans, age less than 18 years, and unavailability or nonviability of Candida culture for susceptibility testing. Patients given fluconazole for more than 24 h before the culture date or given their first dose more than 2 days after the culture date were excluded from this analysis. Data collected on all patients were fluconazole start and stop dates, dose, and route; Candida species type and date of culture; demographics (age, gender, race, weight, and past medical and surgical history); Acute Physiology and Chronic Health Evaluation (APACHE) II scores (intensive care unit [ICU] patients only), use of corticosteroids, presence of central venous catheters, total parenteral nutrition, requirement for hemodialysis or mechanical ventilation, admission type (surgical or nonsurgical), and location of patient at time of culture (ICU or non-ICU). Laboratory information included data to calculate APACHE II scores, white blood cell count, and maximum temperature within the 24 h prior to collection of blood culture (13). Mortality was assessed at hospital discharge. Other outcome variables collected included admission and discharge dates from the hospital and ICU. Onset of symptoms was defined as the day the blood culture was obtained. This study was approved by the institutional review board at each participating institution.
Blood culture technique and susceptibility testing.
Trained nurses or phlebotomists obtained all blood cultures after sterile disinfection of a peripheral vein or central venous catheter. All blood samples were inoculated into aerobic media and processed in the clinical microbiology laboratory of each institution using automated culture techniques (Vitek). Species identification was confirmed using the Vitek YBC system or with the API 20C Aux method (BioMerieux Vitek, Inc., Hazelwood, Mo.). The isolates were subcultured on Sabouraud dextrose agar for viability and stored in Microbank vials (Pro-Lab Diagnostic, Toronto, Ontario, Canada) at −70°C until use. The CLSI M27-A2-recommended quality control isolates were used for susceptibility testing with each batch and included ATCC 6258 (Candida krusei) and ATCC 22019 (Candida parapsilosis) (23).
Fluconazole stock solutions (1,000 μg/ml) were prepared using deionized water, and susceptibility testing was performed using the CLSI M27-A2 broth microdilution method. This method involves use of RPMI 1640-2% dextrose buffered with 0.165 M 3-(N-morpholino)propanesulfonic acid adjusted to pH 7.0 (7, 9, 18). Drug-containing microplates were prepared in batches through serial doubling dilutions of fluconazole (0.5 μg/ml to 256.0 μg/ml), parafilmed, stored at −70°C, and used within 2 weeks of preparation. Candida isolates were grown on Sabouraud dextrose agar overnight and used to prepare a 0.5 McFarland organism suspension diluted to a 2× inoculum. One hundred microliters of the inocula was then dispensed into each microwell of the thawed fluconazole-containing microplate to yield the appropriate concentration of drug and microorganism in each microwell. The plates were incubated at 35°C, and the MICs were interpreted at 24 and 48 h based on a marked reduction in turbidity.
Statistical analysis.
Values are expressed as means ± standard deviations (SD) for continuous variables and as percentages of the group from which they were derived for categorical variables. Susceptibility of fluconazole was categorized based on breakpoints provided by the CLSI (15). A fluconazole MIC of ≤8 μg/ml was considered susceptible, 16 to 32 μg/ml was considered dose-dependent susceptible, and ≥64 μg/ml was considered resistant. Fluconazole dosewn/MIC and AUC/MIC ratios were calculated by dividing the fluconazole dose (mg/kg of body weight) or AUC by the MIC at 24 h (MIC24) and MIC48. Fluconazole AUC values were calculated by dividing individual fluconazole doses by individual estimated fluconazole clearances. Fluconazole clearance was estimated using a previously published regression equation that modeled fluconazole clearance as a function of creatinine clearance (31). Fluconazole AUC/MIC and dosewn/MIC ratio data were transformed to log10 to approximate a normal distribution prior to statistical analysis. The correlation between mortality rates and susceptibility breakpoints was assessed using chi-square analysis. Logistic regression was used to assess the associations between AUC/MIC and dosewn/MIC ratios against mortality. Time till initiation of fluconazole (0, 1, or 2 days) was included in mortality analyses, as previous research has shown that a delay in antifungal prescription can impact mortality (10, 20). Significant AUC/MIC and dosewn/MIC breakpoints for mortality were determined using classification and regression tree (CART) analysis (12, 21). All statistical analyses were performed with either SAS software, version 9.1 (SAS Institute, Cary, N.C.), or SYSTAT, version 8.0 (SPSS, Inc., Chicago, IL). All tests were two-tailed, and a P value of <0.05 was considered significant.
RESULTS
Fluconazole susceptibility and clinical data were available for 77 of 109 patients with candidemia treated with fluconazole. Cultures were not available or were nonviable upon regrowth for the remaining 32 isolates. Patient demographics and clinical data are presented in Table 1. Comorbid conditions present in more than 10% of the population included diabetes mellitus (23%), hemodialysis (17%), and solid-organ cancer (13%). The majority of patient were either febrile (42%) or experienced an increased white blood cell count (52%) on the day of blood culture with an average APACHE II score of 19 ± 9. Risk factors for candidemia present on the blood culture day included a central venous catheter (81%); hospitalization in the ICU (38%); and requirement for mechanical ventilation (29%), corticosteroids (13%), or total parenteral nutrition (8%). Fluconazole was initiated in 61 of 77 (79%) patients at the onset of symptoms, 6 of 77 (7%) within 24 h, and 10 of 77 (13%) within 24 to 48 h.
TABLE 1.
Clinical data for 77 patients with candidemia
| Variable | No. (%) of patients |
|---|---|
| Demographicsa | |
| Gender | |
| Male | 41 (53) |
| Female | 36 (47) |
| Race | |
| Caucasian | 27 (35) |
| Hispanic | 28 (36) |
| African-American | 10 (13) |
| Other | 12 (16) |
| Comorbid conditions | |
| Diabetes mellitus | 18 (23) |
| Hemodialysis | 13 (17) |
| Solid-organ cancer | 10 (13) |
| Human immunodeficiency virus/AIDS | 2 (3) |
| Hospital variables collected on day of blood cultureb | |
| Febrile (temp > 100.5°F) | 32 (42) |
| Increased white blood cell count (>12.0 × 109/liter) | 40 (52) |
| Hospitalized in ICU | 29 (38) |
| Central venous catheter | 62 (81) |
| Corticosteroid treatment | 10 (13) |
| Total parenteral nutrition | 6 (8) |
| Mechanical ventilation | 22 (29) |
Mean age (years) ± SD, 51 ± 15; mean weight (kg) ± SD, 78 ± 18.
Mean APACHE II score ± SD, 19 ± 9.
The most commonly isolated Candida species were C. albicans (64%), C. glabrata (14%), C. parapsilosis (8%), C. tropicalis (6%), and C. lusitaniae (4%); other Candida species were also isolated. The in vitro susceptibility of Candida to fluconazole at 24 hours is displayed in Table 2. The 48-h fluconazole MICs were within one doubling dilution of the 24-h MIC for ∼90% of isolates. However, five isolates (three C. tropicalis isolates and two C. albicans isolates) had a 48-h MIC that was at least 16-fold higher at 48 h compared to 24 h due to high-trailing-growth isolates. The MIC at which 50% of isolates were inhibited (MIC50) and MIC90 for all species were 0.5 and 8 μg/ml, respectively. MICs were lowest for C. albicans and highest for C. glabrata at 24 hours and 48 hours. Fluconazole doses ranged from 150 to 800 mg. The fluconazole dosewn/MIC ratio ranged from 0.035 to 35.56 and averaged 12.06 ± 10.31. The dosewn/MIC24 ratio was lowest for C. glabrata (1.88 ± 2.20; range, 0.3 to 7.9) and highest for C. albicans (15.39 ± 9.95; range, 0.035 to 35.6) although a wide range of dosewn/MIC ratios were noted (P = 0.0004). The dosewn/MIC48 ratio was lowest for C. glabrata (1.51 ± 2.34; range, 0.30 to 7.92) and highest for C. albicans (13.17 ± 10.47; range, 0.019 to 35.6) (P = 0.0008). As illustrated in Table 2, mortality rates by species were as follows: C. albicans, 16.3%; C. glabrata, 36.4%; C. parapsilosis, 0%; C. tropicalis, 0%; C. lusitaniae, 33.3%.
TABLE 2.
In vitro susceptibility to fluconazole, AUC/MIC, dosewn/MIC, and mortality according to common isolated species at 24 h
| Candida sp. (n) | MIC50 (μg/ml) (range) | Mean AUC/MIC ± SD (range) | Mean dosewn/MIC ± SD (range) | Mortality rate (%) |
|---|---|---|---|---|
| C. albicans (49) | 0.25 (0.25-128) | 946 ± 764 (0.58-3,097) | 15.4 ± 10.0 (0.035-35.6) | 16.3 |
| C. glabrata (11) | 4.0 (0.25-16.0) | 182 ± 427 (11.2-430) | 1.9 ± 2.2 (0.30-7.9) | 36.4 |
| C. parapsilosis (6) | 1.25 (0.25-2.0) | 635 ± 500 (59.9-1,050) | 10.7 ± 11.0 (1.18-26.7) | 0 |
| C. tropicalis (5) | 1.0 (0.25-4.0) | 657 ± 788 (58.6-1,774) | 11.4 ± 12.2 (1.23-32.7) | 0 |
| C. lusitaniae (3) | 2.0 (1.0-4.0) | 232 ± 214 (27.8-462.2) | 3.3 ± 1.6 (0.78-12.9) | 33.3 |
Correlation between fluconazole dosewn/MIC and mortality.
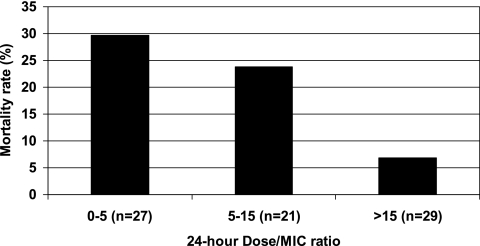
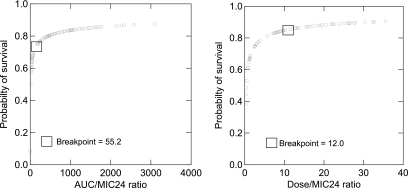
Seventy-three of 77 (95%) isolates were susceptible according to CLSI breakpoints, 2 of 77 (2.5%) were dose-dependent susceptible, and 2 of 77 (2.5%) were resistant (Table 3). Although mortality rates were higher among patients infected with nonsusceptible Candida species (50%) compared to susceptible (18%) species, these results were not statistically significant after controlling for time to initiation of fluconazole (P = 0.17). Fluconazole dosewn/MIC24 ratio was significantly higher for survivors (13.3 ± 10.5; range, 0.035 to 35.6) compared to nonsurvivors (7.0 ± 8.0; range, 0.037 to 27.6), controlling for time to initiation of fluconazole (P = 0.0345). Although dosewn/MIC48 was higher for survivors (10.7 ± 10.5; range, 0.019 to 35.6) compared to nonsurvivors (6.1 ± 8.1; range, 0.019 to 27.6), these results were not statistically significant (P = 0.15). Mortality rates stratified by dosewn/MIC ratio using 24-hour MICs are shown in Fig. 1. Mortality rates declined significantly with increased fluconazole dosewn/MIC24 ratios (P = 0.0272) from 30% for dosewn/MIC ratios between 0 and 5, 23% to 25% for ratios between 5 and 15, 10% for ratios between 15 and 20, and 5% for ratios above 20. Similar results were observed using the fluconazole dosewn/MIC48 ratio; however, these results were not statistically significant (P = 0.27). A breakpoint of 12.0 for dosewn/MIC24 ratio was observed using CART analysis (P= 0.007; Fig. 2).
TABLE 3.
Mortality rates stratified by fluconazole breakpoint
| Susceptibility | MIC breakpoint (μg/ml) | No. of patients | Mortality
|
|||
|---|---|---|---|---|---|---|
| 24-h MICa
|
48-h MICa
|
|||||
| n | % | n | % | |||
| Susceptible | ≤8 | 73 | 13 | 18 | 13 | 18 |
| Dose-dependently susceptible | 16-32 | 2 | 1 | 50 | 1 | 50 |
| Resistant | ≥64 | 2 | 1 | 50 | 1 | 50 |
P = 0.1731 by chi-square analysis, controlling for time to initiation of fluconazole.
FIG. 1.
Mortality rate stratified by tertiles and fluconazole dosewn/MIC at 24 h (P = 0.03 using logistic regression controlling for time to initiation of fluconazole therapy).
FIG. 2.
Probability of survival as a function of the AUC/MIC24 and dosewn/MIC24 ratio for 77 patients with candidemia given fluconazole. CART analysis showed that the AUC/MIC24 breakpoint was 55.2 (P = 0.008) and the dosewn/MIC24 breakpoint was 12.0 (P = 0.007).
Correlation between fluconazole AUC/MIC and mortality.
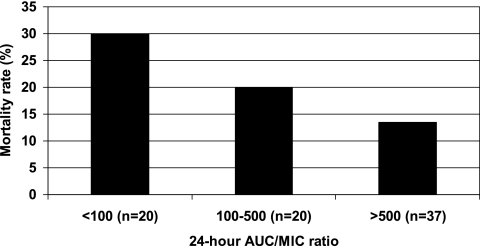
Although fluconazole AUC/MIC24 and AUC/MIC48 were higher in survivors compared to nonsurvivors, these results were not statistically significant when controlled for time to fluconazole initiation. The AUC/MIC24 ratios were 775 ± 739 (range, 0.58 to 3,097) for survivors and 589 ± 715 (range, 0.91 to 2,340) for nonsurvivors (P = 0.09). The AUC/MIC48 ratios were 614 ± 724 (range, 0.58 to 3,097) for survivors and 496 ± 687 (range, 0.46 to 2,340) for nonsurvivors (P = 0.3). As illustrated in Fig. 3, the mortality rates stratified by AUC/MIC24 were 13.5%, 20.0%, and 30.0% for ratios of ≥500, 100 to 499, and <100, respectively. However, an AUC/MIC of ≥500 was also not found to be a statistically significant predictor of survival (P = 0.10). A breakpoint of 55.2 for the AUC/MIC24 ratio was observed using CART analysis (P = .008; Fig. 2).
FIG. 3.
Mortality rate stratified by tertiles and fluconazole AUC/MIC at 24 h (P = 0.09 using logistic regression controlling for time to initiation of fluconazole therapy).
DISCUSSION
In 1997, the CLSI published guidelines for the susceptibility of yeast via broth macrodilution and microdilution methods (22). The use and rationale for yeast susceptibility testing have been recently reviewed (27, 29). The breakpoints were originally derived primarily from data correlating in vitro antifungal susceptibility to clinical outcomes in human immunodeficiency virus-positive patients with oropharyngeal and esophageal candidiasis treated with fluconazole (30). Since that time, five different studies involving more than 600 patients have evaluated the effect of susceptibility testing on clinical outcomes (5, 8, 15, 30, 32). Lee et al. evaluated the clinical correlation of fluconazole MICs by the CLSI broth macrodilution method for 32 non-AIDS patients with hematogenous and deep-seated candidiasis given 400 mg of fluconazole intravenously with dosage adjustment for renal dysfunction (15). Although only two isolates were defined as resistant by the CLSI criteria, both isolates contributed to clinical failure. In a study of 242 patients with Candida bloodstream infections, clinical success rates decreased from 70% (144 of 206 patients) in patients with susceptible isolates to 64% (16 of 25 patients) for dose-dependently susceptible isolates to 55% (6 of 11 patients) with resistant isolates (32). A dose-response relationship has also been noted in ICU patients with candidiasis, where the mortality rate was threefold greater in a group receiving 5 mg/kg versus 10 mg/kg of fluconazole (11).
Perhaps the most convincing clinical evidence of a pharmacodynamic relationship between fluconazole dose and outcomes involved 32 patients treated with various doses of fluconazole (8). In that study, a fluconazole dose/MIC ratio of >50 was associated with a 74% chance of mycologic eradication compared to an 8% chance of success with fluconazole dosewn/MIC ratios of ≤50. The present study expands on this previous work by evaluating the relationship between fluconazole AUC/MIC and dosewn/MIC ratios and mortality in hospitalized, nonneutropenic patients with candidemia. The dose/MIC relationship was calculated based on weight-normalized values, making direct comparison between the two studies difficult. However, weight normalization was critical in our study given that two-thirds of our population were over 70 kg and 25% were over 90 kg. Consistent with previous studies our patient population had significant comorbdities and high APACHE II scores. The Candida species distribution was also consistent with national prevalence rates, and fluconazole resistance (MIC ≥ 64 μg/ml) was only noted in two isolates. The fluconazole dosewn/MIC24 ratio was identified as a statistically significant variable associated with mortality. Although a trend was noted, our relatively small sample size likely prevented the identification of a statistically significant relationship between fluconazole AUC/MIC24 ratio and mortality. The optimal AUC/MIC ratio calculated using CART analysis of 55 correlates positively with a previous nonneutropenic murine model of systemic candidiasis that identified an AUC/MIC ratio of 44 as predictive of efficacy (4, 16). Taken together, our data along with previously published studies support a concentration-dependent pharmacodynamic profile for fluconazole that has significant clinical impact.
This present study as well as previous studies are limited by small sample sizes in their ability to provide an interpretive pharmacodynamic breakpoint value. Thirty-two isolates were not available for susceptibility testing in this study, and further prospective studies will be required to assure that the study results were not influenced by selection bias. Clinical outcomes for patients with candidemia are also influenced by host factors such as age, severity of illness, and comorbid conditions (28). Larger studies will be required to assess the influence of these variables on the pharmacodynamic optimization of antifungals. The 48-hour MIC determinations were also shown to not be as statistically significant, likely due to the trailing effect seen at 48 hours for five isolates. Likewise, extrapolation of these results to other antifungal agents will require further research. Also, given that susceptibility testing results are not available for 24 to 48 h, the ability to use breakpoint values to prospectively guide patient therapy is limited until faster susceptibility techniques become available. However, optimization of antibiotic treatment against bacterial bloodstream infections using average MIC50 or MIC90 values has been described (19). Population fluconazole MICs could be used to optimize fluconazole dosing based on appropriate pharmacodynamic parameters. For this to happen, a large multicenter study to assess the appropriate interpretive pharmacodynamic breakpoint against Candida species will be required.
In conclusion, increased fluconazole dosewn/MIC values were associated with decreased mortality in 77 nonneutropenic patients with candidemia. Given the high mortality rates regardless of in vitro susceptibility, these data also assert the need for higher fluconazole doses to manage C. glabrata-related relative to C. albicans-related candidemia. Prospective studies that include a large number of patients with C. glabrata-related candidemia are necessary to validate these results. This improved understanding will invariably help clinicians select and optimize antifungal therapy against this common, deadly, and expensive fungal disease. For now, clinicians should appreciate the strong possibility that underdosing fluconazole for less-susceptible Candida isolates increases the risk of mortality.
Acknowledgments
This study was supported by a research grant from Merck & Co., Inc.
Manjunath P. Pai has received research support from Pfizer, Inc., Astellas, and Enzon Pharmaceuticals. Robin S Turpin is an employee of Merck & Co., Inc. Kevin W. Garey has received past research support from Merck & Co., Inc.
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Andes, D., K. Marchillo, R. Conklin, G. Krishna, F. Ezzet, A. Cacciapuoti, and D. Loebenberg. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacodynamics of a new triazole, ravuconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., and M. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniadou, A., H. A. Torres, R. E. Lewis, J. Thornby, G. P. Bodey, J. P. Tarrand, X. Y. Han, K. V. Rolston, A. Safdar, I. I. Raad, and D. P. Kontoyiannis. 2003. Candidemia in a tertiary care cancer center: in vitro susceptibility and its association with outcome of initial antifungal therapy. Medicine (Baltimore) 82:309-321. [DOI] [PubMed] [Google Scholar]
- 6.Bustamante, C. I. 2005. Treatment of Candida infection: a view from the trenches! Curr. Opin. Infect. Dis. 18:490-495. [DOI] [PubMed] [Google Scholar]
- 7.Canton, E., J. Peman, A. Carrillo-Munoz, A. Orero, P. Ubeda, A. Viudes, and M. Gobernado. 1999. Fluconazole susceptibilities of bloodstream Candida sp. isolates as determined by National Committee for Clinical Laboratory Standards method M27-A and two other methods. J. Clin. Microbiol. 37:2197-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clancy, C. J., V. L. Yu, A. J. Morris, D. R. Snydman, and M. H. Nguyen. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuenca-Estrella, M., C. B. Moore, F. Barchiesi, J. Bille, E. Chryssanthou, D. W. Denning, J. P. Donnelly, F. Dromer, B. Dupont, J. H. Rex, M. D. Richardson, B. Sancak, P. E. Verweij, and J. L. Rodriguez-Tudela. 2003. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin. Microbiol. Infect. 9:467-474. [DOI] [PubMed] [Google Scholar]
- 10.Garey, K. W., M. Rege, M. P. Pai, D. E. Mingo, K. J. Suda, R. S. Turpin, and D. T. Bearden. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25-31. [DOI] [PubMed] [Google Scholar]
- 11.Graninger, W., E. Presteril, B. Schneeweiss, B. Teleky, and A. Georgopoulos. 1993. Treatment of Candida albicans fungaemia with fluconazole. J. Infect. 26:133-146. [DOI] [PubMed] [Google Scholar]
- 12.Highet, V. S., A. Forrest, C. H. Ballow, and J. J. Schentag. 1999. Antibiotic dosing issues in lower respiratory tract infection: population-derived area under inhibitory curve is predictive of efficacy. J. Antimicrob. Chemother. 43(Suppl. A):55-63. [DOI] [PubMed] [Google Scholar]
- 13.Knaus, W. A., E. A. Draper, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 14.Kovacicova, G., Y. Krupova, M. Lovaszova, A. Roidova, J. Trupl, A. Liskova, J. Hanzen, P. Milosovic, M. Lamosova, L. Macekova, Z. Szovenyiova, A. Purgelova, T. Obertik, J. Bille, and V. Krcmery. 2000. Antifungal susceptibility of 262 bloodstream yeast isolates from a mixed cancer and non-cancer patient population: is there a correlation between in-vitro resistance to fluconazole and the outcome of fungemia? J. Infect. Chemother. 6:216-221. [DOI] [PubMed] [Google Scholar]
- 15.Lee, S. C., C. P. Fung, J. S. Huang, C. J. Tsai, K. S. Chen, H. Y. Chen, N. Lee, L. C. See, and W. B. Shieh. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie, A., G. L. Drusano, P. Banerjee, Q. F. Liu, W. Liu, P. Kaw, M. Shayegani, H. Taber, and M. H. Miller. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie, A., Q. F. Liu, G. L. Drusano, W. Liu, M. Mayers, E. Anaissie, and M. H. Miller. 1998. Pharmacokinetic studies of fluconazole in rabbits characterizing doses which achieve peak levels in serum and area under the concentration-time curve values which mimic those of high-dose fluconazole in humans. Antimicrob. Agents Chemother. 42:1512-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lozano-Chiu, M., S. Arikan, V. L. Paetznick, E. J. Anaissie, and J. H. Rex. 1999. Optimizing voriconazole susceptibility testing of Candida: effects of incubation time, end point rule, species of Candida, and level of fluconazole susceptibility. J. Clin. Microbiol. 37:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maglio, D., and D. P. Nicolau. 2004. The integration of pharmacokinetics and pathogen susceptibility data in the design of rational dosing regimens. Methods Find Exp. Clin. Pharmacol. 26:781-788. [DOI] [PubMed] [Google Scholar]
- 20.Morrell, M., V. J. Fraser, and M. H. Kollef. 2005. Delaying the empirical treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouton, J. W. 2002. Breakpoints: current practice and future perspectives. Int. J. Antimicrob. Agents 19:323-331. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A2, 2nd ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.Pai, M. P., and S. L. Pendland. 2003. Antifungal susceptibility testing in teaching hospitals. Ann. Pharmacother. 37:192-196. [DOI] [PubMed] [Google Scholar]
- 25.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., and D. J. Diekema. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., D. J. Diekema, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rentz, A. M., M. T. Halpern, and R. Bowden. 1998. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin. Infect. Dis. 27:781-788. [DOI] [PubMed] [Google Scholar]
- 29.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982-989. [DOI] [PubMed] [Google Scholar]
- 30.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 31.Sobue, S., K. Tan, G. Layton, V. Leclerc, and A. Weil. 2004. The effects of renal impairment on the pharmacokinetics and safety of fosfluconazole and fluconazole following a single intravenous bolus injection of fosfluconazole. Br. J. Clin. Pharmacol. 57:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takakura, S., N. Fujihara, T. Saito, T. Kudo, Y. Iinuma, and S. Ichiyama. 2004. Clinical factors associated with fluconazole resistance and short-term survival in patients with Candida bloodstream infection. Eur. J. Clin. Microbiol. Infect. Dis. 23:380-388. [DOI] [PubMed] [Google Scholar]