Abstract
Voriconazole (VRC) is an antifungal drug that effectively treats keratitis caused by yeasts and molds when administered orally. We retrospectively evaluated clinical outcomes and plasma and aqueous humor drug concentrations in five patients with fungal keratitis and one patient with posttraumatic endophthalmitis who were treated with VRC. VRC was administered either topically (1% eye drops every hour) or orally (400 mg twice a day). Plasma and aqueous humor samples from affected eyes were taken 12 h after oral administration or 1 h after eye drop application. The drug concentration was measured by liquid chromatography with UV or mass spectrometric detection. All six patients responded well to VRC treatment. The VRC concentration ranged from 2.93 to 3.40 mg/liter in the aqueous humor and from 3.20 to 4.20 mg/liter in the plasma after combined oral and topical treatment. Topical administration alone resulted in highly variable trough VRC concentrations of 0.61 to 3.30 mg/liter in the aqueous humor. VRC concentrations were above the MIC for Candida albicans Aspergillus fumigatus and clinical improvement was seen in all four patients with C. albicans and A. fumigatus keratitis. Combined orally and topically administered VRC resulted in aqueous humor drug concentrations of ≥2.93 mg/liter, which is above the VRC MIC for most fungi. Topical VRC treatment resulted in an aqueous humor drug concentration >0.61 mg/liter, which is above the MIC for most Candida species. The results from this small series of patients suggest that both topical and combined systemic and topical VRC therapy can be effective in treating fungal keratitis. Furthermore, the data provide preliminary support for initiation of VRC treatment with a combined topical and systemic administration until the causative fungus and its MIC are identified.
Voriconazole (VRC) is a new, broad-spectrum antifungal agent that is effective against yeasts and molds. Excellent clinical results have been achieved following VRC treatment in cases of fungal keratitis and endophthalmitis caused by Aspergillus spp. (7), Scedosporium apiospermum (6, 24), Paecilomyces lilacinus (2), Candida spp. (4), and Fusarium spp. (8, 17).
Administered orally in the absence of inflammatory changes, VRC concentrations in the aqueous humor of 1.13 ± 0.57 mg/liter have been reported after a dose of 400 mg twice within 12 h in humans (5), which is within the therapeutic range for many fungal isolates (10, 20, 22). In a human case of fungal keratitis treated with oral VRC 200 mg twice a day (b.i.d.), the aqueous humor concentration of VRC was 1.8 mg/liter (12).
Due to its small molecular mass (349 Da), VRC appears to be well-suited for topical administration as eye drops. When administered topically to noninflamed rabbit eyes, 0.01% (100 μg/ml) VRC eye drops b.i.d. resulted in high aqueous humor drug concentrations with wide ranges (14.56 ± 12.90 mg/liter) (25). We are unaware of any published reports of VRC concentrations in human aqueous humor following topical administration. Furthermore, the degree of systemic drug absorption in a topically treated eye is not known.
The goal of this retrospective study was to report plasma and aqueous humor concentrations of VRC in human keratitis patients following topical, oral, and combined treatment regimens.
MATERIALS AND METHODS
Six patients with fungal eye infections that were unresponsive to conventional treatment with fluconazole and amphotericin B were subsequently treated with VRC (Fig. 1). All patients gave their informed consent for a treatment regimen that had not been established at that time and that required ocular drug concentration analysis. Five of the patients were diagnosed with fungal keratitis and one with posttraumatic endophthalmitis. The underlying ocular pathology in individuals with fungal keratitis was corneal foreign bodies (n = 2), a chronic neurotrophic ocular surface problem (n = 1), chronic bullous keratopathy (n = 1), and a contaminated corneal transplant (n = 1). In the absence of therapeutic algorithms, the decision for combined or topical VRC treatment was based on individual clinical judgment (severity of initial corneal infiltrate and visual potential of the affected eye).
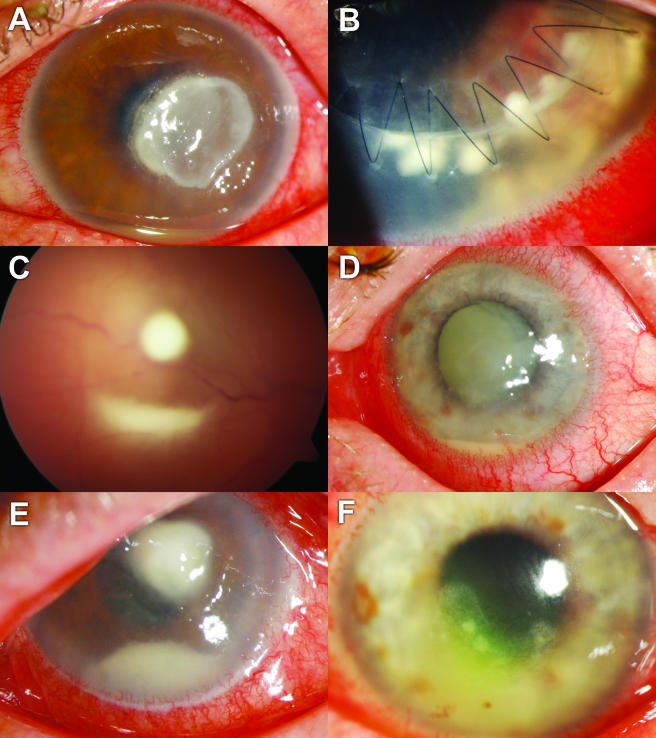
FIG. 1.
Clinical presentation at the time when VRC treatment was initiated. (A) Case 1, A. fumigatus keratitis after a corneal foreign body injury. (B) Case 2, C. albicans-infected corneal graft. (C) Case 3, culture-negative, posttraumatic endophthalmitis. (D) Case 4, C. albicans-infected neurotrophic lesion. (E) Case 5, keratitis with corneal smears positive for fungi after corneal foreign body injury. (F) Case 6, C. albicans-infected chronic bullous keratopathy.
Corneal scrapings were obtained from all patients for direct microscopic examination and inoculation of culture medium to identify causative organisms and to determine the MICs of VRC. The treatment regimen for topical therapy consisted of 1% (10 mg/ml) VRC eye drops hourly. The combined topical and systemic administration consisted of a rather high dosage of oral VRC twice daily (400 mg b.i.d., i.e., approximately 6 mg/kg of body weight) and 1% (10 mg/ml) VRC eye drops hourly. Eye drops were prepared by reconstituting lyophilized powder with sterile deionized water. The eye drops were administered every hour by an ophthalmic nurse to ensure maximal compliance and optimal administration.
To assess whether antifungal target MICs were achieved, we measured plasma and aqueous humor concentrations of VRC. In patients who received topical VRC alone, 100-μl samples of aqueous humor were obtained at the end of the 1-h dosing interval immediately before administration of the subsequent dose. In patients who received combined oral and topical VRC, samples were obtained at the common trough of both treatment regimens (i.e., 12 h after the previous oral dose and 60 min after the previous topical administration and just before a subsequent oral and topical dose). To prevent a possible contamination of the needle tip used to sample the aqueous humor by VRC from the tear film, the ocular surface was rinsed four times with balanced salt solution (Alcon, Switzerland) and twice with 1% povidone-iodine prior to sample collection. Samples were immediately frozen and stored at −20°C. To assess the clinical course, a single ophthalmologist (M.A.T.) examined the patients at regular intervals, and ocular lesions were photographed.
Plasma concentrations of VRC were determined by high-performance liquid chromatography as previously reported (16). Aqueous humor concentrations of VRC were determined using liquid chromatography coupled to mass spectrometry (LC/MS). Briefly, samples were spiked with an internal standard (UK-115794) and directly injected into the LC/MS system. The LC system consisted of a Luna C18 column (3 μm; 50 by 2 mm at 40°C) and an isocratic mobile phase using 0.02 M ammonium acetate, including 1% acetic acid and acetonitrile (65:35 vol:vol) at a flow rate of 0.35 ml/min. The injection volume was 10 μl. An atmospheric pressure chemical ionization source (4.5 kV; 400°C) was used for ionization, and the mass spectrometer worked in the selected ion-monitoring mode at m/z 350 (VRC, [M + H]+) and m/z 348 (internal standard, [M + H]+). The limit of quantification was 0.05 mg/liter, and the calibration range was 0.05 to 10 μg/ml. Correlation coefficients were always expressed as r2 values of >0.99.
RESULTS
The clinical and microbiological characteristics of all cases are shown in Table 1. In three patients, fungal cultures from corneal scrapings revealed Candida albicans with an MIC of <0.008 mg/liter for VRC. One case revealed Aspergillus fumigatus with an MIC of 0.25 mg/liter. In another case, fungal cultures remained negative despite corneal smears being positive for fungi by periodic acid-Schiff staining. Cultures also remained negative in a case of exogenous endophthalmitis that was assumed to be of fungal etiology. All corneal infiltrates regressed within 2 to 6 weeks of initiation of VRC treatment.
TABLE 1.
Voriconazole concentrations during different treatment regimens and clinical and microbiological characteristics of all patientsa
| Treatment | Case no. | Diagnosis | Fungus (MIC90 [mg/liter]) for VRC | Approximate corneal surface area without epithelium (%) | Duration of VRC treatment (days) until VRC measurement | VRC in plasma (mg/liter) measured by HPLC/UV | VRC in aqueous humor (mg/liter) measured by LC/MS | Total duration of oral VRC treatment (days) | Patient's wt (kg) |
|---|---|---|---|---|---|---|---|---|---|
| VRC 2× 400 mg/day orally and 1% topical VRC | 1 | Corneal foreign body | A. fumigatus (0.25) | 90 (epithelial debridement) | 35 | 4.2 | 3.40 | 245 | 55 |
| 3 | Posttraumatic endophthalmitis | Culture neg. | 0 | 1.5 | 3.2 | 2.93 | 11 | 72 | |
| 1% Topical VRC | 1b | Corneal foreign body | A. fumigatus (0.25) | 20 | 49 | ND | 0.61 | n.a. | 55 |
| 2c | Contaminated corneal transplant | C. albicans (<0.008) | 90 (epithelial debridement) | 4 | ND | 0.90 | 128 | 85 | |
| 4 | Infected neurotrophic lesion | C. albicans (<0.008) | 20 | 1.5 | ND | 3.30 | n.a. | 58 | |
| 5 | Corneal foreign body | Culture neg. | <10 | 1.5 | n.m. | 1.05 | n.a. | 106 | |
| 6 | Infected bullous keratopathy | C. albicans (<0.008) | <10 | 11 | n.m. | 0.95 | n.a. | 73 |
HPLC, high-performance liquid chromatography; ND, not detectable by HPLC/UV (<0.2 mg/liter); neg., negative; n.m., not measured; n.a., not applicable. All patients were Caucasians, and both treatments were applied hourly.
Case 1, results are from 2 weeks after reducing treatment to topical voriconazole only.
Case 2, data collected only while patient was under topical therapy.
After 5 weeks of combined oral and topical VRC, treatment in case 1 (A. fumigatus) was reduced to topical treatment only. Despite aqueous humor VRC concentrations of 0.61 mg/liter, which exceeded the MIC (0.25 mg/liter), the exacerbation of stromal keratitis was noted after 2 weeks of topical treatment alone. However, the keratitis regressed following reintroduction of oral VRC. After 7 months of continuous oral VRC, the antifungal treatment was stopped and the eye remained quiet. A corneal transplant was performed 1 year later.
In case 2, a patient who had received a corneal transplant contaminated with Candida albicans (MIC < 0.008 mg/liter), the initial response to topical VRC treatment was positive, but due to concerns about the recurrence that was observed in case 1, an oral regimen of VRC was instituted. The eye remained stable after 4 months of treatment, and the patient received a second corneal graft for optical rehabilitation.
The patient in case 3, a farmer presenting with endophthalmitis of suspected fungal etiology, received a vitrectomy with amphotericin B irrigation followed by oral VRC (400 mg b.i.d.) and hourly VRC eye drops. The vitreoretinal inflammation regressed, and after 4 days, a small intraretinal foreign body was discovered. The patient underwent a second vitrectomy for the removal of the foreign body. Subsequently, the eye became quiet within 3 days and VRC treatment was stopped after 1 week.
In case 4, a patient with neurotrophic keratitis superinfected with C. albicans (MIC < 0.008 mg/liter), treatment with topical VRC alone was successful and was discontinued after 6 weeks. However, 2 months after recovery from the Candida infection, bacterial keratitis necessitated the evisceration of the previously blind eye. Histology and microbiology of the eviscerated eye confirmed complete fungal eradication.
In case 5, a patient with a foreign body injury and corneal smears positive for fungi by periodic acid-Schiff staining, the corneal infiltrates initially enlarged despite intensive topical VRC treatment. Microbiological cultures remained negative. After 6 weeks of a combined antibacterial and antifungal treatment (hourly topical 0.3% ofloxacin and topical 1% VRC), the eye became quiet and VRC treatment was stopped 2 weeks later.
In case 6, a patient with bullous keratopathy superinfected with C. albicans (MIC < 0.008 mg/liter), the corneal infiltrates regressed under topical treatment alone. However, the cornea had to be covered with a conjunctival flap because of persistent epithelial surface problems. VRC treatment was stopped at the time of surgery, and the eye remained quiet thereafter.
Plasma and aqueous humor concentrations of VRC are summarized in Table 1. VRC was detectable in the aqueous humor of all patients. Combined oral and topical administration of 400 mg b.i.d. and hourly 1% eye drops resulted in aqueous humor drug concentrations of 2.93 and 3.40 mg/liter. Topical administration of 1% VRC eye drops resulted in aqueous humor drug concentrations of 0.61 to 3.30 mg/liter (median, 0.95 mg/liter), with no detectable VRC in the plasma of the three cases tested. The size of the fungal ulcer and the epithelial defect had no obvious effect on VRC penetration into the anterior chamber after topical administration (Table 1). However, the route of administration seemed to be important because the highest concentration (3.40 mg/liter) was found in case 1, a patient who received both oral and topical VRC. In this patient, aqueous humor VRC concentrations were higher during periods of combined treatment than during periods of topical treatment alone (0.61 mg/liter).
Three of the patients who received oral treatment reported minor visual sensations (e.g., flashes) that did not require any change in the treatment regimen. Two patients with long-term oral VRC treatment complained of light-induced skin rashes after 4 and 7 months of treatment. The rashes disappeared within 2 weeks of discontinuation of oral VRC.
DISCUSSION
The clinical context of this study is characterized by the presence of a serious fungal infection, the failure of conventional therapy regimens, and the administration of an alternative therapy whose efficacy has not yet been established and validated. Since the number of patients included was small, only preliminary conclusions can be drawn.
In the five patients with fungal keratitis, VRC drug concentrations in the aqueous humor following combined oral and topical administration exceeded the MIC for common fungi, particularly Candida. In these patients, VRC concentrations in the aqueous humor were 85 and 90% of the corresponding VRC concentration in plasma. This exceeds the VRC concentrations of 52 and 53% reported in two previously published, single-case reports using oral treatment alone (7, 12). Aqueous humor drug concentrations in our patients were considerably higher than those reported in patients with noninflamed eyes who received 400 mg VRC twice within 12 h (1.13 ± 0.57 mg/liter) (5). These differences in intraocular drug concentration may be due to the inflamed states of the eyes in our study. The inflammation may have disturbed the blood-eye barrier and allowed the drug to penetrate more readily from the iris vessels into the aqueous humor. An alternative explanation for the higher drug concentrations in our study is that the measurements in our population were performed after repeated drug administration for at least 36 h, allowing more time for drug distribution.
Therapeutic target tissue concentrations are not established for VRC, and also the therapeutic range in plasma is not well defined. Earlier studies in patients with Candida infection, a fungus rather sensitive to VRC, revealed no evidence for a relationship between plasma VRC and effectiveness (9), possibly because VRC concentrations in current treatment regimens consistently reach maximum effective concentrations for Candida. However, for patients with a progression of fungal infections with higher MIC (i.e., Aspergillus, Scedosporium, and Blastomyces), a recent retrospective study suggested that the outcome is worse if plasma trough concentrations do not exceed 2 mg/liter (21). Hence, higher plasma (and tissue) VRC concentrations are desired in the treatment of the most challenging fungal infections.
Clinical improvement was observed in all five keratitis patients after switching to VRC, which is in agreement with previous reports (4, 6, 7, 24). In our study, patients received an oral dose of 400 mg VRC b.i.d., which is a relatively high maintenance dose. We selected the dose of 400 mg VRC b.i.d. because, with this dose, VRC aqueous humor concentrations exceeding MICs for Aspergillus have been reported (5) and because higher-than-regular doses have been reported to be effective for treating fungal infections in the central nervous system (11, 18). As the blood-eye barrier is as effective as the blood-brain barrier in drug exclusion, the high VRC dose was selected as a rescue regimen in these potentially blinding infections.
VRC eye drops, prepared as recommended for intravenous drug application, were tolerated by all patients. When VRC was administered topically, aqueous humor drug concentrations were more variable and generally lower than after combined oral and topical treatment but still in excess of the MIC of VRC for C. albicans (0.008 mg/liter). As expected, the quantity of drug administered topically did not result in detectable circulating VRC concentrations. A regimen of hourly eye drop administration was chosen according to an earlier experience with fluconazole (1). In the present study, all patients were treated as inpatients and VRC eye drops were administered by an ophthalmic nurse to ensure maximal compliance. For the treatment of fungi, such as A. fumigatus (MIC, 0.25 to 0.5 mg/liter), Fusarium solani (MIC, 4.0 mg/liter), or Scedosporium spp. (MICs, 0.25 and 4.0 mg/liter), the drug concentration recorded within the aqueous humor after topical administration alone may not reliably achieve the required MIC (3, 19). Therefore, it is reasonable to initiate treatment of suspected fungal keratitis with oral or combined oral and topical administration of VRC. Once the causative fungal strain has been identified and its MIC determined, treatment may be reduced to topical VRC alone if topical eye solutions are available and adherence to the demanding regimen is guaranteed. Systemic VRC is more expensive but also more convenient for patients due to the ease of administration (b.i.d. versus every hour). As with other antifungal drugs, the duration of treatment depends primarily on the severity of the keratitis and the individual clinical response.
Drug concentrations in the aqueous humor following hourly administration of 1% VRC eye drops were 10 to 15 times lower than drug concentrations in healthy rabbit eyes after treatment with a VRC solution that was 100 times less concentrated (i.e., 100 μg/ml b.i.d.) (25). This difference in intraocular drug bioavailability suggests that it is not feasible to extrapolate ocular penetration data from animal experiments to human situations. Moreover, because rabbits have a very low blink rate and a large epithelial surface (nictitating membrane), the penetration of lipophilic and nonirritating drugs like VRC may be facilitated. Furthermore, it is not clear that the ocular surface in the experimental rabbit study was flushed as thoroughly as in our patients prior to sampling, and contamination with VRC may have occurred, resulting in apparently high ocular concentrations.
In humans, the corneal epithelium prevents the penetration of certain antifungal drugs and the removal of the surface epithelium is recommended to improve the penetration of drugs such as amphotericin B (13, 14). In the present study, VRC concentrations in the aqueous humor depended neither on the size of the epithelial defect (Table 1) nor on epithelial removal, which was performed repeatedly with case 2. This suggests that epithelial debridement is not necessary for VRC penetration. This advantage of VRC eye drops may be explained by the fact that VRC is a rather small, lipophilic molecule (molecular mass, 349 Da; degree of lipophilicity [log P], 1.8) with a maximal water solubility of 0.2 mg/ml and a large volume of distribution. To increase water solubility to 20 mg/ml, the commercially available intravenous drug solution is formulated with sulfobutyl ether-β-cyclodextrin (Captisol; CyDex, Inc., KS). Sulfobutyl ether-β-cyclodextrin is a large molecule (molecular mass, 2,163 Da) that binds lipophilic drugs in its center (15, 23). Upon intravenous administration, the active drug molecule dissociates from the sulfobutyl ether-β-cyclodextrin carrier. When VRC is administered to corneas with intact epithelium, it is likely that VRC dissociates from the cyclodextrin carrier in the tear film, which enables VRC to penetrate the cornea by taking advantage of its own lipophilicity. In the case of a large epithelial defect, VRC is expected to penetrate more rapidly than an antifungal like amphotericin B because of its smaller size. In the case of an epithelial defect, even the nondissociated VRC-sulfobutyl ether-β-cyclodextrin complex is small enough to penetrate the corneal stroma. Therefore, it could be hypothesized that the VRC concentration in the corneal stroma of the five patients with documented or suspected fungal keratitis was higher than that in the aqueous humor where the drug and its carrier dissociate.
In summary, in all five cases, fungal keratitis resolved after the introduction of VRC. In contrast to amphotericin B, corneal penetration of topically administered VRC did not depend on the state of the epithelial surface. However, intraocular drug concentration following topical administration of VRC alone may be below the MIC required for the treatment of less susceptible fungi. Therefore, initial therapy with combined oral and topical administration of VRC is recommended until the causative fungus and its MIC are identified.
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Abbasoğlu, O. E., B. M. Hosal, B. Sener, N. Erdemoglu, and E. Gursel. 2001. Penetration of topical fluconazole into human aqueous humor. Exp. Eye Res. 72:147-151. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. L., S. Mitra, R. Salouti, T. A. Pham, and H. R. Taylor. 2004. Fungal keratitis caused by Paecilomyces lilacinus associated with a retained intracorneal hair. Cornea 23:516-521. [DOI] [PubMed] [Google Scholar]
- 3.Arikan, S., V. Paetznick, and J. H. Rex. 2002. Comparative evaluation of disk diffusion with microdilution assay in susceptibility testing of caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 46:3084-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granados, J. M., N. Puerto, and M. J. Carrilero. 2004. Efficiency of voriconazole in fungal keratitis caused by Candida albicans. Arch. Soc. Esp. Oftalmol. 79:565-568. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 5.Hariprasad, S. M., W. F. Mieler, E. R. Holz, H. Gao, J. E. Kim, J. Chi, and R. A. Prince. 2004. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch. Ophthalmol. 122:42-47. [DOI] [PubMed] [Google Scholar]
- 6.Hernáandez Prats, C., F. Llinares Tello, A. Burgos San Jose, J. Selva Otaolaurruchi, and J. P. Ordovas Baines. 2004. Voriconazole in fungal keratitis caused by Scedosporium apiospermum. Ann. Pharmacother. 38:414-417. [DOI] [PubMed] [Google Scholar]
- 7.Kim, J. E., S. L. Perkins, and G. J. Harris. 2003. Voriconazole treatment of fungal scleritis and epibulbar abscess resulting from scleral buckle infection. Arch. Ophthalmol. 121:735-737. [DOI] [PubMed] [Google Scholar]
- 8.Klont, R. R., C. A. Eggink, A. J. Rijs, P. Wesseling, and P. E. Verweij. 2005. Successful treatment of Fusarium keratitis with cornea transplantation and topical and systemic voriconazole. Clin. Infect. Dis. 40:e110-e112. [DOI] [PubMed] [Google Scholar]
- 9.Lutsar, I., M. R. Hodges, K. Tomaszewski, P. F. Troke, and N. D. Wood. 2003. Safety of voriconazole and dose individualization. Clin. Infect. Dis. 36:1087-1088. [DOI] [PubMed] [Google Scholar]
- 10.Marangon, F. B., D. Miller, J. A. Giaconi, and E. C. Alfonso. 2004. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am. J. Ophthalmol. 137:820-825. [DOI] [PubMed] [Google Scholar]
- 11.Nesky, M. A., E. C. McDougal, and J. E. Peacock, Jr. 2000. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 31:673-677. [DOI] [PubMed] [Google Scholar]
- 12.Nulens, E., C. Eggink, A. J. Rijs, P. Wesseling, and P. E. Verweij. 2003. Keratitis caused by Scedosporium apiospermum successfully treated with a cornea transplant and voriconazole. J. Clin. Microbiol. 41:2261-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Day, D. M., W. S. Head, R. D. Robinson, and J. A. Clanton. 1986. Bioavailability and penetration of topical amphotericin B in the anterior segment of the rabbit eye. J. Ocul. Pharmacol. 2:371-378. [DOI] [PubMed] [Google Scholar]
- 14.O'Day, D. M., W. S. Head, R. D. Robinson, and J. A. Clanton. 1986. Corneal penetration of topical amphotericin B and natamycin. Curr. Eye Res. 5:877-882. [DOI] [PubMed] [Google Scholar]
- 15.Okimoto, K., R. A. Rajewski, K. Uekama, J. A. Jona, and V. J. Stella. 1996. The interaction of charged and uncharged drugs with neutral (HP-β-CD) and anionically charged (SBE7-β-CD) β-cyclodextrins. Pharm. Res. 13:256-264. [DOI] [PubMed] [Google Scholar]
- 16.Pennick, G. J., M. Clark, D. A. Sutton, and M. G. Rinaldi. 2003. Development and validation of a high-performance liquid chromatography assay for voriconazole. Antimicrob. Agents Chemother. 47:2348-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis, A., R. Sundmacher, K. Tintelnot, H. Agostini, H. E. Jensen, and C. Althaus. 2000. Successful treatment of ocular invasive mould infection (fusariosis) with the new antifungal agent voriconazole. Br. J. Ophthalmol. 84:932-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz, S., D. Milatovic, and E. Thiel. 1997. Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute leukaemia. Br. J. Haematol. 97:663-665. [DOI] [PubMed] [Google Scholar]
- 19.Serrano Mdel, C., A. Valverde-Conde, M. M. Chavez, S. Bernal, R. M. Claro, J. Peman, M. Ramirez, and E. Martin-Mazuelos. 2003. In vitro activity of voriconazole, itraconazole, caspofungin, anidulafungin (VER002, LY303366) and amphotericin B against aspergillus spp. Diagn. Microbiol. Infect. Dis. 45:131-135. [DOI] [PubMed] [Google Scholar]
- 20.Shah, K. B., T. G. Wu, K. R. Wilhelmus, and D. B. Jones. 2003. Activity of voriconazole against corneal isolates of Scedosporium apiospermum. Cornea 22:33-36. [DOI] [PubMed] [Google Scholar]
- 21.Smith, J., N. Safdar, V. Knasinski, W. Simmons, S. M. Bhavnani, P. G. Ambrose, and D. Andes. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swinne, D., M. Watelle, and N. Nolard. 2005. In vitro activities of voricon-azole, fluconazole, itraconazole and amphotericin B against non Candida albicans yeast isolates. Rev. Iberoam Micol. 22:24-28. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38(Suppl. 1):335-347. [PubMed] [Google Scholar]
- 24.Wu, Z., H. Ying, S. Yiu, J. Irvine, and R. Smith. 2002. Fungal keratitis caused by Scedosporium apiospermum: report of two cases and review of treatment. Cornea 21:519-523. [DOI] [PubMed] [Google Scholar]
- 25.Zhou, L., R. D. Glickman, N. Chen, W. E. Sponsel, J. R. Graybill, and K. W. Lam. 2002. Determination of voriconazole in aqueous humor by liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. B 776:213-220. [DOI] [PubMed] [Google Scholar]