Abstract
The current treatments for leishmaniasis are unsatisfactory due to their toxic side effects, high costs, and increasing problems with drug resistance. Thus, there is an urgent need for alternative drugs against leishmaniasis. Different approaches have been used to identify novel pharmacophores against Leishmania sp. parasites, and one strategy has been the analysis of naturally occurring plant-derived compounds, including naphthylisoquinoline alkaloids. In the present study, we examined the abilities of these alkaloids to inhibit the growth of Leishmania major promastigotes and evaluated their effects on macrophages, dendritic cells, and fibroblasts. Furthermore, we determined the efficacy of selected compounds in decreasing the infection rate of macrophages and regulating their production of cytokines and nitric oxide. Our results demonstrate that the naphthylisoquinoline alkaloids ancistrocladiniums A and B (compounds 10 and 11) and the synthetic isoquinolinium salt (compound 14) were effective against intracellular amastigotes in the low submicromolar range, while toxicity against mammalian cells was observed at concentrations that were significantly higher than those needed to impair parasite replication. The activities of compounds 11 and 14 were mainly directed against the amastigote stage of L. major. This effect was not associated with the stimulation of host macrophages to produce nitric oxide or secrete cytokines relevant for the leishmanicidal function. In conclusion, our data suggest that ancistrocladiniums A and B (compounds 10 and 11) and the synthetically prepared isoquinolinium salt (compound 14) are promising candidates to be considered as lead compounds for leishmanicidal drugs.
Toxic pentavalent antimonials constitute the mainstay treatment for leishmaniasis. Second-line drugs, such as pentamidine and amphotericin B, display high liver and heart toxicities and cause uncomfortable side effects, including fever (14, 30). Therefore, the chemotherapy of leishmaniasis requires close clinical control. Moreover, antileishmanial treatments remain extremely expensive for countries in which the disease is endemic. Misuse of these compounds has increased the frequency of chemoresistant cases and contributes to the rising incidence of human immunodeficiency virus-Leishmania coinfections (16, 32, 41).
Paramomycin was originally identified as an antileishmanial drug in the 1960s (16). Its oral bioavailability is poor, and the development of the parenteral formulation has been slow. However, phase III trials are currently ongoing in India and East Africa (16). Miltefosine, an inhibitor of sterol biosynthesis, has been approved as the first oral drug for the treatment of visceral leishmaniasis in India (17, 26) and has been used in clinical trials to cure cutaneous leishmaniasis in Colombia (42). Cure rates of visceral leishmaniasis reach 94 to 97% (26), with few side effects. Unfortunately, relapses in miltefosine-treated patients infected with Leishmania amazonensis have been reported (41) and, at least in vitro, Leishmania parasites develop resistance to miltefosine (18, 34, 39). These issues emphasize that the development of oral, effective, safer, and cheaper chemotherapeutic agents for treating leishmaniasis is not only desirable but fundamental to control this tropical disease.
Different approaches have been used to identify novel pharmacophores against protozoan organisms, and one promising scheme is the analysis of naturally occurring plant-derived compounds (15, 19, 25, 27, 40), including naphthylisoquinoline alkaloids, isolated from tropical lianas belonging to the plant families Ancistrocladaceae and Dioncophyllaceae (5, 6). This class of naturally occurring biaryls was initially described as exhibiting strong growth-inhibiting activities in vitro against Plasmodium falciparum and Plasmodium berghei (20, 21, 23) and in vivo against P. berghei (22). Efficacy against Trypanosoma brucei (11) and Leishmania donovani has also been reported (8-10). These findings suggest that naphthylisoquinoline alkaloids constitute an interesting class of antiparasitic compounds that should be pharmacologically explored.
In the present study, we compared the activities of 11 isolated naphthylisoquinoline alkaloids and three synthetic analogs (Fig. 1A) against the growth of Leishmania major promastigotes, J774.1 macrophages, and NIH 3T3 fibroblasts and determined their effects on the survival of bone marrow-derived dendritic cells (BMDC) and peritoneal macrophages. In addition, we evaluated the efficacy of selected compounds in decreasing the infection rate of peritoneal macrophages containing L. major amastigotes. The results demonstrate that the naphthylisoquinoline alkaloids ancistrocladinium A (compound 10) and ancistrocladinium B (compound 11) and a synthetically prepared isoquinolinium salt (compound 14) are promising candidates to be considered as lead compounds for leishmanicidal drugs since they are effective against intracellular amastigotes at concentrations in the low submicromolar range, while toxicity against various mammalian cells is observed only at higher concentrations.
FIG. 1.
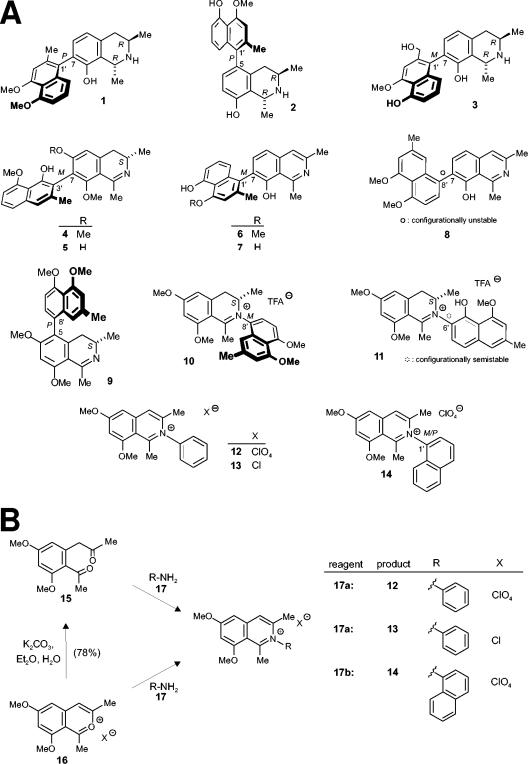
Substances tested for activity against L. major. (A) Structures of the naphthylisoquinoline alkaloids dioncophyllines A (compound 1) and C (compound 2), dioncopeltine A (compound 3), ancistrocladidine (compound 4), ancistroheynine B (compound 5), ent-dioncophylleine A (compound 6), 5′-O-demethyl-ent-dioncophylleine A (compound 7), dioncophylleine D (compound 8), ancistroealaine A (compound 9), ancistrocladiniums A (compound 10) and B (compound 11), and the synthetically prepared analogs (compounds 12 to 14). Note that the naphthylisoquinolinium salt (compound 14) occurs as a racemic mixture of atropisomers at the rotationally hindered N,C axis. (B) Synthesis of naphthylisoquinolinium salts 12 to 14. Naphthylisoquinoline alkaloid analogs compounds 12, 13, and 14) were synthesized from compounds 15 and 16. TFA, trifluoroacetic acid.
MATERIALS AND METHODS
Materials.
Starting materials for the synthesis of compounds were purchased from Sigma-Aldrich (Taufkirchen, Germany) and used without any further purification. Sephadex LH-20 was obtained from Amersham Bioscience (Uppsala, Sweden). Organic solvents were distilled and dried prior to use, using standard procedures. l-Glutamine was obtained from Biochrom (Berlin, Germany); Click RPMI 1640 medium, Dulbecco's modified Eagle medium (DMEM), and HEPES buffer were from Invitrogen (Karlsruhe, Germany); diethanol amine [NH(CH2CH2OH)2] and magnesium chloride (MgCl2) were from Merck (Darmstadt, Germany); fetal calf serum (FCS) was from PAA Laboratories (Linz, Austria); granulocyte-macrophage colony-stimulating factor (GM-CSF) was from PeproTech (London, United Kingdom); acridine orange, ammonium chloride (NH4Cl), amphotericin B, dimethyl sulfoxide (DMSO), ethidium bromide, gelatin, gentamicin, 2-mercaptoethanol, N-(1-naphthyl)ethylenediamine dihydrochloride, nonessential amino acids, penicillin, phosphoric acid, sodium hydrogen carbonate (NaHCO3), sodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), sulfanilamide, streptomycin, and Tween 20 were from Sigma-Aldrich; and Alamar Blue was from Trinova Biochem (Gieβen, Germany).
General experimental procedures.
Melting points were determined on a Reichert-Jung Thermovar hot plate (Reichert Optische Werke, Vienna, Austria) and are uncorrected. The nuclear magnetic resonance (NMR) spectra (1H, 400 MHz, and 13C, 100 MHz) were recorded on a Bruker AMX 400 (Bruker BioSpin, Rheinstetten, Germany), using deuterochloroform (δ 7.26 and 77.01; Roth, Karlsruhe, Germany) and methanol-d4 (δ 3.31 and 49.15; Roth) as the solvents and the internal 1H and 13C standards. Electron ionization mass spectra were determined on a Finnigan MAT 8200 mass spectrometer (Thermo Electron Corporation, Whatman) in the positive mode (70 eV). The combustion analyses were performed on a CHNS 932 analyzer (Leco Instruments, St. Joseph, MI).
Isolation of naphthylisoquinoline alkaloids.
Previously described methods were used to isolate the different naphthylisoquinoline alkaloids: dioncophyllines A (compound 1) and C (compound 2) and dioncopeltine A (compound 3) from Triphyophyllum peltatum (3, 4, 6); ancistrocladidine (compound 4) and ancistroheynine B (compound 5) from Ancistrocladus heyneanus (12); ent-dioncophylleine A (compound 6), 5′-O-demethyl-ent-dioncophylleine A (compound 7) and dioncophylleine D (compound 8) from Ancistrocladus benomensis (13), and ancistroealaine A (compound 9) from Ancistrocladus ealaensis (8). Ancistrocladiniums A (compound 10) and B (compound 11) were obtained from the leaves of an as-yet-undescribed Congolese Ancistrocladaceae species. The CH2Cl2/MeOH extract was submitted to liquid-liquid separation, fast centrifugal chromatography, and preparative high-performance liquid chromatography to yield compounds 10 and 11.
Synthesis of naphthylisoquinoline alkaloid analogs 12 to 14.
Naphthylisoquinoline alkaloid analogs (compounds 12, 13, and 14) were synthesized from compounds 15 and 16. The diketone (compound 15) and benzopyrylium salt (compound 16) were prepared according to a synthetic pathway described previously (2) (Fig. 1B).
N-Phenyl-6,8-dimethoxy-1,3-dimethylisoquinolinium perchlorate (compound 12).
The new N-phenyl-6,8-dimethoxy-1,3-dimethylisoquinolinium perchlorate (compound 12) was obtained by treatment of 315 mg (0.99 mmol) 6,8-dimethoxy-1,3-dimethyl-3-benzopyrylium perchlorate (compound 16) with 92 mg (0.99 mmol) aniline (compound 17a) in 4 ml glacial acetic acid for 5 h at room temperature. The yellow solid was filtered and washed with diethyl ether (Et2O). The mother liquor was diluted with methanol and purified using a Sephadex-LH20 column with methanol as the eluent. Crude products were combined and recrystallized from a mixture of methanol-Et2O and n-hexane to afford 291 mg (0.74 mmol, 75%) of the isoquinolinium perchlorate (compound 12) as yellow needles. Melting point, 279°C; 1H-NMR (400 MHz, CDCl3) δ = 2.21 (3H, s, CH3), 2.77 (3H, s, CH3), 3.88 (3H, s, OCH3), 3.92 (3H, s, CH3), 6.62 (1H, d, J = 2.3 Hz, Ar-H), 6.85 (1H, d, J = 2.3 Hz, Ar-H), 7.18 (2H, dd, J = 6.3 Hz, J = 1.4 Hz, Ar-H), 7.50 (3H, m, Ar-H), 7.72 (1H, s, Ar-H); 13C-NMR (100 MHz, CDCl3) δ = 21.94 (CH3), 23.26 (CH3), 56.50 (OCH3), 56.60 (OCH3), 98.90 (Ar-C), 102.5 (Ar-C), 115.1 (Ar-C), 122.5 (Ar-C), 125.9 (Ar-C), 131.1 (Ar-C), 139.0 (Ar-C), 142.2 (Ar-C), 143.8 (Ar-C), 145.5 (Ar-C), 158.7 (Ar-C), 161.3 (Ar-C), 167.5 (Ar-C), 184.8 (Ar-C); mass specrometry (MS) (electron impact [EI]) m/z (%) = 295 [M + H]+ (2), 294 [M]+ (9), 278 (62), 262 (100), 234 (22), 139 (11), 109 (13), 44 (30); combustion analysis (C19H20ClNO6): calculated CHN content (calcd) C 57.95, H 5.12, N 3.56; found C 57.93, H 5.16, N 3.51.
N-Phenyl-6,8-dimethoxy-1,3-dimethylisoquinolinium chloride (compound 13).
After addition of 300 mg (1.27 mmol) diketone (compound 15) to 118 mg (1.27 mmol) aniline (compound 17a), dissolved in 5 ml acetonitrile and 1 ml hydrochloric acid (HCl), the reaction mixture was refluxed for 5 h. The solvent was evaporated, and the residue was dissolved in 1 ml of methanol. The crude product was purified by chromatography on Sephadex-LH20 material with methanol as the eluent. Recrystallization from methanol-Et2O gave 182 mg (0.55 mmol, 55%) N-phenyl-6,8-dimethoxy-1,3-dimethylisoquinolinium chloride (compound 13) as a yellow amorphous solid. Melting point, 191°C; 1H-NMR (400 MHz, methanol-d4) δ = 2.28 (3H, s, CH3), 2.90 (3H, s, CH3), 4.08 (3H, s, OCH3), 4.11 (3H, s, CH3), 6.97 (1H, d, J = 2.27 Hz, Ar-H), 7.13 (1H, d, J = 2.27 Hz, Ar-H), 7.50 (2H, m, Ar-H), 7.76 (3H, m, Ar-H), 7.98 (1H, s, Ar-H); 13C-NMR (100 MHz, methanol-d4) δ = 22.2 (CH3), 23.9 (CH3), 57.3 (OCH3), 57.4 (OCH3), 102.7 (Ar-C), 105.1 (Ar-C), 118.5 (Ar-C), 126.5 (Ar-C), 129.8 (Ar-C), 133.4 (Ar-C), 137.7 (Ar-C), 143.5 (Ar-C), 146.4 (Ar-C), 148.1 (Ar-C), 163.3 (Ar-C), 165.6 (Ar-C), 169.0 (Ar-C), 169.1 (Ar-C), 171.5 (Ar-C); MS (EI) m/z (%) = 295 [M + H]+ (2), 294 [M]+ (9), 278 (62), 262 (100), 234 (22), 139 (11), 109 (13), 44 (30); combustion analysis (C19H20ClNO2) calcd. C 69.19, H 6.11, N 4.25; found C 69.07, H 6.28, N 4.03.
N,1′-Naphthyl-6,8-dimethoxy-1,3-dimethylisoquinolinium perchlorate (compound 14).
The isoquinolinium salt (compound 14) was produced by stirring a mixture of 300 mg (0.94 mmol) 6,8-dimethoxy-1,3-dimethyl-2-benzopyrylium perchlorate (compound 16) and 130 mg (0.95 mmol) 1-aminonaphthalene (compound 17b) in 4 ml glacial acid for 8 h at room temperature. The yellow solid was filtered and washed with Et2O. The mother liquor was diluted with methanol and purified by chromatography on Sephadex-LH20 material with methanol as the eluent. The crude products were combined and recrystallized from a mixture of methanol, Et2O, and n-hexane to afford 262 mg (0.59 mmol, 59%) of the isoquinolinium perchlorate (compound 14) as yellow needles. Melting point, 224°C; 1H-NMR (400 MHz, CDCl3) δ = 2.33 (3H, s, CH3), 2.81 (3H, s, CH3), 4.05 (3H, s, OCH3), 4.12 (3H, s, CH3), 6.75 (1H, s, Ar-H), 6.94 (1H, d, J = 7.9 Hz, Ar-H), 7.37 (1H, br, Ar-H), 7.55 (1H, t, J = 7.4 Hz, Ar-H), 7.63 (1H, t, J = 7.4 Hz, Ar-H), 7.76 (2H, br, Ar-H), 8.05 (1H, d, J = 8.0 Hz, Ar-H), 8.15 (1H, d, J = 7.4 Hz, Ar-H), 8.32 (1H, br, Ar-H); 13C-NMR (100 MHz, CDCl3) δ = 21.84 (CH3), 23.23 (CH3), 57.23 (OCH3), 57.47 (OCH3), 100.2 (Ar-C), 116.1 (Ar-C), 120.6 (Ar-C), 124.1 (Ar-C), 126.3 (Ar-C), 126.8 (Ar-C), 128.2 (Ar-C), 129.8 (Ar-C), 130.0 (Ar-C), 132.0 (Ar-C), 134.9 (Ar-C), 135.9 (Ar-C), 143.4 (Ar-C), 145.1 (Ar-C), 159.8 (Ar-C), 162.1 (Ar-C), 168.3 (Ar-C); MS (EI) m/z (%) = 344 [M]+ (10), 343 [M-1]+ (40), 328 [M-CH3]+ (41), 312 (100), 202 (11), 134 (11), 127 (9), 44 (24); combustion analysis (C23H22ClNO6) calcd. C 62.24, H 5.00, N 3.16; found C 61.82, H 5.07, N 3.09.
Parasites.
The cloned virulent L. major isolate MHOM/IL/81/FE/BNI was maintained by passage in BALB/c mice (6 to 8 weeks old) (Charles River Breeding Laboratories, Sulzfeld, Germany). Promastigotes were grown in blood agar cultures at 26°C in 5% CO2 with 95% humidity. For the experiments described here, promastigotes were washed twice with phosphate-buffered saline (PBS) and suspended at 1 × 108 cells ml−1 in Click RPMI 1640 medium supplemented with 10% FCS, 2 mM l-glutamine, 10 mM HEPES buffer, pH 7.2, 100 μg ml−1 penicillin, 160 μg ml−1 gentamicin, 7.5% NaHCO3, and 5 × 10−5 M 2-mercaptoethanol (complete medium).
Cells and cell lines.
The macrophage cell line J774.1 was maintained in complete medium. For the experimental procedures, cells were detached from the flasks with a rubber policeman, washed twice with PBS, and suspended at 2 × 106 cells ml−1 in complete medium. Resident peritoneal exudate cells were obtained from BALB/c mice by peritoneal washing with 5 ml of ice-cold complete medium. The cells were subsequently incubated for 10 min with ice-cold TAC buffer (10 mM NH4Cl in PBS, pH 7.2) to eliminate erythrocytes, centrifuged, and suspended at 2 × 106 cells ml−1 in complete medium. Macrophages constituted the major population of peritoneal exudate cells (36). BMDC were generated from bone marrow progenitors of BALB/c mice as described previously (29, 37). Briefly, bone marrow cells were cultured in Click RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 10 mM HEPES buffer (pH 7.2), 60 μg ml−1 penicillin, and 20 μg ml−1 gentamicin, in the presence of 200 U ml−1 GM-CSF. Cultures were fed with GM-CSF on days 3, 6, and 8. At day 10, the nonadherent cells were collected and washed with PBS. Cells were suspended at 2 × 106 cells ml−1 in complete medium. These cells show typical dendritic cell morphology with a myeloid dendritic cell phenotype (37). The mouse embryo fibroblast cell line NIH 3T3 was grown to 80 to 90% confluence in DMEM supplemented with 10% FCS, 0.1 mM nonessential amino acids, 100 U ml−1 penicillin, and 100 U ml−1 streptomycin on gelatin-coated (0.1% in PBS) cell culture dishes at 37°C in 5% CO2 at 95% humidity. For the experimental procedures, cells were detached from the flasks with a rubber policeman, washed with PBS, and suspended in DMEM at 2 × 106 cells ml−1.
Analysis of in vitro antiproliferative activity.
Promastigotes were seeded into 96-well plates in complete medium without phenol red (200 μl), in the absence or presence of increasing concentrations of naphthylisoquinoline alkaloids. They were then incubated for 24 h at 26°C in 5% CO2 with 95% humidity. Following the addition of 20 μl of Alamar Blue, the plates were incubated again and the optical densities (OD) measured 24 and 48 h later with a Multiskan Ascent enzyme-linked immunosorbent assay (ELISA) reader (Thermo Electron Corporation) using a test wavelength of 540 nm and a reference wavelength of 630 nm (31). Absorbance in the absence of compounds was set as 100% of growth (31). J774.1 macrophages, peritoneal macrophages, and BMDC were cultured in 200 μl of complete medium without phenol red, and fibroblasts were cultured in DMEM, in the absence or presence of increasing concentrations of naphthylisoquinoline alkaloids, for 24 h at 37°C in 5% CO2 with 95% humidity. Following the addition of 20 μl of Alamar Blue, the plates were incubated again and the OD were measured 24, 48, and 72 h later as described previously (1). Amphotericin B was used as a reference compound and positive control. The effects of cell density, incubation time, and concentration of DMSO were examined in control experiments. The final concentration of DMSO in the medium never exceeded 1% (vol/vol) and had no effect on the proliferation of extracellular or intracellular parasites. For each experiment, each drug concentration was assayed in duplicate wells.
Analysis of macrophage infection rate.
Freshly isolated peritoneal macrophages (4 × 105 cells ml−1) were allowed to adhere in vitro for 2 h at 37°C in 5% CO2 with 95% air humidity. Nonadherent cells were removed by extensive washing with complete medium. Subsequently, adherent cells were infected for 24 h with stationary-phase L. major promastigotes at a parasite/macrophage ratio of 15 to 1, in a final volume of 0.5 ml of complete medium. After removal of extracellular parasites by thorough rinsing with fresh complete medium, cells were incubated further for 48 h in the absence of drugs or in the presence of increasing concentrations of compounds. Amphotericin B was used as a reference compound and positive control. Intracellular parasites were quantified by staining with acridine orange and ethidium bromide and analyzed by fluorescence microscopy at 495 nm, as described previously (35).
Statistical analysis.
Data on the antiproliferative activity of the compounds (from at least two experiments) were analyzed with Thermo Electron Ascent software (Thermo Electron Corporation) and Microsoft Excel. OD values at 48 h were used to calculate the 50% inhibitory concentrations (IC50) via linear interpolation (24). The index included in Table 1 was calculated as the ratio of the IC50 value for macrophages to the IC50 for L. major. Data on infection rate are expressed as mean values ± standard errors of the mean of at least three experiments in which 300 macrophages were analyzed for each drug concentration. The program Graph-pad was used to fit the data to nonlinear regression and to determine the concentration that decreases the infection rate to 50% (the 50% effective concentration [EC50]). Differences in IC50 and EC50 values of treated or untreated macrophages were tested for statistical significance by the unpaired Student's t test using the Graph-pad program.
TABLE 1.
In vitro activities of naphthylisoquinoline alkaloids and synthetic analogs against L. major promastigotes and J774.1 macrophages
| Compound | IC50 (μM)a
|
||
|---|---|---|---|
| L. major | J774.1 | Index | |
| 1 | 46.99 ± 20.70 | ND | ND |
| 2 | 36.49 ± 3.55 | 32.83 ± 0.69 | <1 |
| 3 | >100 | >100 | ND |
| 4 | >100 | >100 | ND |
| 5 | 33.01 ± 4.29 | 29.90 ± 0.28 | <1 |
| 6 | 32.30 ± 0.00 | 34.83 ± 0.00 | 1.07 |
| 7 | >100 | >100 | ND |
| 8 | >100 | >100 | ND |
| 9 | >100 | >100 | ND |
| 10 | 4.90 ± 2.93 | 31.76 ± 4.75 | 6.48 |
| 11 | 1.24 ± 0.15 | 11.21 ± 2.09 | 9.04 |
| 12 | 26.58 ± 24.50 | 39.20 ± 6.69 | 1.47 |
| 13 | 46.99 ± 26.10 | 43.70 ± 18.60 | <1 |
| 14 | 2.91 ± 2.37 | 10.15 ± 6.70 | 3.48 |
Experiments with parasites and macrophages were performed in parallel. ND, not determined. The index is calculated as the ratio of the IC50 values for macrophages to the IC50 for L. major.
RESULTS
Cytotoxicity against L. major promastigotes and the macrophage cell line J774.1.
The activities of various naturally occurring naphthylisoquinoline alkaloids and their synthetic analogs were tested in vitro using L. major promastigotes and the macrophage cell line J774.1. The results in Table 1 show that dioncophylline C (compound 2), ancistroheynine B (compound 5), ent-dioncophylleine A (compound 6), and N-phenyl-6,8-dimethoxy-1,3-dimethylisoquinolinium chloride (compound 13) inhibited the growth of J774.1 macrophages (IC50, 32.83 ± 0.69, 29.90 ± 0.28, 34.83 ± 0.00, and 43.70 ± 18.60 μM, respectively) at concentrations similar to those needed to inhibit the proliferation of parasites. In contrast, compound 12 inhibited the growth of J774.1 macrophages (IC50, 39.20 ± 6.69 μM) at concentrations higher than those needed to inhibit the proliferation of L. major (Table 1), but the compound displayed low antileishmanial activity (IC50, 26.58 ± 24.50 μM). However, compounds 10, 11, and 14 displayed high antileishmanial activity (IC50, 4.90 ± 2.93, 1.24 ± 0.15, and 2.91 ± 2.37 μM, respectively) and were toxic against the J774.1 macrophage cell line at higher concentrations than those needed to inhibit the parasite cell growth (IC50, 31.76 ± 4.75, 11.21 ± 2.09, and 10.15 ± 6.70 μM, respectively). The IC50 for amphotericin B, which was used as a reference compound, was 2.51 ± 1.36 μM for L. major promastigotes, without affecting the growth of J774.1 macrophages. Together, these results demonstrate that ancistrocladiniums A (compound 10) and B (compound 11) and the synthetic analog (compound 14) are effective against L. major promastigotes at concentrations in the low micromolar range, while they impair the growth of J774.1 macrophages at concentrations that are at least threefold higher. The data also suggest that the efficacy of these compounds against L. major is similar to that of amphotericin B, although they are more toxic against the macrophage cell line J774.1.
Cytotoxicity against macrophages, BMDC, and NIH 3T3 fibroblasts.
As the in vitro cytotoxicity indexes [IC50(J774.1)/IC50(L. major)] of compounds 10, 11 and 14 were higher than 3, we also analyzed their cytotoxicity against cells of the NIH 3T3 fibroblast line, freshly isolated peritoneal macrophages, and in vitro-generated BMDC. The different cell types were treated with increasing concentrations of naphthylisoquinoline alkaloids, and the cytotoxicity of the compounds was evaluated using the Alamar blue method. The IC50 values against macrophages and BMDC were 41.68 ± 13.13 μM and 36.01 ± 13.23 μM for compound 10, 16.93 ± 5.10 μM and 20.10 ± 3.51 μM for compound 11, and 15.50 ± 6.00 μM and 27.40 ± 4.01 μM for compound 14. The IC50 values against NIH 3T3 fibroblasts were 10.62 ± 0.42 μM for compound 11 and 15.00 ± 2.05 μM for compound 14. These results indicate that naphthylisoquinoline alkaloids impair the growth of fibroblasts as well as freshly prepared macrophages and BMDC at concentrations that are similar to those interfering with the proliferation of the J774.1 macrophage cell line: i.e., at concentrations that are 3- to 20-fold higher than those needed to impair the growth of parasites.
Decrease of the infection rate of peritoneal macrophages.
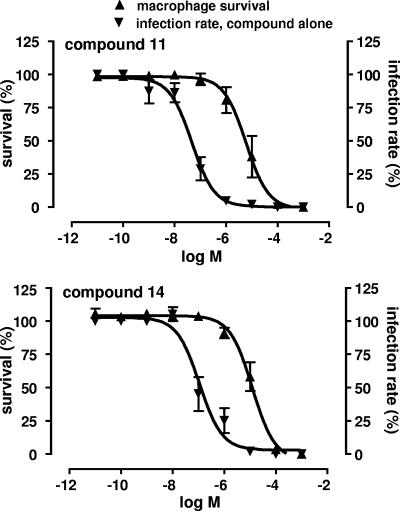
The results described above encouraged us to analyze the activities of compounds 10, 11, and 14 against intracellular L. major amastigotes. To this end, freshly isolated peritoneal macrophages were infected with L. major for 24 h and then treated with increasing concentrations of the compounds for 48 h. Figure 2 presents a summary of the data for macrophage survival and decrease in infection rate after treatment of the cells with compounds 11 and 14. Macrophages that were not treated with compounds had infection rates of 20 to 30%, which were consistent within each experiment. The infection rates of untreated peritoneal macrophages were normalized to 100% for further analysis of the results. Macrophage survival was affected by compounds 10, 11, and 14 at concentrations higher than those needed to decrease the infection rate, as described in the previous section. A 50% decrease of the infection rate was obtained at concentrations of 1.31 ± 0.50 μM (confidence interval, 0.8 to 2.12 μM; r2 = 0.978), 0.05 + 0.02 μM (confidence interval, 0.027 to 0.069 μM; r2 = 0.948), and 0.11 ± 0.03 μM (confidence interval, 0.064 to 0.188 μM; r2 = 0.949) for compounds 10 (data not shown), 11, and 14, respectively (Fig. 2). The EC50 for amphotericin B, which was used as a reference compound, was 0.075 ± 0.019 μM (confidence interval, 0.039 to 0.139 μM; r2 = 0.997). These results demonstrate that a significant decrease in macrophage infection rate is obtained at concentrations of compounds 11 and 14 that are 25-fold lower than those needed to inhibit the growth of L. major promastigotes. Furthermore, the efficacy of the agents against intracellular amastigotes is similar to that of amphotericin B.
FIG. 2.
Dose-response curves showing the effects of compounds ancistrocladinium B (compound 11) (upper panel) and the synthetic analog 14 (lower panel) on the survival and infection rate of peritoneal macrophages. Macrophages were infected with stationary-phase L. major promastigotes for 24 h. After removal of extracellular parasites, the cells were incubated further for 48 h in the absence or presence of increasing concentrations of drugs. Macrophage survival and infection rate were normalized to 100%.
DISCUSSION
Chemotherapy against leishmaniasis is mainly based on the antimonial compounds sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime). The mode of action of antimonials is poorly understood, and their toxicity causes serious side effects that often result in patients deserting the treatment. Furthermore, there is a worldwide escalating frequency of chemoresistance to antimonials. Thus, affordable alternative drugs against leishmaniasis are desperately needed. Testing of novel pharmacophores against protozoan organisms has led to the identification of naphthylisoquinoline alkaloids.
The bioactivities of the naturally occurring plant-derived naphthylisoquinoline alkaloids have previously been evaluated against Plasmodium, Trypanosoma, and L. donovani (7, 8, 11, 20, 21, 23). Here we show that they are also active against L. major. Notably, this is the first report addressing the cytotoxic activities of naphthylisoquinoline alkaloids against important host cells of Leishmania: i.e., macrophages and dendritic cells. The results presented herein demonstrate that selected compounds of this class are effective against intracellular amastigotes at concentrations in the low submicromolar range, while toxicity against various mammalian cells is observed only at manifold higher concentrations.
Ancistroealaine A (compound 9), one of the most active naphthylisoquinoline alkaloids against L. donovani (6), was inactive against L. major. The IC50 activity of compound 9 against L. donovani promastigotes was determined to be 4.10 μg ml−1 (8). The same range of concentrations is needed for compounds 10 (2.61 μg ml−1), 11 (1.52 μg ml−1), and 14 (1.29 μg ml−1) to reach the IC50 against L. major promastigotes.
The results for all compounds tested (compounds 1 to 14) reveal the IC50 values against L. major to extend over more than three logarithmic ranges, despite great structural similarities. In general, the naphthylisoquinoline alkaloids, having a C,C-biaryl axis connecting the naphthyl and isoquinoline moieties (compounds 1 to 9), display weak or no leishmanicidal activity, whereas the isolated alkaloids 10 and 11, which are N,C coupled and thus equipped with a hetero-biaryl axis, exhibit significantly higher leishmanicidal activities. Both are quaternary alkaloids, and thus cationic isoquinolinium salts, here with trifluoroacetic acid as the counter-ion.
Compared to the alkaloids 10 and 11, the synthetic analogs 12 to 14 are equipped with a fully aromatic isoquinolinium part and a simplified aryl moiety. The most promising synthetic structure was found to be the naphthalene-substituted isoquinolinium perchlorate 14, which exhibited an activity 10 times higher than that of the phenyl isoquinolinium perchlorate 12. The less active phenyl analogs 12 and 13 differ in their activities against L. major by a factor of 2. This difference may be a result of the different counter-ion that stabilizes each structure, an observation that strongly suggests that the counter-ion may have an effect on the leishmanicidal activity.
Our results demonstrate that ancistrocladiniums A (compound 10) and B (compound 11), the synthetic analog 14, and amphotericin B, herein used as a reference compound, are effective against L. major promastigotes at similar concentrations. However, ancistrocladiniums A (compound 10) and B (compound 11) and isoquinolinium perchlorate 14 were more toxic against J774.1 macrophages and peritoneal macrophages than amphotericin B. Compounds 10, 11, and 14 also displayed moderate toxicities against BMDC. Together, these data suggest that structural modifications of the lead compounds should be performed in order to decrease their cytotoxicity, assisted by toxicity-guided quantitative structure-activity relationship (QSAR) investigations (43). This work is in progress.
Since the activity of the compounds against L. major promastigotes is not necessarily predictive of their activity against the intracellular amastigote form, we assayed the efficacy of compounds 10, 11, and 14 in decreasing the infection rate of peritoneal macrophages parasitized with L. major. Our results reveal that 25-fold lower concentrations of compounds 11 or 14 are needed to decrease the infection rate of peritoneal macrophages by 50% than to inhibit promastigote growth by 50%. The effective concentrations of compounds 11 or 14 are similar to the concentrations of amphotericin B needed to decrease the infection rate of peritoneal macrophages by 50% (18, 28), demonstrating that despite the higher toxicities of compounds 11 and 14 against peritoneal macrophages, their efficacy against amastigotes is similar to that of amphotericin B.
Macrophages are important target cells in the therapy of leishmaniasis because they are critical for the clearance of intracellular parasites via the production of cytokines and reactive nitrogen metabolites (38). We recently demonstrated that the activity of another class of novel antileishmanial pharmacophores, the cysteine protease inhibitors aziridine-2,3-dicarboxylates 13b and 13e, is based not only on a direct leishmanicidal effect but also on the modulation of cytokine secretion and nitric oxide production by infected macrophages (36). Therefore, it was of interest to analyze the production of proinflammatory cytokines by macrophages treated with naphthylisoquinoline alkaloids in the present study. Surprisingly, while exposure of L. major-infected macrophages to compounds 11 or 14 alone did not cause significant changes in the levels of interleukin 1β (IL-1β), IL-6, and tumor necrosis factor alpha, these compounds strongly reduced the release of IL-6 and tumor necrosis factor alpha in response to bacterial lipopolysaccharide or the cytokine gamma interferon, two types of macrophage stimulators (data not shown). These findings showed that compounds 11 and 14 do not induce cytokine secretion by macrophages but rather interfere with the cells' cytokine response to potent stimulators and, therefore, suggest that the compounds may help to limit the extent of the inflammatory response and to restore homeostasis of the host cell. Moreover, compounds 11 and 14 did not induce the production of nitric oxide by infected macrophages (data not shown). Together, these results strongly suggest that the significant decrease in macrophage infection rate induced by naphthylisoquinoline alkaloids is based on a direct effect of the compounds on the intracellular parasites. This differs from the antileishmanial action of the cysteine protease inhibitors aziridine-2,3-dicarboxylates, which involves both a direct leishmanicidal effect and the alteration of the cytokine activity and nitric oxide production by host macrophages (36), and emphasizes the importance of analyzing the mechanism of action of drug candidates. An apoptosis-like death pathway may be the possible mode of action of the isoquinolinium salts. In this context, it should be noted that compounds 10, 11, and 14 share structural features with miltefosine, which has been proposed to induce an apoptosis-like death pathway in the parasite (33).
A question that remains to be answered is why low inhibitor concentrations decreased the infection rate of macrophages but did not kill promastigotes (Table 1 and Fig. 2). This effect might be the consequence of an increased sensitivity of amastigotes or a selective activity of the naphthylisoquinolines against amastigotes. Alternatively, it may be caused by a selective uptake mechanism at the level of the macrophage plasma membrane with enhanced concentrations of the compounds reaching the parasitophorous vacuole within the host cell.
Together, our results indicate that the naphthylisoquinolines 11 and 14 are effective against intracellular amastigotes at submicromolar concentrations and most likely act directly on the parasites. With regard to structural features, these results demonstrate the importance of the quaternary nitrogen atom and its counter-ion, which clearly increase the antileishmanial activity. These findings are helpful for the QSAR-guided design, selection, and synthesis of novel synthetic naphthylisoquinoline alkaloid-related molecules with anti-infective activity.
Acknowledgments
The authors gratefully acknowledge financial support from the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 630) and the Fonds der Chemischen Industrie (fellowship of T. Gulder and supplies).
The authors are grateful to Calin Apetrei and Simon Berzel for help with cell isolation; Christina De Witt and Christine Hambrecht for technical assistance; and Marcela Fajardo-Moser, Ulrich Körner, and Katharina Remer for fruitful discussions.
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Ahmed, S. A., R. M. Gogal, and J. F. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 2.Bringmann, G., and J. R. Jansen. 1985. Einfache Synthesen nützlicher Diketo-Bausteine für biomimetische Isochinolin- und Naphthalin-Synthesen. Liebigs Ann. Chem. 11:2116-2125. [Google Scholar]
- 3.Bringmann, G., M. Rübenacker, P. Vogt, H. Busse, L. Aké Assi, K. Peters, and H. G. von Schnering. 1991. Dioncopeltine A and dioncolactone A: alkaloids from Triphyophyllum peltatum. Phytochemistry 30:1691-1696. [Google Scholar]
- 4.Bringmann, G., M. Rübenacker, R. Weirich, and L. Aké Assi. 1992. Dioncophylline C from the roots of Triphyophyllum peltatum, the first 5,1′-coupled Dioncophyllaceae alkaloid. Phytochemistry 31:4019-4024. [Google Scholar]
- 5.Bringmann, G., and F. Pokorny. 1995. The naphthylisoquinoline alkaloids, p. 127-271. In G. A. Cordell (ed.), The alkaloids, vol. 46. Academic Press, New York, NY. [Google Scholar]
- 6.Bringmann, G., G. François, L. Aké Assi, and J. Schlauer. 1998. The alkaloids of Triphyophyllum peltatum (Dioncophyllaceae). Chimia 52:18-28. [Google Scholar]
- 7.Bringmann, G., W. Saeb, R. God, M. Schäfer, G. François, K. Peters, E. M. Peters, P. Proksch, K. Hostettmann, and L. Aké Assi. 1998. 5′-O-Demethyldioncophylline A, a new antimalarial alkaloid from Triphyophyllum peltatum. Phytochemistry 49:1667-1673. [DOI] [PubMed] [Google Scholar]
- 8.Bringmann, G., A. Hamm, C. Günther, M. Michel, R. Brun, and V. Mudogo. 2000. Ancistroealaines A and B, two new bioactive naphthylisoquinolines, and related naphthoic acids from Ancistrocladus ealaensis. J. Nat. Prod. 63:1465-1470. [DOI] [PubMed] [Google Scholar]
- 9.Bringmann, G., K. Messer, R. Brun, and V. Mudogo. 2002. Ancistrocongolines A-D, new naphthylisoquinoline alkaloids from Ancistrocladus congolensis. J. Nat. Prod. 65:1096-1101. [DOI] [PubMed] [Google Scholar]
- 10.Bringmann, G., M. Dreyer, J. H. Faber, P. W. Dalsgaard, D. Stærk, J. Jaroszewski, H. Ndangalasi, F. Mbago, R. Brun, M. Reichert, K. Maksimenka, and S. B. Christensen. 2003. Ancistrotanzanine A, the first 5,3′-coupled naphthylisoquinoline alkaloid, and two further, 5,8′-linked related compounds from the newly described species Ancistrocladus tanzaniensis. J. Nat. Prod. 66:1159-1165. [DOI] [PubMed] [Google Scholar]
- 11.Bringmann, G., V. Hoerr, U. Holzgrabe, and A. Stich. 2003. Antitrypanosomal naphthylisoquinoline alkaloids and related compounds. Pharmazie 58:343-346. [PubMed] [Google Scholar]
- 12.Bringmann, G., M. Dreyer, M. Michel, F. S. K. Tayman, and R. Brun. 2004. Ancistroheynine B and two further 7,3′-coupled naphthylisoquinoline alkaloids from Ancistrocladus heyneanus. Phytochemistry 65:2903-2907. [DOI] [PubMed] [Google Scholar]
- 13.Bringmann, G., M. Dreyer, H. Kopff, H. Rischer, M. Wohlfarth, H. A. Hadi, R. Brun, H. Meimberg, and G. Heubl. 2005. Ent-dioncophylleine A and related dehydrogenated naphthylisoquinoline alkaloids, the first Asian Dioncophyllaceae-type alkaloids, from the “new” plant species Ancistrocladus benomensis. J. Nat. Prod. 68:686-690. [DOI] [PubMed] [Google Scholar]
- 14.Chia, J. K. S., and E. J. McManus. 1990. In vitro tumor necrosis factor induction assay for analysis of febrile toxicity associated with amphotericin B preparations. Antimicrob. Agents Chemother. 34:906-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croft, S. L., A. T. Evans, and R. A. Neal. 1985. The activity of plumbagin and other electron carriers against Leishmania donovani and Leishmania mexicana amazonensis. Ann. Trop. Med. Parasitol. 79:651-653. [DOI] [PubMed] [Google Scholar]
- 16.Croft, S. L., K. Seifert, and V. Yardley. 2006. Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 123:399-410. [PubMed] [Google Scholar]
- 17.Desjeux, P. 2004. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27:305-318. [DOI] [PubMed] [Google Scholar]
- 18.Escobar, P., S. Matu, C. Marques, and S. L. Croft. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Trop. 81:151-157. [DOI] [PubMed] [Google Scholar]
- 19.Fournet, A., A. A. Barrios, V. Munoz, R. Hocquemiller, and A. Cave. 1992. Effect of natural naphthoquinones in BALB/c mice infected with Leishmania amazonensis and L. venezuelensis. Trop. Med. Parasitol. 43:219-222. [PubMed] [Google Scholar]
- 20.François, G., G. Bringmann, C. Dochez, C. Schneider, G. Timperman, and L. Aké Assi. 1995. Activities of extracts and naphthylisoquinoline alkaloids from Triphyophyllum peltatum, Ancistrocladus abbreviatus and Ancistrocladus barteri against Plasmodium berghei (Anka strain) in vitro. J. Ethnopharmacol. 46:115-120. [DOI] [PubMed] [Google Scholar]
- 21.François, G., G. Timperman, J. Holenz, L. Aké Assi, T. Geuder, L. Maes, J. Dubois, M. Hanocq, and G. Bringmann. 1996. Naphthylisoquinoline alkaloids exhibit strong growth-inhibiting activities against Plasmodium falciparum and P. berghei—in vitro-structure-activity relationships of dioncophylline C. Ann. Trop. Med. Parasitol. 90:115-123. [DOI] [PubMed] [Google Scholar]
- 22.François, G., G. Timperman, W. Eling, L. Aké Assi, J. Holenz, and G. Bringmann. 1997. Naphthylisoquinoline alkaloids against malaria: evaluation of the curative potentials of dioncophylline C and dioncopeltine A against Plasmodium berghei in vivo. Antimicrob. Agents Chemother. 41:2533-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.François, G., G. Timperman, T. Steenackers, L. Aké Assi, J. Holenz, and G. Bringmann. 1997. In vitro inhibition of liver forms of the rodent malaria parasite Plasmodium berghei by naphthylisoquinoline alkaloids—structure-activity relationships of dioncophyllines A and C and ancistrocladine. Parasitol. Res. 83:673-679. [DOI] [PubMed] [Google Scholar]
- 24.Huber, W., and J. C. Koella. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55:257-261. [DOI] [PubMed] [Google Scholar]
- 25.Iwu, M. M., J. F. Jackson, and B. G. Schuster. 1994. Medicinal plants in the fight against leishmaniasis. Parasitol. Today 10:65-68. [DOI] [PubMed] [Google Scholar]
- 26.Jha, T. K., S. Sundar, C. P. Thakur, P. Bachmann, J. Karbwang, C. Fischer, A. Voss, and J. Berman. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 341:1795-1800. [DOI] [PubMed] [Google Scholar]
- 27.Kayser, O., A. F. Kiderlen, H. Laatsch, and S. L. Croft. 2000. In vitro leishmanicidal activity of monomeric and dimeric naphthoquinones. Acta Trop. 77:307-314. [DOI] [PubMed] [Google Scholar]
- 28.Larabi, M., V. Yardley, P. M. Loiseau, M. Appel, P. Legrand, A. Gulik, C. Bories, S. L. Croft, and G. Barratt. 2003. Toxicity and antileishmanial activity of a new stable lipid suspension of amphotericin B. Antimicrob. Agents Chemother. 47:3774-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Röβner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 30.McGuire, T. R., W. J. Trickler, L. Smith, E. B. Hoie, and D. W. Miller. 2005. Release of TNF-α and IL-1β from porcine brain endothelium corresponds to the pyrogenic potential of three marketed formulations of amphotericin. Inflamm. Res. 54:375-379. [DOI] [PubMed] [Google Scholar]
- 31.Mikus, J., and D. Steverding. 2000. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 48:265-269. [DOI] [PubMed] [Google Scholar]
- 32.Ouellette, M., J. Drummelsmith, and B. Papadopoulou. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Update 7:257-266. [DOI] [PubMed] [Google Scholar]
- 33.Paris, C., P. M. Loiseau, C. Bories, and J. Bréard. 2004. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 48:852-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Victoria, J. M., F. J. Pérez-Victoria, A. Parodi-Talice, I. A. Jiménez, A. G. Ravelo, S. Castanys, and F. Gamarro. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 45:2468-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponte-Sucre, A. I., Y. Campos, M. Fernández, H. Moll, and A. Mendoza-León. 1998. Growth and survival of Leishmania sp. are impaired by ion channel blockers. Exp. Parasitol. 88:11-16. [DOI] [PubMed] [Google Scholar]
- 36.Ponte-Sucre, A., R. Vicik, M. Schultheis, T. Schirmeister, and H. Moll. 2006. Aziridine-2,3-dicarboxylates: peptidomimetic cysteine protease inhibitors with antileishmanial activity. Antimicrob. Agents Chemother. 50:2439-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramírez-Pineda, J. R., A. Fröhlich, C. Berberich, and H. Moll. 2004. Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J. Immunol. 172:6281-6289. [DOI] [PubMed] [Google Scholar]
- 38.Scott, P. 2003. Development and regulation of cell-mediated immunity in experimental leishmaniasis. Immunol. Res. 27:489-498. [DOI] [PubMed] [Google Scholar]
- 39.Seifert, K., S. Matu, J. Pérez-Victoria, S. Castanys, F. Gamarro, and S. L. Croft. 2003. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int. J. Antimicrob. Agents 22:380-387. [DOI] [PubMed] [Google Scholar]
- 40.Sepulveda-Boza, S., and B. K. Cassels. 1996. Plant metabolites active against Trypanosoma cruzi. Planta Med. 62:98-105. [DOI] [PubMed] [Google Scholar]
- 41.Singh, S., and R. Sivakumar. 2004. Challenges and new discoveries in the treatment of leishmaniasis. J. Infect. Chemother. 10:307-315. [DOI] [PubMed] [Google Scholar]
- 42.Soto, J., J. Toledo, P. Gutierrez, R. S. Nicholls, J. Padilla, J. Engel, C. Fischer, A. Voss, and J. Berman. 2001. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin. Infect. Dis. 33:57-61. [DOI] [PubMed] [Google Scholar]
- 43.Stiefl, N., G. Bringmann, C. Rummey, and K. Baumann. 2003. Evaluation of extended parameter sets for the 3D-QSAR technique MaP: implications for interpretability and model quality exemplified by antimalarially active naphthylisoquinoline alkaloids. J. Comp.-Aided Mol. Des. 17:347-365. [DOI] [PubMed] [Google Scholar]