Abstract
In this exploratory study, the human immunodeficiency virus (HIV) protease inhibitor atazanavir was detected in seminal plasma in 15 out of 15 HIV-infected men taking an atazanavir-containing regimen. However, this penetration was limited and variable, and the median seminal/blood plasma ratio was only 0.1.
Human immunodeficiency virus type 1 (HIV-1) RNA concentrations in blood plasma and seminal plasma decrease during successful highly active antiretroviral therapy (HAART) (1, 10, 16), but the concentrations of HIV-1 RNA in blood plasma and seminal plasma do not always show a parallel response (3, 17). Especially during (a)symptomatic local inflammation/infection, leukocytospermia, and advanced-stage HIV infection circumstances may be favorable for (more) local HIV production (12), and one concern with current antiretroviral therapy is that the protease inhibitors, with the exception of indinavir and amprenavir, show poor penetration into seminal plasma (2, 5, 8, 12, 13). Poor penetration of antiretroviral drugs into an anatomical site could lead to independent HIV-1 RNA replication during antiretroviral therapy, allowing the local selection or development of drug-resistant strains (6, 9, 12).
Atazanavir is a new HIV protease inhibitor that allows once-daily dosing. Since data on the penetration of atazanavir in semen are currently lacking, we determined atazanavir concentrations in blood and seminal plasma of 15 HIV-1-infected men using an atazanavir-containing HAART regimen.
Between December 2003 and January 2006, these men were recruited from the HIV outpatient clinic of the Academic Medical Center, Amsterdam, The Netherlands. Men were eligible if they were using atazanavir as part of their HAART for at least 6 weeks and were compliant with their therapy. Exclusion criteria were symptoms of a genital infection and a vasectomy. The study was approved by the local Medical Ethics Committee, and all patients gave written informed consent.
Semen was produced by masturbation, and the ejaculate was collected in a sterile container. Within 1 hour the semen sample was centrifuged at 1,200 × g for 10 min and the supernatant, consisting of seminal plasma, was stored at −20°C until analysis of drug levels.
A venous blood sample was taken within 2 hours before or after semen collection, for the measurement of atazanavir concentrations in blood plasma. Time of last intake of the drugs, production of the semen sample, and collection of the blood were recorded. Atazanavir concentrations in heparinized blood plasma were measured using high-performance liquid chromatography. Atazanavir concentrations in seminal plasma were measured using high-performance liquid chromatography coupled with tandem mass spectrometry as described previously (4). Sample pretreatment consisted of protein precipitation with 50% methanol in acetonitrile using 100 μl of blood plasma or seminal plasma. Chromatographic separation of atazanavir from endogenous compounds was established with reversed-phase chromatography on an Inertsil ODS3 column (50- × 2.0-mm inside diameter; particle size, 5 μm). A quick stepwise gradient using an acetate buffer (pH 5) and methanol was applied at a flow rate of 0.5 ml/minute in a total run time of 5.5 min. The column outlet was connected to the mass spectrometer inlet through a postcolumn splitter (1:4). Drug concentrations measured in the range of 0.01 to 10 μg/ml were validated. Previously determined intra- and interday coefficients of variation were less than 3.8%, and accuracies were within ±7.3% (4).
The 15 patients used the atazanavir-containing regimen for a median duration of 31 (range, 12 to 68) weeks. Two men were using unboosted 400 mg atazanavir once daily, nine men were using 300 mg atazanavir once daily boosted with 100 mg ritonavir, and four men were using 400 mg atazanavir once daily boosted with 100 mg ritonavir, the last in the case of an efavirenz- or nevirapine-containing regimen. In three patients atazanavir was started as first-line therapy; in the other patients previous regimens were changed because of side effects or virological failure. At the time of the study visit all patients had a blood plasma HIV-1 RNA concentration below 500 copies/ml, with the exception of one patient who had a temporary “viral blip.” All but one patient had hyperbilirubinemia (mean total bilirubin, 38 [standard deviation, 19.0] μmol/liter; reference value, 0 to 17 μmol/liter).
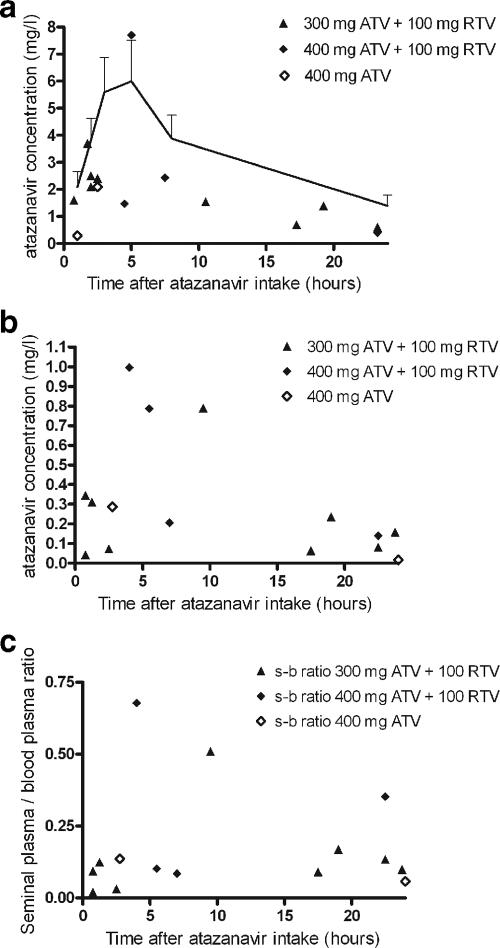
Blood plasma atazanavir levels were in the lower range for all patients, especially for those using unboosted atazanavir (Fig. 1a). None of the patients used a proton pump inhibitor or rifampin.
FIG. 1.
(a and b) Atazanavir (ATV) concentration in blood plasma (a) and seminal plasma (b) versus time after medication intake. (c) Seminal/blood plasma ratio. The dosage of atazanavir is indicated in the upper right corner. In panel a the population curve for once-daily atazanavir (300 mg)-ritonavir (RTV; 100 mg) is given (15).
Atazanavir was detected in all seminal plasma samples (Fig. 1b). The atazanavir concentration ranged from 0.02 to 0.99 mg/liter (median, 0.21 mg/liter; interquartile range, 0.07 to 0.35 mg/liter). The exact degree of protein binding in seminal plasma is not known. However, the concentration of albumin in seminal plasma is approximately the same as in serum (7, 14). A weak correlation was observed between the seminal plasma and the blood plasma concentration of atazanavir (ρ = 0.46, P = 0.08; Spearman's rank). The median seminal/blood plasma ratio was only 0.10 (interquartile range, 0.08 to 0.17) (Fig. 1c). No clear relationship was observed between the seminal/blood plasma ratio and the time since atazanavir intake (ρ = 0.20, P = 0.46; Spearman's rank).
In summary, atazanavir was detected in all seminal plasma samples. As 86% of atazanavir is bound to human serum protein (11), our findings are in agreement with previous studies that describe good penetration into the seminal plasma of antiretroviral drugs with a protein binding of less than or equal to 90% (12). However, in most patients the penetration of atazanavir was low, as the median seminal/blood plasma ratio was only 0.10. The gold standard approach to calculate a ratio is to conduct area-under-the-curve studies in blood and semen and to report that ratio. The reported seminal/blood plasma ratios should therefore be interpreted with some caution.
Thus, it appears that atazanavir, in contrast to most other protease inhibitors, penetrates into seminal plasma. However, this penetration is limited and variable.
Acknowledgments
This research has been funded by grant number 7003 from AIDS Fonds Netherlands, which is a not-for-profit charity organization.
The funding source had no influence on the analysis of the data or on the content of the manuscript.
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Barroso, P. F., M. Schechter, P. Gupta, M. F. Melo, M. Vieira, F. C. Murta, Y. Souza, and L. H. Harrison. 2000. Effect of antiretroviral therapy on HIV shedding in semen. Ann. Intern. Med. 133:280-284. [DOI] [PubMed] [Google Scholar]
- 2.Chaudry, N. I., J. J. Eron, O. J. Naderer, A. S. Pereira, M. B. Wire, S. A. Fiscus, and A. D. Kashuba. 2002. Effects of formulation and dosing strategy on amprenavir concentrations in the seminal plasma of human immunodeficiency virus type 1-infected men. Clin. Infect. Dis. 35:760-762. [DOI] [PubMed] [Google Scholar]
- 3.Craigo, J. K., B. K. Patterson, S. Paranjpe, K. Kulka, M. Ding, J. Mellors, R. C. Montelaro, and P. Gupta. 2004. Persistent HIV type 1 infection in semen and blood compartments in patients after long-term potent antiretroviral therapy. AIDS Res. Hum. Retrovir. 20:1196-1209. [DOI] [PubMed] [Google Scholar]
- 4.Crommentuyn, K. M., H. Rosing, M. J. Hillebrand, A. D. Huitema, and J. H. Beijnen. 2004. Simultaneous quantification of the new HIV protease inhibitors atazanavir and tipranavir in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 804:359-367. [DOI] [PubMed] [Google Scholar]
- 5.Ghosn, J., M. L. Chaix, G. Peytavin, E. Rey, J. L. Bresson, C. Goujard, C. Katlama, J. P. Viard, J. M. Treluyer, and C. Rouzioux. 2004. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1-infected men. AIDS 18:1958-1961. [DOI] [PubMed] [Google Scholar]
- 6.Ghosn, J., J. P. Viard, C. Katlama, M. de Almeida, R. Tubiana, F. Letourneur, L. Aaron, C. Goujard, D. Salmon, M. Leruez-Ville, C. Rouzioux, and M. L. Chaix. 2004. Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pre-treated HIV-infected men. AIDS 18:447-457. [DOI] [PubMed] [Google Scholar]
- 7.Hafez, E. S. E. 1976. Human semen and fertility regulation in men. The C. V. Mosby Company, St. Louis, MO.
- 8.Isaac, A., S. Taylor, P. Cane, E. Smit, S. E. Gibbons, D. J. White, S. M. Drake, S. Khoo, and D. J. Back. 2004. Lopinavir/ritonavir combined with twice-daily 400 mg indinavir: pharmacokinetics and pharmacodynamics in blood, CSF and semen. J. Antimicrob. Chemother. 54:498-502. [DOI] [PubMed] [Google Scholar]
- 9.Kim, L. U., M. R. Johnson, S. Barton, M. R. Nelson, G. Sontag, J. R. Smith, F. M. Gotch, and J. W. Gilmour. 1999. Evaluation of sperm washing as a potential method of reducing HIV transmission in HIV-discordant couples wishing to have children. AIDS 13:645-651. [DOI] [PubMed] [Google Scholar]
- 10.Leruez-Ville, M., E. Dulioust, D. Costabliola, D. Salmon, A. Tachet, L. Finkielsztejn, M. De Almeida, B. Silbermann, D. Sicard, P. Jouannet, and C. Rouzioux. 2002. Decrease in HIV-1 seminal shedding in men receiving highly active antiretroviral therapy: an 18 month longitudinal study (ANRS EP012). AIDS 16:486-488. [DOI] [PubMed] [Google Scholar]
- 11.Le Tiec, C., A. Barrail, C. Goujard, and A. M. Taburet. 2005. Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin. Pharmacokinet. 44:1035-1050. [DOI] [PubMed] [Google Scholar]
- 12.Lowe, S. H., S. U. Sankatsing, S. Repping, F. V. Veen, P. Reiss, J. M. Lange, and J. M. Prins. 2004. Is the male genital tract really a sanctuary site for HIV? Arguments that it is not. AIDS 18:1353-1362. [DOI] [PubMed] [Google Scholar]
- 13.Pereira, A. S., L. M. Smeaton, J. G. Gerber, E. P. Acosta, S. Snyder, S. A. Fiscus, R. R. Tidwell, R. M. Gulick, R. L. Murphy, and J. J. Eron, Jr. 2002. The pharmacokinetics of amprenavir, zidovudine, and lamivudine in the genital tracts of men infected with human immunodeficiency virus type 1 (AIDS clinical trials group study 850). J. Infect. Dis. 186:198-204. [DOI] [PubMed] [Google Scholar]
- 14.Rumke, P., N. van Amstel, E. N. Messer, and P. D. Bezemer. 1974. Prognosis of fertility of men with sperm agglutinins in the serum. Fertil. Steril. 25:393-398. [DOI] [PubMed] [Google Scholar]
- 15.Taburet, A. M., C. Piketty, C. Chazallon, I. Vincent, L. Gerard, V. Calvez, F. Clavel, J. P. Aboulker, and P. M. Girard. 2004. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 48:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor, S., N. M. Ferguson, P. A. Cane, R. M. Anderson, and D. Pillay. 2001. Dynamics of seminal plasma HIV-1 decline after antiretroviral treatment. AIDS 15:424-426. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, H., G. Dornadula, M. Beumont, L. Livornese, Jr., B. Van Uitert, K. Henning, and R. J. Pomerantz. 1998. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N. Engl. J. Med. 339:1803-1809. [DOI] [PubMed] [Google Scholar]