Abstract
This trial was aimed to estimate the pharmacokinetic interaction between voriconazole and methadone at steady state in male patients on methadone therapy and to characterize the safety and tolerability profile during the coadministration. Twenty-three patients on individualized methadone therapy (30 to 100 mg once daily) were enrolled into this randomized, patient- and investigator-blind, placebo-controlled, parallel-group study. Methadone pharmacokinetic samples were collected from patients receiving methadone alone as the baseline before they were randomized to coadminister either 200 mg voriconazole twice daily (BID) (400-mg BID loading doses on the first day) (n = 16) or matching placebo (n = 7) for the next 5 days. Pharmacokinetic samples for methadone and voriconazole were collected on the last day of voriconazole dosing. The safety data were collected throughout the study. Voriconazole increased the steady-state exposure of pharmacologically active enantiomer (R)-methadone: the mean area under the concentration-time curve from 0 to 24 h (AUC0-24) was increased by 47.2% (90% confidence intervals [CI]: 37.7%, 57.4%), and the mean peak concentration (Cmax) was increased by 30.7% (90% CI: 22.2%, 39.8%). The magnitude of increase in (S)-methadone exposure was greater than that of (R)-methadone: the AUC0-24 was increased by 103.4% (90% CI: 85.0%, 123.6%), and the Cmax was increased by 65.4% (90% CI: 52.6%, 79.2%). Methadone appeared to have no effect on the steady-state voriconazole pharmacokinetics compared to the historical data for voriconazole alone. Methadone patients receiving voriconazole showed no signs or symptoms of significant opioid withdrawal or overdose. Coadministration of 200 mg voriconazole BID with methadone was generally safe and well tolerated. Nevertheless, caution should be exercised when voriconazole is coadministered with methadone due to the increase in (R)-methadone exposure, which in turn may require a dose reduction of methadone.
Subjects most susceptible to serious fungal infections are typically immunocompromised patients, which include, but are not limited to, human immunodeficiency virus-AIDS patients. Drug abuse by injection also accounts for a high percentage of AIDS cases in adults and adolescents, and therefore, a proportion of human immunodeficiency virus-AIDS patients receive methadone for the treatment of opiate abstinence syndrome (4, 10). Because of the risk of fungal infections in an addict population, it is possible that voriconazole would be used in patients receiving methadone maintenance therapy.
Voriconazole is a broad-spectrum triazole antifungal agent approved for the primary treatment of acute invasive aspergillosis and as a salvage therapy for serious fungal infections caused by Scedosporium apiospermum and Fusarium species as well as for candidemia in nonneutropenic patients (VFEND [voriconazole] package insert; Pfizer Inc., New York, NY). It was reported that in vitro voriconazole is 4- to 16-fold more active than fluconazole and 2- to 8-fold more active than itraconazole against Candida species including C. krusei and C. glabrata (2, 22). In common with other triazole antifungal agents, voriconazole inhibits fungal cytochrome P450 (CYP)-dependent 14-α-sterol demethylase, an essential enzyme in the synthesis of ergosterol (6, 11, 23). Results of in vitro and in vivo studies have shown that voriconazole is metabolized by the cytochrome P450 isozymes CYP2C19, CYP2C9 and, to a lesser extent, CYP3A4, and it also inhibits the activity of CYP2C19, CYP2C9, and, to a lesser extent, CYP3A4, possibly through the saturation of active sites (17, 24, 25, 29). The major metabolite of voriconazole is the N-oxide metabolite, which has minimal antifungal activity and accounts for more than 70% of the metabolites in plasma. CYP2C19 is significantly involved in the metabolism of voriconazole and exhibits genetic polymorphism, which accounts for a considerable proportion of the intersubject variability in voriconazole pharmacokinetics. In this study, however, patients were not genotyped for CYP2C19 because the main focus of the study was to evaluate the effect of voriconazole on methadone pharmacokinetics in a crossover design in which each patient was his own control. Furthermore, the prevalence of poor CYP2C19 metabolizers is low (3 to 5%) in Caucasians and blacks (the study population) (VFEND package insert) (31).
Methadone hydrochloride is a synthetic μ-opioid receptor agonist that is widely used in the prevention of opiate abstinence syndrome and also as an analgesic in patients with severe pain (21). Methadone is typically administered as a racemic mixture of (R)- and (S)-methadone, but only (R)-methadone is pharmacologically active and responsible for most opioid activity (18). Since methadone has very large intersubject variability in its pharmacokinetics (i.e., variable protein binding, variable enzymatic activities, etc.) and pharmacodynamics (receptor affinity), the dosing regimen for patients on methadone therapy is individualized as the standard practice (3, 10, 21). In general, because of the high morbidity and mortality associated with opioid dependence, methadone is usually used at a daily dose within the range of 30 to 100 mg (median, 70 mg) (7, 8, 10). Methadone is metabolized primarily by CYP3A4 and to a lesser extent by CYP2D6 through N-demethylation to form an inactive metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (3, 9, 10). Many in vitro and in vivo studies suggested that other CYP enzymes may also be involved in methadone metabolism, including CYP1A2, CYP2B6, CYP2C8, CYP2C9, and CYP2C19, and some have demonstrated a stereoselectivity preference, such as CYP2C8 and CYP2D6 towards stereoselective metabolism of (R)-methadone and CYP2B6 towards (S)-methadone (3, 10, 15, 28). Methadone has not been shown to be an inducer or inhibitor of CYP3A4 or other isozymes associated with voriconazole metabolism. It has been reported that methadone may inhibit the activity of CYP2D6 (30). Although methadone and voriconazole share the CYP3A4 metabolic pathway, it is not likely that methadone would have an effect on voriconazole pharmacokinetics based on the knowledge that CYP3A4 substrates and inhibitors, such as erythromycin and indinavir, have no effect on voriconazole pharmacokinetics in humans (VFEND package insert).
It is possible that the inhibition of the metabolic activity of CYP3A4, CYP2C9, and CYP2C19 by voriconazole may cause a clinically relevant increase in (R)-methadone exposure leading to methadone toxicity, and this may paradoxically be interpreted as opioid withdrawal. The adverse effects and toxicity of methadone are similar to those described for morphine, including respiratory depression, nausea, vomiting, dizziness, mental clouding, dysphoria, pruritus, constipation, increased pressure in the biliary tract, urinary retention, and hypotension (3, 10, 21). Two of the major concerns with methadone overdose are respiratory depression and potential QT prolongation, since these effects could increase the risk of mortality in methadone patients. Careful monitoring of electrocardiograms (ECGs) and respiration rates (RR) was conducted in this study.
The primary objectives of this study were to estimate the effect of multiple oral therapeutic doses of voriconazole on the steady-state pharmacokinetics of methadone in patients on methadone therapy and to characterize the safety and tolerability profile of methadone and voriconazole treatment. The secondary objective was to estimate the effect of methadone treatment on the steady-state pharmacokinetics of voriconazole.
MATERIALS AND METHODS
Study design.
This study was a randomized, patient- and investigator-blind, sponsor-open, placebo-controlled, parallel-group, multiple-dose study of 23 male patients on methadone therapy for the prevention of opiate abstinence syndrome. This study was blinded with respect to voriconazole treatment. Patients who had been receiving a once-daily (QD) oral methadone dose (30 to 100 mg) for at least 30 days and who met the screening inclusion criteria were enrolled into the study after they signed the informed consent. Patients continued to receive their individualized methadone doses in the morning throughout the study. On study day 2, serial blood samples were collected for methadone measurement, and patients were assigned to one of two groups based on a 2:1 randomization code: group 1 (voriconazole) (n = 16) or group 2 (placebo) (n = 7). On study days 3 to 7, in addition to their methadone dose, patients also received oral voriconazole twice daily (BID) (400 mg BID on day 3 and 200 mg BID on days 4 to 7) or matching placebo. Serial blood samples for methadone, voriconazole, and its N-oxide metabolite measurement were collected on day 7. All patients were confined to the clinical research unit (CRU) at the Cincinnati VA Medical Center for 7 days, and patients returned for a follow-up safety visit at 7 to 10 days after the last dose of voriconazole or placebo before final discharge from the study. The study protocol was approved by the Cincinnati Addiction Research Center's Institutional Review Board and the R & D Committee of the Cincinnati VA Medical Center.
Study population.
All male patients on methadone were otherwise healthy, were 18 to 55 years old with a body mass index (BMI) of between 18 and 30 kg/m2, and weighed >50 kg. Healthy was defined as no clinically significant abnormalities other than those associated with a patient population receiving methadone therapy for the prevention of opiate abstinence syndrome. Patients were excluded if they had known hypersensitivity to azoles, positive urine drug screen (except for methadone or its metabolites), or evidence of liver disease or if they were dependent on or abusing alcohol. Patients were prohibited from taking medications known to be inhibitors, inducers, or substrates of the CYP3A4 enzyme or to interact with voriconazole. No consumption of grapefruit or grapefruit-containing products within 7 days before the first dose of voriconazole was allowed.
Drug administration and sample collection.
Voriconazole (VFEND; Pfizer) and matching placebo tablets were supplied to the CRU by Pfizer (New York, NY). Methadone hydrochloride (Dolophine hydrochloride; Roxane) was supplied by the CRU pharmacy under the conditions for distribution and use of methadone products described in the Code of Federal Regulations, Title 21, Section 291.505. While confined to the CRU, patients were fasted at least 4 h before any safety laboratory evaluations and 8 h prior to the start of drug administration. Patients continued without food for at least 1 h after dosing.
Blood samples (5 ml) for (R)- and (S)-methadone measurements were collected into heparinized tubes on days 2 and 7 at 1, 2, 3, 4, 6, 8, 10, 12, 16, and 24 h postdose as well as prior to the morning methadone dose on days 1 to 2 and 4 to 7 (trough concentrations [Cmin]). Blood samples (5 ml) for measurement of voriconazole and its N-oxide metabolite were collected on day 7 at predose and 1, 2, 3, 4, 6, 8, 10, and 12 h after the morning dose. All blood samples were centrifuged at 1,700 × g for about 10 min at approximately 4°C, and the harvested plasma was stored at approximately −20°C within 1 h of collection until analysis.
Analytical methods.
CEDRA Corporation (Austin, TX) analyzed plasma samples of (R)- and (S)-methadone using a validated liquid chromatography (LC) atmospheric pressure ionization tandem mass spectrometry (MS/MS) assay (ATM-415; CEDRA) (26). Methadone-D9 was used as the internal standard. The plasma samples (0.250 ml) were extracted via solid-phase extraction and eluted with a methanol-triethylamine solution. An aliquot of the dried reconstituted extract was analyzed using SCIEX API 3000 LC/MS/MS apparatus equipped with a chiral column. The dynamic range of the assay for methadone was 5 to 500 ng/ml per enantiomer. PPD Development (Richmond, VA) analyzed plasma samples for voriconazole and its N-oxide metabolite using a previously validated LC/MS/MS assay (27). The plasma samples (0.100 ml) were extracted using a solid-phase extraction procedure followed by LC/MS/MS separation and detection. The dynamic range of the assay for voriconazole was 10 to 2,500 ng/ml, and that for its N-oxide metabolite was 20 to 5,000 ng/ml. The accuracy of the quality control samples used during sample analysis ranged from −0.550% to 1.10%, with a precision of ≤5.66% for voriconazole, and for the N-oxide metabolite, the accuracy of the quality control samples ranged from −3.75% to 1.25%, with a precision of ≤10.2%. Samples were analyzed within the established long-term stability period.
Pharmacokinetic analysis.
Pharmacokinetic analysis was performed with WinNonlin v.3.2 (Pharsight, Mountain View, CA) using standard noncompartmental methods. Maximum observed plasma concentrations (Cmax), time to reach Cmax (Tmax), and trough concentrations (Cmin) for (R)-methadone, (S)-methadone, voriconazole, and the N-oxide metabolite were estimated directly from concentration-time data. The area under the plasma concentration-time curve (AUC) during the dosing interval (τ) (AUC from 0 to 24 h [AUC0-24] for methadone and AUC0-12 for voriconazole and the N-oxide metabolite) was estimated using linear/log-trapezoidal approximation. The approximate linearity of methadone steady-state pharmacokinetics at daily doses ranging from 7.5 mg to 130 mg was demonstrated previously (13). For the purpose of presenting mean pharmacokinetic profiles and mean parameter values at a given dose, the methadone concentrations were dose normalized to a 100-mg equivalent dose. Dose normalization of the data does not affect the ratios of AUC0-24 and Cmax, since each patient was his own control for ratio calculations.
Safety assessment.
Assessments included repeated safety laboratory tests (hematology, chemistry, and urinalysis) on days 1 and 7; physical examinations on days 1, 5, and 8 (before discharge from CRU); vital signs (supine heart rate [HR], blood pressure [BP], and RR) and 12-lead ECGs on days 2, 3, 6, and 8 (before discharge from CRU); and continuous adverse-event monitoring. All these assessments were also measured at screening and at follow-up visits. Multiple measures of vital signs and 12-lead ECGs were at predose and 2, 4, and 8 h after the morning dose on days 2 (triplicate baseline values), 3, and 6. In addition, in order to help gauge the severity of symptoms and monitor changes in clinical status during the study, all patients were queried daily for signs and symptoms commonly seen in patients with opioid withdrawal using the Clinical Institute Narcotic Assessment (CINA) scale, including at the follow-up visit (14, 16).
Statistical analysis. (i) Sample size determination.
It was determined that a sample size of 12 patients completing the voriconazole treatment (group 1) was sufficient to provide 80% power to detect a 20% difference in (R)-methadone steady-state pharmacokinetic parameters with a 90% confidence interval (CI) range (19). These calculations were based on the intersubject coefficient of variation (55%) of the steady-state AUC0-24 for (R)-methadone (13).
(ii) Pharmacokinetic parameters.
AUC0-τ and Cmax for methadone (dose normalized), voriconazole, and the N-oxide metabolite are presented as arithmetic means and standard deviations (SD), and Tmax is presented as median and range. The median and range are also displayed for methadone AUC0-24, Cmax, and Cmin values without dose normalization and for voriconazole and the N-oxide metabolite AUC0-12 and Cmax values. Natural log-transformed dose-normalized AUC0-24 and Cmax of methadone were analyzed using a mixed-effects analysis of variance model with the SAS MIXED procedure using SAS v.8.2 (SAS Institute Inc., Cary, NC). Restricted maximum likelihood estimation was used. Treatment was specified as the fixed effect with a random effect for patients within a group. The point estimates of the adjusted mean treatment differences (day 7 − day 2) and their respective 90% CIs around the differences were calculated. These estimated treatment differences and their respective confidence limits were anti-log (exponent) transformed to the ratios of the adjusted geometric means (day 7/day 2) and their respective 90% CIs around the ratios. No formal statistical analysis was performed on exposure parameters of voriconazole.
(iii) Safety data.
All the safety data were summarized descriptively. ECGs (Bazett corrected QT [QTcB] and Fridericia corrected QT [QTcF]) and vital signs (supine BP, HR, and RR) were qualitatively described and categorized relative to the change from the mean of day 2 triplicate values (methadone alone) for those on days 3 and 6 (methadone plus voriconazole or placebo), where day 2 measurements served as the time-matched baseline. A linear mixed-effects model for repeated measures was used to model the change from baseline in RR and ECG data for all nominal time points obtained on days 3 and 6, respectively, for each treatment regimen with SAS v.8.2. This model had the treatment as the fixed effect and the baseline as a covariate. This model also compared the within-treatment-group differences between the voriconazole group and the placebo group. Appropriate linear contrasts were used to obtain point estimates of mean differences of interest, and their 95% CIs of the mean differences were constructed. No adjustments were made for multiple comparisons.
RESULTS
Twenty-three male patients receiving an individualized daily methadone dose ranging from 32 to 100 mg entered and completed this study. All patients were included in the pharmacokinetic and safety analyses. Two groups (voriconazole and placebo groups) had similar demographics and baseline characteristics (Table 1). The median methadone daily dose in the voriconazole group (n = 16) was 85 mg, with a mean age of 44 years and a mean weight of 87 kg. In the placebo group (n = 7), the median methadone daily dose was 80 mg, with a mean age of 44 years and a mean weight of 90 kg. Two patients with a BMI higher than 30 kg/m2 were allowed to participate in this study and were balanced in each group.
TABLE 1.
Demographic characteristics at baseline
| Characteristic | Value for group
|
|
|---|---|---|
| Voriconazole (n = 16) | Placebo (n = 7) | |
| Race (no. Caucasian/no. black) | 12/4 | 7/0 |
| Age (yr) [mean ± SD (range)] | 44.0 ± 9.2 (19-54) | 44.3 ± 6.7 (33-55) |
| Wt (kg) [mean ± SD (range)] | 87.2 ± 18.3 (65.3-135.2) | 89.9 ± 12.5 (74.8-108.9) |
| BMIa (kg/m2) [mean ± SD (range)] | 27.3 ± 5.6 (20.6-44.0) | 26.6 ± 2.9 (23.0-30.8) |
| Methadone daily dose (mg) [median (range)] | 85 (55-100) | 80 (32-100) |
Two patients had a BMI higher than 30 kg/m2 (44.0 and 30.8 kg/m2).
Effect of voriconazole on steady-state methadone pharmacokinetics.
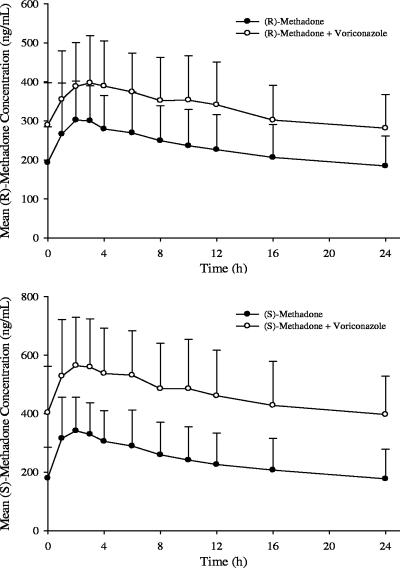
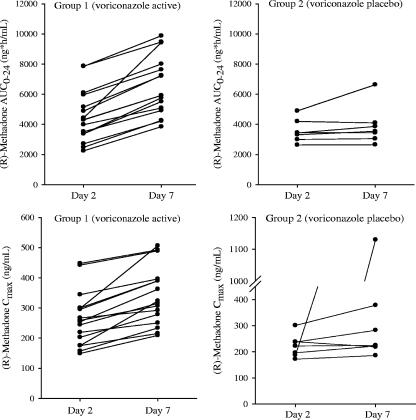
The dose-normalized mean steady-state (R)- and (S)-methadone concentration-time profiles following a continuous QD dose of methadone alone (day 2) and coadministration with 200 mg voriconazole BID (day 7) are shown in Fig. 1. The methadone concentrations were higher following coadministration with voriconazole. As shown in Fig. 2 (left panels), there was a consistent increase in individual steady-state exposure parameters (AUC0-24 and Cmax) of (R)-methadone during coadministration with voriconazole. Increases in the adjusted geometric mean (R)-methadone AUC0-24 and Cmax were 47.2% (90% CI: 37.7%, 57.4%) and 30.7% (90% CI: 22.2%, 39.8%), respectively, following coadministration with voriconazole. A similar trend was observed for (S)-methadone, and increases in the adjusted geometric mean (S)-methadone AUC0-24 and Cmax were 103.4% (90% CI: 85.0%, 123.6%) and 65.4% (90% CI: 52.6%, 79.2%), respectively (Table 2). The 90% CIs of (R)- and (S)-methadone AUC0-24 and Cmax ratios fell out of the 80% to 125% equivalence interval (Table 2). It is evident that the magnitude of the increase in (S)-methadone exposure is approximately 50% higher than that of (R)-methadone. In addition, Table 3 presents the median and range of methadone AUC0-24, Cmax, and Cmin without dose normalization.
FIG. 1.
Mean steady-state methadone plasma concentration-time profiles following continuous QD dose of methadone alone and coadministered with 200 mg voriconazole BID. Concentrations were normalized to a 100-mg methadone dose. Error bars represent standard deviations.
FIG. 2.
Individual steady-state (R)-methadone exposure parameters (AUC0-24 and Cmax) following continuous QD dose of methadone alone and coadministered with 200 mg voriconazole BID or matching placebo. Day 2, methadone alone; day 7, methadone plus voriconazole or placebo. In the lower right panel, the highest Cmax of (R)-methadone on day 7 was the 1-h concentration in the patient receiving 32 mg methadone plus placebo.
TABLE 2.
Summary of dose-mormalized steady-state methadone pharmacokinetic parameters following continuous QD dose of methadone alone (day 2) and coadministration with 200 mg voriconazole BID (day 7)a
| Parameter | Value for groupb
|
Geometric mean ratio (%) (day 7/day 2) | 90% CIs | Range of increase (%) | |
|---|---|---|---|---|---|
| Methadone alone (day 2) | Methadone + voriconazole (day 7) | ||||
| (R)-Methadone | |||||
| AUC0-24 (ng · h/ml) [mean (SD)] | 5,540 (2,100) | 7,980 (2,390) | 147.2 | 137.7, 157.4 | 20-113 |
| Cmax (ng/ml) | 322 (109) | 417 (117) | 130.7 | 122.2, 139.8 | 9-85 |
| Tmax (h) [median (range)] | 2.5 (1.0-4.0) | 3.0 (1.0-6.0) | |||
| (S)-Methadone | |||||
| AUC0-24 (ng · h/ml) [mean (SD)] | 5,730 (2,580) | 11,200 (3,580) | 203.4 | 185.0, 223.6 | 51-203 |
| Cmax (ng/ml) | 366 (128) | 596 (166) | 165.4 | 152.6, 179.2 | 34-151 |
| Tmax (h) [median (range)] | 2.0 (1.0-3.0) | 2.5 (1.0-6.0) | |||
n = 16.
AUC0-24 and Cmax were dose normalized to 100 mg.
TABLE 3.
Steady-state methadone pharmacokinetic parameters following continuous QD dose of 30 to 100 mg methadone alone (day 2) and coadministration with 200 mg voriconazole BID (day 7)a
| Treatment | Median (range)
|
||
|---|---|---|---|
| AUC0-24 (ng · h/ml) | Cmax (ng/ml) | Cmin (ng/ml) | |
| Methadone alone (day 2) | |||
| (R)-Methadone | 4,300 (2,240-7,860) | 256 (149-447) | 147 (78.3-287) |
| (S)-Methadone | 4,030 (2,060-7,770) | 287 (143-488) | 116 (48.2-287) |
| Methadone + voriconazole (day 7) | |||
| (R)-Methadone | 5,910 (3,840-9,870) | 319 (209-506) | 219 (128-418) |
| (S)-Methadone | 8,710 (5,190-13,370) | 486 (298-749) | 308 (180-643) |
n = 16.
As expected, in the placebo group, the dose-normalized mean steady-state (R)- and (S)-methadone concentration-time profiles were similar on days 2 and 7 following repeated dosing. As shown in Fig. 2 (right panel), there was no consistent trend for individual (R)-methadone AUC0-24 and Cmax following coadministration with placebo, except that one patient had an unusually high Cmax on day 7. A similar trend was also observed for (S)-methadone. The unusual Cmax (day 7) in this patient was approximately fivefold higher than that on day 2, while his AUC0-24 on day 7 was slightly higher than that on day 2. The reasons for this high Cmax are not clear (sample reassay was conducted). The statistical analyses of exposure parameters in the placebo group were performed with and without this data point. With the exclusion of this data point, the adjusted geometric mean ratios of (R)-methadone AUC0-24 and Cmax (day 7/day 2) were 102.2% and 102.6%, respectively, with their 90% CIs falling in the equivalence acceptance interval (80% to 125%). The statistical results for (S)-methadone were similar to those for (R)-methadone. These results confirmed that the increase in methadone exposure was due to voriconazole. In the placebo group, the intrasubject coefficients of variation for the AUC0-24 and Cmax of (R)-methadone were 13% and 19%, respectively, and those of (S)-methadone were 14% and 19%, respectively.
The (R)- and (S)-methadone steady state was confirmed following QD dosing of methadone alone and coadministration with voriconazole by visual inspection, which was indicated by similar trough levels on days 1 and 2 and on days 6 and 7. In the voriconazole group, the median change in the (R)-methadone Cmin on day 2 from day 1 was −4.5%, ranging from −21% to 50%, and the median change on day 7 from day 6 was 10.5%, ranging from −8.6% to 31%. A similar range was also observed for the (S)-methadone Cmin. The intersubject variability in the methadone Cmin was comparable between the voriconazole and placebo groups. In the placebo group, the median change in the (R)-methadone Cmin on day 2 from day 1 was −7.2%, ranging from −23.9% to 10.5%, and the median change on day 7 from day 1 was 17.6%, ranging from −53% to 33%.
Effect of methadone on steady-state voriconazole pharmacokinetics.
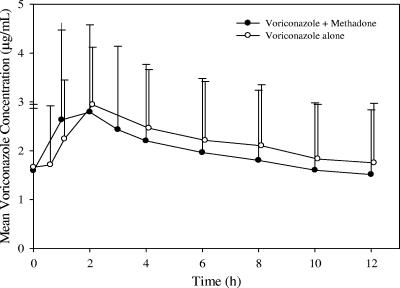
Since the patients were on a continuous methadone dose for the treatment of opiate addiction, it was not ethical to obtain the steady-state voriconazole exposure data in the absence of methadone in these patients. In order to estimate the effect of methadone on voriconazole pharmacokinetics, the historical voriconazole data from a reference study in which the steady-state voriconazole-alone data were obtained from 16 healthy male subjects were used for comparison (20). The mean steady-state voriconazole concentration-time profiles following coadministration with methadone (day 7) appeared to be comparable to those for the historical reference data (Fig. 3). The steady-state exposure parameters of voriconazole and the N-oxide metabolite following coadministration with methadone (day 7) were comparable to those observed in the reference study (Table 4). The intersubject variabilities in the voriconazole AUC0-12 and Cmax were similar in these two studies.
FIG. 3.
Mean steady-state voriconazole plasma concentration-time profile following coadministration of 200 mg voriconazole BID with continuous QD dose of 30 to 100 mg methadone and 200 mg voriconazole BID alone (historical control). The profile for voriconazole alone was obtained from a clinical study of healthy male subjects (20). Error bars represent standard deviations. The time points were separated slightly for presentation purposes.
TABLE 4.
Steady-state pharmacokinetic parameters of voriconazole and its N-oxide metabolite following coadministration of 200 mg voriconazole BID with continuous QD dose of 30 to 100 mg methadone (n = 16) and 200 mg voriconazole BID alone (n = 16)
| Treatment | AUC0-12 (μg · h/ml)
|
Cmax (μg/ml)
|
Tmax (h) [median (range)] | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (range) | Mean (SD) | Median (range) | ||
| Voriconazole | |||||
| With methadone | 24.1 (18.2) | 22.4 (3.34-61.9) | 2.96 (1.82) | 3.10 (0.78-6.14) | 2.0 (1.0-2.0) |
| Alonea | 26.3 (14.0) | 28.5 (5.22-54.4) | 3.06 (1.16) | 3.07 (1.29-5.38) | 2.0 (0.0-4.0) |
| N-Oxide metabolite | |||||
| With methadone | 34.4 (8.66) | 33.8 (18.4-52.0) | 3.26 (0.79) | 3.28 (1.76-4.83) | 4.0 (2.0-12.0) |
| Alonea | 32.6 (8.26) | 32.6 (18.3-45.3) | 3.00 (0.79) | 2.97 (1.68-4.29) | 6.0 (0.5-10.0) |
Pharmacokinetic parameters for voriconazole alone were obtained from a clinical study of healthy male subjects (20).
Safety.
There were no deaths, discontinuations, or dose reductions due to adverse events in this study. During the study period, there were no reports of serious adverse events (SAEs) or severe adverse events. There was one SAE (severe suicide attempt) reported by one patient after 7 days after the last dose of voriconazole. This SAE was not considered treatment related by the investigator but was attributed to cocaine misuse after discharge from the study. A total of 30 adverse events (23 mild and 7 moderate) were reported by 15 out of 23 patients receiving methadone alone on the first 2 days, 62 adverse events (54 mild and 8 moderate) were reported by 15 out of 16 patients receiving methadone plus voriconazole on days 3 to 7 and at the follow-up visit, and 17 adverse events (mild) were reported by 5 out of 7 patients receiving methadone plus placebo on days 3 to 7 and at the follow-up visit (Table 5). Three of the adverse events reported by 3 out of 23 patients on methadone alone were considered treatment related, 23 of the adverse events reported by 10 out of 16 patients on methadone plus voriconazole were considered treatment related, and 7 of the adverse events reported by 3 out of 7 patients on methadone plus placebo were considered treatment related (Table 5). As shown in Table 5, the most frequently reported adverse events were insomnia, back pain, headache, abdominal pain, nausea, somnolence, constipation, and rhinitis. Some of the additional adverse events observed in the voriconazole group are common in patients receiving treatment with voriconazole alone at the therapeutic dose evaluated in this study, such as three patients with six reported mild incidences of abnormal vision (resolved without treatment) (VFEND package insert).
TABLE 5.
Most frequently reported all-causality treatment-emergent signs and symptoms (treatment related)
| All-causality AE (treatment related)a | No. of patients
|
|||
|---|---|---|---|---|
| Voriconazole group
|
Placebo group
|
|||
| Methadone alone (n = 16) | Methadone + voriconazole (n = 16) | Methadone alone (n = 7) | Methadone + placebo (n = 7) | |
| Insomnia | 1 (0) | 5 (1) | 1 (0) | 1 (0) |
| Back pain | 3 (0) | 4 (1) | 0 (0) | 1 (0) |
| Headache | 3 (0) | 2 (0) | 0 (0) | 2 (1) |
| Abdominal pain | 0 (0) | 3 (3) | 1 (0) | 2 (2) |
| Nausea | 1 (0) | 2 (1) | 0 (0) | 2 (1) |
| Somnolence | 2 (0) | 3 (1) | 0 (0) | 0 (0) |
| Constipation | 1 (1) | 2 (1) | 0 (0) | 1 (1) |
| Rhinitis | 1 (1) | 0 (0) | 2 (1) | 1 (0) |
| Abnormal visionb | 0 (0) | 3 (3) | 0 (0) | 0 (0) |
| Total no. of all-causality AEs (treatment related) | 22 (2) | 62 (23) | 8 (1) | 17 (7) |
| Total no. of patients with treatment- related AEs (%) | 2 (12.5) | 10 (62.5) | 1 (14.3) | 3 (42.9) |
If the same patient had more than one occurrence in the same preferred term event category, only the most severe occurrence was noted. Patients were counted only once per treatment in each row.
This event is known to be related to voriconazole treatment.
The majority of the total CINA scores were 0 or 1, and no patient had a total score higher than 7, which indicated that there were no significant signs and symptoms of opioid withdrawal.
There were no clinically significant trends in safety laboratory tests or in mean changes from baseline measurements of BP or HR. There were no differences in postdose changes from baseline values of RR when methadone coadministered with voriconazole was compared with methadone coadministered with placebo, as all of the 95% CIs covered zero. A total of 10 patients had prolonged QT values during the study; however, 9 patients exhibited prolongation of QT and corrected QT (QTc) from their time-matched baselines at variable and inconsistent time points while receiving either methadone plus voriconazole or matching placebo. Table 6 provides a categorical summary of patients with QTcB and QTcF changes from baseline that were between 30 and 60 ms and/or with QTcB and QTcF absolute values of ≥450 ms. These changes were considered within the normal variability for patients on methadone therapy. Additionally, there were no significant mean differences in any postdose changes from baseline values of QTcF or QTcB when methadone coadministered with voriconazole was compared to methadone coadministered with placebo, as all of the 95% CIs contained zero.
TABLE 6.
QTcB and QTcF changes from baselinea
| Parameter | Criterion (ms) | Methadone + voriconazole
|
Methadone + placebo
|
||||
|---|---|---|---|---|---|---|---|
| Total no. of patients | No. of patients that met criteria | % of patients that met criteria | Total no. of patients | No. of patients that met criteria | % of patients that met criteria | ||
| QTcBb | ≥450 | 16 | 5 | 31.3 | 7 | 2 | 28.6 |
| 30-<60d | 16 | 4 | 25.0 | 7 | 1 | 14.3 | |
| QTcFc | ≥450 | 16 | 6 | 37.5 | 7 | 2 | 28.6 |
| 30-<60d | 16 | 4 | 25.0 | 7 | 1 | 14.3 | |
Baseline was the time-matched average of the triplicate values taken at predose and at 2, 4, and 8 h on day 2 (methadone alone).
QTc corrected for heart rate using Bazett's formula: QT/(60/HR)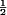 .
.
QTc corrected for heart rate using Fridericia's formula: QT/(60/HR)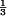 .
.
Maximum increase from baseline.
Overall, the coadministration of 200 mg voriconazole BID in patients on methadone therapy for the treatment of opiate abstinence syndrome was generally safe and well tolerated.
DISCUSSION
The increase in methadone exposure following coadministration with voriconazole was probably due to the inhibition of CYP3A4, CYP2C9, and/or CYP2C19 by voriconazole. It is interesting that there is stereoselective inhibition of methadone metabolism by voriconazole, which resulted in moderate but consistently higher increases in (S)-methadone exposure than in (R)-methadone exposure. It has been reported that CYP3A4 has no stereoselectivity on methadone metabolism (12). In a clinical drug-drug interaction study with paroxetine and methadone, the results suggested that CYP2D6 preferentially metabolized (R)-methadone, and one or more of the other enzymes (CYP1A2, CYP2C9, and CYP2C19) inhibited by paroxetine might have stereoselectivity toward (S)-methadone metabolism (1). Another clinical study with fluvoxamine and methadone suggested that CYP1A2 and CYP2C19 were involved in the metabolism of methadone without stereoselectivity (9). Putting all the information together, CYP2C9 may have stereoselectivity towards (S)-methadone.
The magnitude of the increase in methadone exposure parameters following coadministration with voriconazole does not appeared to be correlated with methadone doses. For instance, four patients who received 100-mg methadone doses had an increase in the (R)-methadone AUC0-24 ranging from 20% to 113%, and two patients who received 55-mg and 60-mg methadone doses had increases in the AUC0-24 of 55% and 71%, respectively. In addition, the relationship between the magnitude of the increase in methadone exposure parameters and the frequency and severity of adverse events was investigated and showed no clear correlation.
Since large intersubject variability in methadone and voriconazole exposures was observed, the following is a discussion of clinical consequences in a few cases in which the extremes of exposures were observed. A 19-year-old Caucasian male receiving a 100-mg methadone dose had the highest increase in (R)-methadone AUC0-24, 113% (9,420 ng · h/ml on day 7), in the presence of voriconazole (AUC0-12, 18.1 μg · h/ml). This patient reported one treatment-related incident (somnolence) on day 3 and had an elevated QTc interval of 478 ms (QTcB) and 467 ms (QTcF) on day 6 (approximately 2 h after the evening dose of voriconazole). Time-matched baseline (day 2) values of 433 ms (QTcB) and 426 ms (QTcF) were recorded for this patient. During the rest of the study, this patient continued to have prolonged QTc intervals of <470 ms, which the investigator considered to be normal. No other adverse events were recorded for this patient; no vital sign measurements or safety laboratory tests were considered to be of potential clinical concern. Three patients had the second-highest increase in (R)-methadone AUC0-24, 71 to 72% (with AUC0-24 achieved on day 7 as 3,840, 4,250, and 5,750 ng · h/ml, respectively), and none of them had treatment-related adverse events. A 50-year-old Caucasian male receiving a 100-mg methadone dose had the lowest increase in (R)-methadone AUC0-24, 20% (7,860 ng · h/ml on day 2 and 9,460 ng · h/ml on day 7), in the presence of voriconazole (AUC0-12, 36.3 μg · h/ml). This patient had three treatment-related adverse events: abnormal vision on day 3, pruritus on days 3 and 4, and agitation on day 6. A 48-year-old Caucasian male receiving a 70-mg methadone dose had the highest exposure to methadone and voriconazole, and the increase in the (R)-methadone AUC0-24 was 26% (7,840 ng · h/ml on day 2 and 9,870 ng · h/ml on day 7) in the presence of voriconazole (AUC0-12, 61.9 μg · h/ml). Based on the exposure values, it is speculated that this patient might be a poor metabolizer of CYP2C19. This patient had two episodes of elevated QTc intervals on days 2 and 3. On day 2 (methadone alone), the QTcB and QTcF values were 594 and 602 ms, respectively, at approximately 2 h postdose. As this was a triplicate measurement and no other measurements recorded at this time were borderline (430 to 450 ms) or prolonged (>450 ms), the investigator attributed this single measurement to a machine error. On day 3, this patient presented with an elevated QTc interval of 520 ms (QTcB) and 526 ms (QTcF) at approximately 2 h after receiving 400 mg voriconazole and 70 mg methadone; this abnormal measurement was considered to be treatment related. Although QTc interval values recorded at the remaining protocol-specified times did not meet the criteria for clinical significance, the investigator did not consider them to be normal, with the exception of the 8-h postdose measurement on day 6 and before discharge from CRU on day 8. This patient also reported a few mild, treatment-related adverse events, such as heart palpitations on days 5, 6, and 7 (approximately 11 h after morning doses), insomnia after the evening voriconazole dose on day 3, and blurred vision of the right eye on day 10. No vital sign measurements were of clinical concern for this patient.
The effect of voriconazole on methadone pharmacokinetics was similar to that of fluconazole, another member of triazole family. Fluconazole increased the methadone AUC0-24 by 35% in patients on methadone therapy (average daily dose of 55 mg) following coadministration with a 200-mg QD dose of fluconazole, where the total methadone was measured (5). The results for fluconazole also suggest that up to a 35% increase in the steady-state methadone AUC would not result in methadone toxicity.
In this study, an attempt was made to estimate the effect of methadone on voriconazole pharmacokinetics by comparing the data with historical voriconazole-alone data from a previous study. However, subjects were not genotyped in both studies. It is well known that a CYP2C19 polymorphism has a significant effect on the systemic exposure of voriconazole, since CYP2C19 is the major enzyme involved in its metabolism. Based on our previous experience, on average, a two- to fourfold-higher systemic exposure in heterozygous extensive metabolizers and poor metabolizers is expected compared to homozygous extensive metabolizers (VFEND package insert). Therefore, comparison of pharmacokinetic profiles among subjects with similar CYP2C19 statuses would have decreased the intersubject variability in voriconazole exposure parameters. In this study, 1 out of 16 patients had very high voriconazole exposure (AUC0-12, 61.9 μg · h/ml), and this patient might be a poor metabolizer of CYP2C19. The potential limitation with respect to high intersubject variability due to a lack of CYP2C19 genotyping data is acknowledged for the comparison with historical voriconazole data. However, the comparison with historical data is valid because of the following reasons: (i) it has not been reported that methadone inhibits or induces CYP2C19 activity (3, 10, 15, 28), and (ii) the intersubject variability and the distribution range of voriconazole exposure observed in this study are similar to those of the historical control. For instance, in the presence of methadone (day 7), the individual voriconazole AUC0-12 ranged from 3.34 to 61.9 μg · h/ml, with a median value of 22.4 μg · h/ml, and the intersubject variabilities in the voriconazole AUC0-12 and Cmax were 75% and 61%, respectively (Table 4). In the reference study, the individual voriconazole AUC0-12 ranged from 5.22 to 54.4 μg · h/ml, with a median value of 28.5 μg · h/ml, and the intersubject variabilities in the voriconazole AUC0-12 and Cmax were 53% and 38%, respectively (Table 4). In addition, based on the data from 236 subjects in clinical phase 1 studies, the average steady-state voriconazole AUC0-12 at a 200-mg BID oral dose was 19.9 μg · h/ml, with intersubject variability of 94% (VFEND package insert). These results indicated that methadone appeared to have no effect on voriconazole pharmacokinetics.
In this study, although the systemic (R)-methadone concentrations were elevated, no patient had evidence of methadone toxicity, which was demonstrated by the safety assessments. In addition, the (R)-methadone concentrations may be slightly decreased after voriconazole discontinuation, and no signs or symptoms of opioid withdrawal (including CINA score) were observed at the follow-up visit (7 to 10 days after the last dose of voriconazole). These safety findings are consistent with the magnitude of pharmacokinetic interactions observed in this trial.
The highest daily methadone dose evaluated was 100 mg in this study. If patients receive much higher methadone doses for pain management, which were not examined in this study, caution should be taken, and a reduction in the methadone dose may be needed, since the probability of concomitant voriconazole causing toxicity in these patients might be higher.
In summary, there was an increase in (R)-methadone exposure (AUC0-24, 47.2%; Cmax, 30.7%), and the coadministration of 200 mg voriconazole BID in male patients receiving methadone was generally safe and well tolerated. Nevertheless, caution should be exercised: careful clinical follow-up of objective signs and subjective symptoms of opioid overdose in methadone patients receiving voriconazole therapy is recommended, and a reduction in the methadone dose may be needed.
Acknowledgments
We sincerely thank all the clinicians and the staff from the Cincinnati Addiction Research Center and the Cincinnati VA Medical Center who were involved in this study. We thank our assay specialist, Penelope Crownover, CEDRA Corporation (Austin, TX), and PPD Development (Richmond, VA) for the analytical assay support.
All the authors are employees of Pfizer, except Eugene Somoza, who was the principal clinical investigator for this study.
This study was sponsored by Pfizer.
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Begre, S., U. von Bardeleben, D. Ladewig, S. Jaquet-Rochat, L. Cosendai-Savary, K. P. Golay, M. Kosel, P. Baumann, and C. B. Eap. 2002. Paroxetine increases steady-state concentrations of (R)-methadone in CYP2D6 extensive but not poor metabolizers. J. Clin. Psychopharmacol. 22:211-215. [DOI] [PubMed] [Google Scholar]
- 2.Belanger, P., C. C. Nast, R. Fratti, H. Sanati, and M. Ghannoum. 1997. Voriconazole (UK-109,496) inhibits the growth and alters the morphology of fluconazole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1840-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulton, D. W., P. Arnaud, and C. L. DeVane. 2001. Pharmacokinetics and pharmacodynamics of methadone enantiomers after a single oral dose of racemate. Clin. Pharmacol. Ther. 70:48-57. [DOI] [PubMed] [Google Scholar]
- 4.Clarke, S. M., F. M. Mulcahy, J. Tjia, H. E. Reynolds, S. E. Gibbons, M. G. Barry, and D. J. Back. 2001. The pharmacokinetics of methadone in HIV-positive patients receiving the non-nucleoside reverse transcriptase inhibitor efavirenz. Br. J. Clin. Pharmacol. 51:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobb, M. N., J. Desai, L. S. Brown, Jr., P. N. Zannikos, and P. M. Rainey. 1998. The effect of fluconazole on the clinical pharmacokinetics of methadone. Clin. Pharmacol. Ther. 63:655-662. [DOI] [PubMed] [Google Scholar]
- 6.Cuenca-Estrella, M., J. L. Rodriguez-Tudela, E. Mellado, J. V. Martinez-Suarez, and A. Monzon. 1998. Comparison of the in-vitro activity of voriconazole (UK-109,496), itraconazole and amphotericin B against clinical isolates of Aspergillus fumigatus. J. Antimicrob. Chemother. 42:531-533. [DOI] [PubMed] [Google Scholar]
- 7.D'Aunno, T., N. Folz-Murphy, and X. Lin. 1999. Changes in methadone treatment practices: results from a panel study, 1988-1995. Am. J. Drug Alcohol Abuse 25:681-699. [DOI] [PubMed] [Google Scholar]
- 8.D'Aunno, T., and H. A. Pollack. 2002. Changes in methadone treatment practices: results from a national panel study, 1988-2000. JAMA 288:850-856. [DOI] [PubMed] [Google Scholar]
- 9.Eap, C. B., G. Bertschy, K. Powell, and P. Baumann. 1997. Fluvoxamine and fluoxetine do not interact in the same way with the metabolism of the enantiomers of methadone. J. Clin. Psychopharmacol. 17:113-117. [DOI] [PubMed] [Google Scholar]
- 10.Eap, C. B., T. Buclin, and P. Baumann. 2002. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin. Pharmacokinet. 41:1153-1193. [DOI] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A. 1998. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J. Clin. Microbiol. 36:198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster, D. J., A. A. Somogyi, and F. Bochner. 1999. Methadone N-demethylation in human liver microsomes: lack of stereoselectivity and involvement of CYP3A4. Br. J. Clin. Pharmacol. 47:403-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster, D. J., A. A. Somogyi, K. R. Dyer, J. M. White, and F. Bochner. 2000. Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. Br. J. Clin. Pharmacol. 50:427-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fultz, J. M., and E. C. Senay. 1975. Guidelines for the management of hospitalized narcotics addicts. Ann. Intern. Med. 82:815-818. [DOI] [PubMed] [Google Scholar]
- 15.Gerber, J. G., R. J. Rhodes, and J. Gal. 2004. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality 16:36-44. [DOI] [PubMed] [Google Scholar]
- 16.Gold, C. G., D. J. Cullen, S. Gonzales, D. Houtmeyers, and M. J. Dwyer. 1999. Rapid opioid detoxification during general anesthesia: a review of 20 patients. Anesthesiology 91:1639-1647. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman, H. L., and R. C. Rathbun. 2002. Review of the safety and efficacy of voriconazole. Expert Opin. Investig. Drugs 11:409-429. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen, K., C. B. Christensen, and L. L. Christrup. 1995. The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 56:PL45-PL50. [DOI] [PubMed] [Google Scholar]
- 19.Kupper, L., and K. Hafner. 1989. How appropriate are popular sample size formulas? Am. Statistician 43:101-105. [Google Scholar]
- 20.Liu, P., G. Foster, R. LaBadie, M. Gutierrez, and A. Sharma. 2005. Pharmacokinetic interaction between voriconazole and efavirenz at steady-state in healthy subjects. Abstr. Clin. Pharmacol. Ther. 77:40. [DOI] [PubMed] [Google Scholar]
- 21.Medical Economics Co., Inc. 2002. Dolophine (methadone hydrochloride) product information, p. 3056-3057. In Physicians' desk reference, 56th ed. Medical Economics Co., Inc., Montdale, NJ.
- 22.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1998. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, Sch 56592, and voriconazole. Antimicrob. Agents Chemother. 42:3242-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., J. Zhang, S. A. Messer, M. E. Brandt, R. A. Hajjeh, C. J. Jessup, M. Tumberland, E. K. Mbidde, and M. A. Ghannoum. 1999. In vitro activities of voriconazole, fluconazole, and itraconazole against 566 clinical isolates of Cryptococcus neoformans from the United States and Africa. Antimicrob. Agents Chemother. 43:169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purkins, L., N. Wood, P. Ghahramani, E. R. Love, M. D. Eve, and A. Fielding. 2003. Coadministration of voriconazole and phenytoin: pharmacokinetic interaction, safety, and toleration. Br. J. Clin. Pharmacol. 56(Suppl. 1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purkins, L., N. Wood, D. Kleinermans, and D. Nichols. 2003. Voriconazole potentiates warfarin-induced prothrombin time prolongation. Br. J. Clin. Pharmacol. 56(Suppl. 1):24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shelton, M. J., D. Cloen, R. DiFrancesco, C. S. Berenson, A. Esch, P. J. de Caprariis, B. Palic, J. L. Schur, C. J. Bugge, A. Ljungqvist, O. Espinosa, and R. G. Hewitt. 2004. The effects of once-daily saquinavir/minidose ritonavir on the pharmacokinetics of methadone. J. Clin. Pharmacol. 44:293-304. [DOI] [PubMed] [Google Scholar]
- 27.Stopher, D. A., and R. Gage. 1997. Determination of a new antifungal agent, voriconazole, by multidimensional high-performance liquid chromatography with direct plasma injection onto a size-exclusion column. J. Chromatogr. B Biomed. Sci. Appl. 691:441-448. [DOI] [PubMed] [Google Scholar]
- 28.Wang, J. S., and C. L. DeVane. 2003. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab. Dispos. 31:742-747. [DOI] [PubMed] [Google Scholar]
- 29.Wood, N., K. Tan, L. Purkins, G. Layton, J. Hamlin, D. Kleinermans, and D. Nichols. 2003. Effect of omeprazole on the steady-state pharmacokinetics of voriconazole. Br. J. Clin. Pharmacol. 56(Suppl. 1):56-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu, D., S. V. Otton, B. A. Sproule, U. Busto, T. Inaba, W. Kalow, and E. M. Sellers. 1993. Inhibition of human cytochrome P450 2D6 (CYP2D6) by methadone. Br. J. Clin. Pharmacol. 35:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie, H. G., C. M. Stein, R. B. Kim, G. R. Wilkinson, D. A. Flockhart, and A. J. Wood. 1999. Allelic, genotypic and phenotypic distributions of S- mephenytoin 4′-hydroxylase (CYP2C19) in healthy Caucasian populations of European descent throughout the world. Pharmacogenetics 9:539-549. [PubMed] [Google Scholar]