Abstract
The pharmacokinetic profiles of azithromycin given as a single-dose regimen (2.0-g extended-release microspheres) were characterized in serum and white blood cells (WBC) and compared with those of a 3-day regimen (a 500-mg immediate-release tablet once daily; total dose, 1.5 g) in an open-label, randomized, parallel-group study of 24 healthy adult subjects. Serial blood samples were collected up to 5 days after the start of dosing for both regimens. Safety assessments were conducted throughout the study. A single 2.0-g dose of azithromycin microspheres achieved significantly higher exposures in serum and WBC during the first 24 h after the start of dosing than a 3-day regimen: an approximately threefold higher area under the curve from time zero to 24 h postdose (AUC0-24) and an approximately twofold higher mean peak concentration on day 1. The single-dose regimen provided total azithromycin exposures in serum and WBC similar to those of the 3-day regimen, as evidenced by the similar AUC0-120 and trough azithromycin concentrations in serum and WBC (mononuclear leukocytes [MNL] and polymorphonuclear leukocytes [PMNL]). For both regimens, the average total azithromycin exposures in MNL and PMNL were approximately 300- and 600-fold higher than those in serum. Azithromycin concentrations in MNL and PMNL remained above 10 μg/ml for at least 5 days after the start of dosing for both regimens. This “front-loading” of the dose on day 1 is safely achieved by the extended-release microsphere formulation, which maximizes the drug exposure at the time when the bacterial burden is likely to be highest.
Azithromycin is an azalide, structurally related to the macrolide family of antibiotics. It acts by binding to the 50S ribosomal subunit of susceptible organisms, thereby interfering with protein synthesis. Azithromycin is approved worldwide as a broad-spectrum antibiotic to treat a variety of community-acquired infections. Azithromycin is distributed extensively to a variety of body tissues and fluids. Due to its dibasic structure, azithromycin is actively taken up by a wide variety of cells, including white blood cells (WBC) and fibroblasts, a pattern different from that of the classic macrolide agents (1, 12, 13). The unique pharmacokinetic (PK) properties of azithromycin make short-course therapy possible. For most adult indications, a total of 1.5 g of immediate-release (IR) azithromycin formulations (Zithromax; Pfizer) is administered in divided doses over a period of 3 or 5 days (500 mg once daily [QD] for 3 days, or 500 mg QD on day 1 and 250 mg QD on days 2 to 5) (Zithromax [azithromycin] package insert; Pfizer Inc., New York, NY). The highest approved single oral dose of an azithromycin IR formulation is 2.0 g for the treatment of gonococcal urethritis (Zithromax package insert). The most common adverse events (AEs) of azithromycin are gastrointestinal (GI) in nature and appear to be dose related. The actual use of the 2.0-g dose is limited due to the high incidence of GI AEs such as nausea (18%), diarrhea/loose stools (14%), and vomiting (7%) (Zithromax package insert). It has been suggested that the GI AEs (i.e., nausea and vomiting) are primarily local in origin and occur shortly after oral dosing of azithromycin, possibly due to the drug's action on the motilin receptors in the upper GI tract, as with other macrolides (i.e., erythromycin) (17, 19, 20).
The development of a single-dose azithromycin regimen for the treatment of respiratory tract infections was based on preclinical findings with animal infection models, which showed improved survival or more rapid bacterial eradication (i.e., mouse pneumonia, acute peritonitis, and neutropenic thigh infection models) when a course of azithromycin was administered as a single dose rather than as divided doses over multiple days (7, 9-11). An extended-release (ER) microsphere formulation was developed to deliver an entire therapeutic course of azithromycin in a single dose with an improved tolerability profile (5). This microsphere formulation was designed to delay the release of azithromycin so that it is released in the lower GI tract slowly, bypassing the upper-GI motilin receptors. The alkalizing agents incorporated in the formulation help raise the pH in the constituted suspension and probably in the stomach to minimize the release of the drug from microspheres in the mouth and stomach, and the microsphere matrix helps control the drug release rate, since the dissolved azithromycin is diffused through the pores formed in situ in the microspheres. This ER formulation does not compromise the oral bioavailability of azithromycin substantially, although it bypasses a small portion of the absorption site(s) in the upper GI tract; it achieved approximately 83% bioavailability relative to the IR formulation (5). The 2.0-g single-dose ER regimen (Zmax; Pfizer) has demonstrated clinical effectiveness and has been approved for the treatment of community-acquired pneumonia and acute bacterial sinusitis in adults (6) (Zmax [azithromycin extended release for oral suspension] package insert; Pfizer Inc., New York, NY).
This is the first clinical pharmacology study to characterize and compare azithromycin PK profiles in serum and WBC between the 2.0-g single-dose ER regimen and the 3-day IR regimen (500-mg tablet QD for 3 days). The study was also designed to evaluate whether saturation of uptake by phagocytes would occur at high doses of azithromycin.
(Part of this research was presented as a poster at the 2005 Interscience Conference on Antimicrobial Agents and Chemotherapy).
MATERIALS AND METHODS
This study was conducted at a single center, PHAROS GmbH, Ulm, Germany, in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with the Independent Ethics Committee, informed consent regulations, and the Good Clinical Practices Guidelines of the International Conference on Harmonization. In addition, all local regulatory requirements were followed, in particular those affording greater protection of the safety of trial participants. Written informed consent was obtained before each subject entered the study.
Subjects.
Healthy adult male and female subjects (ages, 18 to 55 years; body weight, >50 kg; body mass index [BMI], 18 to 30 kg/m2, inclusive) who were willing and able to provide informed consent and to be confined to the Clinical Research Unit (CRU) were included. Subjects were required to be in good health as determined by a detailed medical history, a full physical examination including vital signs, 12-lead electrocardiograms, and clinical laboratory tests (including complete blood count, differential cell count, liver and renal function tests, and urinalysis). Subjects with a WBC count of >120% of the upper limit of normal or <80% of the lower limit of normal were excluded from the study (<2.5 × 103/mm3 or > 17.5 × 103/mm3). Subjects were excluded if they had any conditions possibly affecting drug absorption, if they used prescription or nonprescription drugs or dietary supplements within 7 days prior to the first dose of study medication (excluding contraceptives, hormone replacement therapy, and acetaminophen at ≤2 g/day), if they used herbal supplements within 30 days, or if they had either a known allergy to macrolide antibiotics, a severe allergic reaction to any drug in the past, or a history of intolerance to azithromycin. No consumption of grapefruit or any grapefruit-containing product within 4 days before the first dose of azithromycin was allowed.
Study design.
This was an open-label, randomized, parallel-group study of 24 healthy adult subjects (12 subjects per treatment group). Subjects were randomly assigned to receive either a single-dose ER azithromycin regimen (2.0-g microspheres administered as an oral suspension) or a 3-day IR azithromycin regimen (500-mg tablet QD for 3 days; total dose, 1.5 g). Serial blood samples for measurement of azithromycin concentrations in serum and WBC (mononuclear leukocytes [MNL; monocytes and lymphocytes] and polymorphonuclear leukocytes [PMNL; neutrophils]) were collected up to 5 days after the start of dosing. Due to the limit of the total blood sampling volume, no serum samples were collected following the second day of dosing for the IR regimen, and the azithromycin serum exposure was estimated based on day-1 and day-3 data by using compartmental PK modeling. Sitting vital signs, including blood pressure (BP) and heart rate (HR), and AEs were monitored throughout the study. The total duration of the study was 7 days, and subjects were required to stay in the CRU for approximately 4 days (1 day prior to dosing and 3 days after dosing).
Drug administration.
The 2.0-g azithromycin ER formulation, consisting of microspheres, vehicle blend, sucrose, and alkalizing agents (sodium phosphate and magnesium hydroxide), and the 500-mg azithromycin IR tablet were provided to the CRU by Pfizer (New York, NY). The azithromycin ER formulation was supplied as a powder for oral suspension and was constituted with prescribed quantities of water (60 ml) just prior to dosing. All treatments were administered with 240 ml of water. While confined to the CRU, subjects were fasted at least 4 h before any safety laboratory evaluations and 8 h prior to the start of drug administration. In order to standardize conditions, all subjects were required to refrain from lying down, eating, and drinking beverages during the first 4 h after dosing. Water was allowed freely throughout the study except for the first 2 h after dosing.
Serum sample collection.
For the group receiving the single-dose ER regimen, blood samples (3 ml) were obtained at 0 h (just prior to dosing) and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, 96, and 120 h after the start of dosing (day 1). For the group receiving the 3-day IR regimen, besides the above time points, extra sampling points were added on day 3 (as 0.5, 1, 2, 3, 4, 6, 8, 10, and 12 h after day-3 dosing). Blood samples were kept at room temperature for approximately 30 min until they clotted and were centrifuged at approximately 1,700 × g for 10 min at 4°C. Serum was obtained and stored under at least −20°C within 1 h of collection until analysis.
WBC sample collection and isolation.
Blood samples (30 ml, with EDTA as an anticoagulant) were collected at 0 h (just prior to dosing) and at 2, 4, 8, 12, 24, 28, 36, 48, 52, 60, 72, 96, and 120 h after the start of dosing for both regimens. Blood samples were processed immediately after collection to obtain the MNL and PMNL samples by using a PMN isolation medium (Polymorphprep) with the following steps: density gradient centrifugation followed by purification of MNL and PMNL. Briefly, the blood samples were overlaid on top of two density gradient media (Histopaque 1077 [top] and Histopaque 1119 [bottom]) and centrifuged for 35 min at 700 × g at room temperature. The upper serum layer was discarded, and the upper MNL band (∼7 ml; cells were located mainly on the wall) was collected. Then the intermediate layer above the lower PMNL band was discarded, and the PMNL band (∼7 ml; cells were located mainly on the wall) on Histopaque 1119 was collected. The MNL fraction was purified with phosphate-buffered saline (PBS) by resuspending and centrifuging twice. The PMNL fraction was purified with PBS by resuspending and centrifuging once, followed by red blood cell lysis (twice) and centrifugation. Then the MNL and PMNL pellets were resuspended in 5 ml of PBS and stored under at least −20°C until analysis.
Cell counts, viability, and purity of MNLs and PMNLs.
Türk's solution and trypan blue methods were used to estimate the cell isolation yields in the MNL and PMNL suspensions. After staining with Türk's solution or trypan blue, the cells were counted in a Neubauer chamber under the microscope. The cell counts obtained by the two methods were similar, and the results from the trypan blue method were used for the calculation of cell concentrations.
The viability and purity of MNL and PMNL were reported for each sample, as part of the validation for the WBC isolation process. The trypan blue method provided cell viability results by differentiating the viable cells (transparent and shining) from the nonviable, damaged cells (stained blue). The purity of the isolated cells was determined by Wright's staining method. The nucleus and cytoplasm of these leukocytes take on a characteristic blue or pink coloration with Wright's stain. Under the microscope (magnification, ×10 to ×100), the isolated viable MNL (or PMNL) were counted versus the non-MNL (or non-PMNL) to obtain a differential count used to calculate the purity of the isolated cells. Viabilities of the MNL isolates were ≥95% except for the 8-h time point for one subject, with 93% viability, and the 60-h time point for the first four subjects, with only 22 to 47% viability (probably due to contamination by the disinfectant, isopropanol, since the calculated cellular concentration for MNL appeared to be in the normal range, and these data were included for PK analysis). The viabilities of the PMNL isolates were also ≥95% except for one sample with 94% viability. The purities of the MNL isolates as well as the PMNL isolates were always ≥90%. The high percentages of viability and purity indicated that the isolation process for MNL and PMNL was acceptable.
Analytical methods.
BAS Analytics (West Lafayette, IN) analyzed serum, MNL, and PMNL samples for azithromycin concentrations using a validated high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method. Azithromycin concentrations were determined following a liquid-liquid extraction. For serum samples, 100 μl of D3-azithromycin (internal standard) in acetonitrile-water (1:1, vol/vol) was added to 50 μl of serum, followed by the addition of 1 ml of 0.06 M Na2CO3, 1 ml of purified water, and 2 ml of methyl-t-butyl ether. For the MNL or PMNL samples, 50 μl of internal standard was added to 50 μl of MNL or PMNL samples in a TomTec 96-well format, followed by the addition of 100 μl of 0.5 M Na2CO3 and 500 μl of methyl-t-butyl ether. After brief vortexing (1 min), the samples were centrifuged at 3,000 rpm for 5 min to separate the layers. The upper ether layer was transferred to a clean tube or 96-well plate and evaporated under nitrogen at 40°C. The dried extract was reconstituted with 100 μl (serum) or 200 μl (MNL or PMNL) of the mobile phase (73% 0.05 M ammonium acetate-27% acetonitrile, vol/vol) and vortexed. A 50-μl (serum) or 15-μl (MNL or PMNL) aliquot was injected into an LC-MS/MS system (Bioanalytical Systems PM-80 isocratic pump with an LC-26 on-line vacuum degasser and a MicroMass Quattro LC tandem quadrupole mass spectrometer with an electrospray source) set up with a Dupont Zorbax XDB-8 Eclipse C-8 narrow bore column (150 by 2.1 mm; Agilent Technologies). The mass spectrometer was operated in the positive ionization mode and monitored the transition ions m/z 749.5→591.4 and 752.6→594.4 for azithromycin and D3-azithromycin, respectively. The dynamic range for the serum assay was 10.0 to 250 ng/ml, and the dynamic range for MNL and PMNL suspension was 10.0 to 1,000 ng/ml. Four hundred forty-four samples were analyzed in eight acceptable analytical runs for azithromycin in serum. Three hundred thirty-six samples were analyzed in 12 acceptable analytical runs for azithromycin in MNL and PMNL. The accuracy of the quality control samples used during sample analysis ranged from −2.4% to 3.3%, with a precision of ≤5.2%, for azithromycin in serum; accuracy ranged from 4.5% to 6.0%, with a precision of ≤3.5%, for azithromycin in MNL and PMNL.
Calculation of azithromycin concentrations in WBC.
To obtain the average azithromycin concentration in MNL and PMNL, the concentration measured in the 5-ml cell suspension was divided by the total cell count to produce the amount of azithromycin per cell; then the amount of azithromycin in each cell was divided by the cell volume. The cell volumes were not measured in this study, but literature values were used (421 fl per monocyte, 204 fl per lymphocyte, and 334 fl per granulocyte [neutrophil]) (16). The average of the cell volumes of monocytes and lymphocytes, 312.5 fl, was used for MNL calculation.
Pharmacokinetic compartmental-analysis method.
A mixed-effects modeling approach was employed to estimate the azithromycin concentration-time profile in serum on the second day of dosing for subjects receiving the 3-day IR regimen based on their day-1 and day-3 exposure data. This approach was implemented in the NONMEM program (version V, level 1.1; GloboMax, Hanover, MD, and NONMEM Project Group, University of California at San Francisco) using the first-order conditional estimation and maximum-likelihood estimation and the NM-TRAN preprocessor (4). Four different PK models (the two-compartment or three-compartment model with first-order or zero-order absorption) were evaluated. Model selection was based on the goodness of fit of models to the data using the following criteria: (i) successful minimization, (ii) a significant decrease in the objective function (−2 · log-likelihood) of more than 3.84, which approximates a P value of <0.05 based on the assumption of a χ2 distribution for the distribution of differences of the objective functions for two models differing by 1 degree of freedom, (iii) successful completion of the covariance step, (iv) visual inspection of the diagnostic plots for randomness of population/individual predicted concentrations versus observed concentrations across the identity line, and randomness of individual weighted residuals versus predicted concentrations and time, and (v) precision of the parameter estimates and decreases in both intersubject and residual (intrasubject) variability. After the selection of an appropriate model, the concentration-time profile on day 2 was estimated for each individual with the individual PK parameters (posthoc estimation). Then the estimated individual concentration-time data were used to calculate the area under the concentration-time curve from time 24 to 48 h postdosing (AUC24-48) using the standard noncompartment method as described below.
Pharmacokinetic noncompartmental-analysis method.
The standard noncompartmental pharmacokinetic analyses of the data for azithromycin concentrations in serum, MNL, and PMNL were carried out using WinNonlin, version 3.2 (Pharsight, Mountain View, CA). The maximum observed concentration (Cmax) of azithromycin and the time to reach Cmax (Tmax) were estimated directly from the experimental data. AUC0-24, AUC24-48, AUC48-120, and AUC0-120 were estimated using the linear/log trapezoidal approximation. The serum AUC0-120 for the 3-day regimen was calculated as the sum of the observed AUC0-24, estimated AUC24-48, and observed AUC48-120.
Safety.
Safety assessments included safety laboratory tests, limited physical examinations, sitting vital signs (BP and HR), and AEs. During the study period, AEs were assessed by spontaneous reporting, by staff observation, and by asking the subjects to respond to nonleading questions at 1, 3, 6, 12, 24, 48, 72, 96, and 120 h after the start of dosing. The investigator recorded all the clinically significant changes in physical-examination findings and abnormal objective-test findings (e.g., laboratory) as AEs. If the AE persisted, follow-up was required until resolution or stabilization occurred. Any AEs occurring following the start of dosing and within the standard lag time of 35 days for azithromycin were counted as treatment emergent.
Statistical analysis. (i) Sample size determination.
The objective of this study was to estimate the exposure of a single 2.0-g dose of ER azithromycin relative to the 3-day IR tablet regimen (total dose, 1.5 g) in a parallel design. The sample size was empirically determined. With 24 subjects (12 subjects per treatment group), if the estimated ratio of serum AUC0-120 (ER/3-day IR) was 0.9, the 90% confidence interval (CI) would be no wider than 71.2% to 113.8%, with a tolerance probability of 0.80 (15). This calculation was based on the assumption that the within-group coefficient of variation for serum AUC with the 3- and 5-day IR regimens was 30%, which was estimated from an earlier study (3).
(ii) Pharmacokinetic parameters.
The primary PK parameters evaluated and compared between the ER and IR regimens were day-1 and total azithromycin exposures (AUC0-24 and AUC0-120) for serum, MNL, and PMNL. The secondary parameters compared were the respective exposure ratios: the ratio of MNL AUC to serum AUC and the ratio of PMNL AUC to serum AUC. Similar comparisons were also performed for Cmax on day 1. The natural log-transformed PK parameters (AUC0-24, AUC0-120, and Cmax) were analyzed using a one-way analysis-of-variance model with treatment as the only effect, with a SAS mixed procedure (SAS Institute, Cary, NC). Geometric means, their ratios (ER regimen/IR regimen), and 90% CIs around the ratios were obtained by anti-Log transforming estimates obtained on the log scale. A two-sided P value of <0.05 was considered significant.
(iii) Safety.
Safety data were summarized descriptively for each treatment, and no formal statistical analysis was performed.
RESULTS
Subjects.
Twenty-four healthy subjects were enrolled and completed the study. All subjects were included in PK and safety analyses. In the group receiving the single-dose ER regimen, there were 2 male and 10 female subjects. The mean age was 42.3 years (range, 24 to 55 years), the mean BMI was 22.8 kg/m2 (range, 19.1 to 26.3 kg/m2), and the mean weight was 63.8 kg (range, 50.2 to 90.6 kg). In the group receiving the 3-day IR regimen, there were 1 male and 11 female subjects. The mean age was 33.9 years (range, 23 to 47 years), the mean BMI was 24.0 kg/m2 (range, 19.4 to 28.9 kg/m2), and the mean weight was 67.2 kg (range, 52.0 to 81.0 kg). All subjects were Caucasians.
Serum pharmacokinetics.
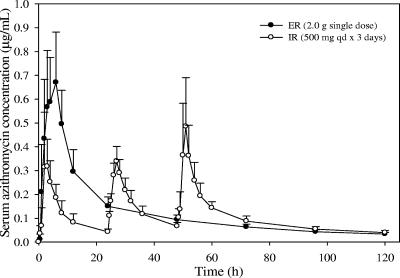
As expected, the serum azithromycin concentrations on day 1 for the ER regimen were significantly higher than those for the IR regimen (Table 1). The mean serum azithromycin concentration-time profiles for the ER and IR regimens are displayed in Fig. 1. The geometric mean azithromycin AUC0-24 and Cmax ratios (ER/IR regimen) were 300% and 184%, respectively (P < 0.00001), demonstrating that the ER regimen achieved approximately three- and twofold-higher AUC0-24 and Cmax on day 1 in serum, respectively (Tables 2 and 3). The total systemic exposure in serum over 5 days following the start of dosing (AUC0-120) was similar for the ER and IR regimens, as indicated by the geometric mean AUC0-120 ratio (108%) and its 90% CI (92.34%, 125.44%) (Table 2).
TABLE 1.
Azithromycin pharmacokinetic parameters in serum, MNL, and PMNL following administration of a single-dose ER regimen versus a 3-day IR regimen to healthy subjectsa
| Regimen | AUC0-24 (μg · h/ml) | AUC0-120 (μg · h/ml) | Cmax (μg/ml) | Median (range) Tmax (h) | Ct=120d (μg/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum
|
||||||||||
| Single-dose ER | 7.87 (1.87) | 14.8 (3.16) | 0.725 (0.224) | 3.5 (1.0-6.0) | 0.03 (0.007) | |||||
| 3-day IRb | 2.67 (0.92) | 13.7 (3.18) | 0.405 (0.161) | 2.0 (2.0-3.0) | 0.04 (0.009) | |||||
| MNL
|
||||||||||
| Single-dose ER | 1,790 (540) | 4,710 (1,100) | 116 (40.2) | 8.0 (4.0-24.0) | 16.2 (5.51) | |||||
| 3-day IRc | 647 (237) | 3,890 (1,330) | 72.7 (30.0) | 52.0 (2.0-52.0) | 20.2 (7.72) | |||||
| PMNL
|
||||||||||
| Single-dose ER | 2,080 (650) | 10,000 (2,690) | 146 (66.0) | 12.0 (4.0-96.0) | 81.7 (23.3) | |||||
| 3-day IRc | 704 (188) | 7,830 (1,790) | 114 (44.2) | 60.0 (8.0-120) | 83.3 (22.6) | |||||
The ER regimen consisted of a single 2.0-g dose; the IR regimen consisted of 500 mg QD for 3 days. Each group included 12 subjects. Except for Tmax, pharmacokinetic parameters are arithmetic means (standard deviations).
For the IR regimen, serum AUC0-24, Cmax, and Tmax represent the values on day 1.
For the IR regimen, Cmax in WBC represents the overall maximum across the entire dosing period; the mean Cmax values in MNL and PMNL on day 1 were 53.9 and 42.9 μg/ml, respectively.
Azithromycin concentration at 120 h after the start of dosing.
FIG. 1.
Mean azithromycin serum concentration-time profiles following administration of a 2.0-g single-dose ER regimen versus a 3-day IR regimen (500 mg QD for 3 days) to healthy subjects (n = 12 per group). The profile on day 2 for the 3-day azithromycin IR regimen was estimated based on pharmacokinetic modeling. Error bars, standard deviations.
TABLE 2.
Summary of statistical analyses of azithromycin AUC0-24 and AUC0-120 in serum, MNL, and PMNL following administration of a single-dose ER regimen versus a 3-day IR regimen to healthy subjectsa
| PK parameter (μg · h/ml) | Adjusted geometric mean
|
B/A ratio (%) | 90% Confidence interval (%) | ||
|---|---|---|---|---|---|
| A (IR) | B (ER) | ||||
| Day 1 | |||||
| Serum AUC0-24 | 2.55 | 7.66 | 300.13 | (247.99, 363.23) | |
| MNL AUC0-24 | 608.93 | 1721.20 | 282.66 | (223.99, 356.69) | |
| PMNL AUC0-24 | 682.19 | 1992.85 | 292.12 | (239.25, 356.68) | |
| MNL/serum AUC0-24 | 238.59 | 224.71 | 94.18 | (80.28, 110.48) | |
| PMNL/serum AUC0-24 | 267.30 | 260.17 | 97.33 | (82.80, 114.42) | |
| Five days |
|
||||
| Serum AUC0-120 | 13.40 | 14.42 | 107.62 | (92.34, 125.44) | |
| MNL AUC0-120 | 3697.79 | 4580.18 | 123.86b | (100.84, 152.15) | |
| PMNL AUC0-120 | 7646.49 | 9668.50 | 126.44c | (106.14, 150.64) | |
| MNL/serum AUC0-120 | 275.93 | 317.57 | 115.09 | (100.64, 131.61) | |
| PMNL/serum AUC0-120 | 570.59 | 670.38 | 117.49d | (103.50, 133.37) | |
The ER regimen consisted of a single 2.0-g dose; the IR regimen consisted of 500 mg QD for 3 days. Each group included 12 subjects.
P = 0.08.
P = 0.03.
P = 0.04.
TABLE 3.
Summary of statistical analyses of azithromycin Cmax in serum, MNL, and PMNL on day 1 following administration of a single-dose ER regimen versus a 3-day IR regimen to healthy subjectsa
| Cmax (μg/ml) | Adjusted geometric mean
|
B/A ratio (%) | 90% Confidence interval (%) | |
|---|---|---|---|---|
| A (IR) | B (ER) | |||
| Serum | 0.38 | 0.69 | 183.84 | (144.07, 234.60) |
| MNL | 48.78 | 109.92 | 225.33 | (170.02, 298.64) |
| PMNL | 39.70 | 134.46 | 338.72 | (253.90, 451.86) |
| MNL/serum | 129.10 | 158.23 | 122.57 | (92.92, 161.68) |
| PMNL/serum | 105.05 | 193.55 | 184.24 | (135.01, 251.43) |
The ER regimen consisted of a single 2.0-g dose; the IR regimen consisted of 500 mg QD for 3 days. Each group included 12 subjects.
Compartmental modeling of the azithromycin exposure in serum was performed. Based on goodness-of-fit plots as well as fitted individual concentration-time profiles, the two-compartment model with zero-order absorption employing a proportional residual-error model (intraindividual) was selected as the final model to estimate the individual concentration-time profile on day 2 for the IR regimen. The two-compartment model with first-order absorption also showed acceptable estimation except that the zero-order absorption model showed a slightly better fit around the peak concentrations. The three-compartment model with either first-order or zero-order absorption failed to fit the data due to an inability to complete the covariance step successfully. This was probably due to the complexity of the model and insufficient observed concentration data.
Though the majority of the concentration-time profile, especially the elimination phase, was well characterized by the final model, the peak concentrations were underestimated, indicating that the zero-order absorption rate was not optimal for the description of the absorption phase of the azithromycin tablet formulation. The mean PK parameter estimates and standard errors of the final model are summarized in Table 4. The relative standard errors of parameter estimates indicated that most of the parameters were estimated well except for the intersubject variability in the volume of the peripheral compartment (ηVp/F) and the absorption rate (ηR1) (Table 4). In addition, the estimated mean AUC24-48 was 3.98 μg · h/ml, which was between the observed mean values of AUC0-24 and AUC48-72 (2.67 and 4.27 μg · h/ml). This also indicated that the estimation based on the compartmental modeling described above was acceptable.
TABLE 4.
Mean pharmacokinetic parameter estimates and relative standard errors for azithromycin in serum following administration of a 3-day IR regimen to healthy subjectsa
| Parameterb | Estimatec | % RSEd |
|---|---|---|
| CL/F (liters/h) | 122 | 16 |
| Vc/F (liters) | 518 | 13 |
| Vp/F (liters) | 3,400 | 10 |
| Q (liters/h) | 102 | 12 |
| R1 (mg/h) | 184 | 3 |
| ηCL/F | 33% | 93 |
| ηVc/F | 100% | 37 |
| ηVp/F | 6% | 492 |
| ηQ | 48% | 33 |
| ηR1 | 10% | 695 |
| ɛ | 30% | 31 |
The IR regimen consisted of a 500-mg tablet given QD for 3 days. The analysis used a two-compartment model with the zero-order absorption employing a proportional residual-error model.
CL/F, apparent clearance; Vc/F, apparent volume of the central compartment; Vp/F, apparent volume of the peripheral compartment; Q, intercompartmental clearance; R1, zero-order absorption rate; η, intersubject variability; ɛ, residual (intrasubject) variability.
Estimates of η and ɛ are expressed as the square root of the estimated variance in a percentage.
Relative standard error, calculated as (standard error/estimate) × 100, indicating how well the parameter is known.
WBC pharmacokinetics.
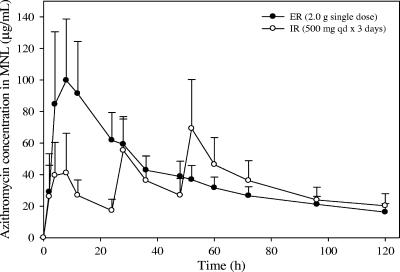
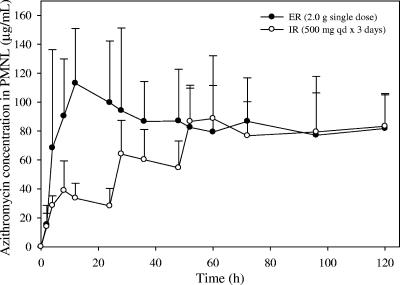
The mean azithromycin concentrations in MNL and PMNL on day 1 for the single-dose ER regimen were significantly higher than those for the 3-day IR regimen (Fig. 2 and 3). The geometric mean AUC0-24 ratios (ER/IR regimen) for MNL and PMNL were 283% and 292%, respectively (P < 0.00001), indicating that approximately threefold-higher exposure was achieved on day 1 in WBC with the single-dose regimen (Table 2). Additionally, the ER regimen achieved day-1 Cmax values in MNL and PMNL more than twofold higher than those for the IR regimen, with geometric mean ratios of 225% and 339%, respectively (P < 0.00001) (Table 3).
FIG. 2.
Mean azithromycin concentration versus time profiles in MNL following administration of a 2.0-g single-dose ER regimen versus a 3-day IR regimen (500 mg QD for 3 days) to healthy subjects (n = 12 per group). Error bars, standard deviations.
FIG. 3.
Mean azithromycin concentration versus time profiles in PMNL following administration of a 2.0-g single-dose ER regimen versus a 3-day IR regimen (500 mg QD for 3 days) to healthy subjects (n = 12 per group). Error bars, standard deviations.
Compared to the 3-day IR regimen, the single-dose ER regimen has slightly higher mean total azithromycin exposures (AUC0-120) in MNL and PMNL, with the lower bound of 90% CIs exceeding 100% (Table 2). The trough concentrations measured at 120 h (5 days) after the start of dosing (Ct=120) in serum and WBC were similar for the ER and IR regimens (Table 1). The average Ct=120 in MNL for the single-dose ER regimen and the 3-day IR regimen were 16.2 μg/ml and 20.2 μg/ml, respectively, and those in PMNL were 81.7 μg/ml and 83.3 μg/ml, respectively. This demonstrated that azithromycin concentrations in WBC remained above 10 μg/ml for at least 120 h after the start of dosing for both the ER and IR regimens.
The ER and IR regimens had similar MNL/serum and PMNL/serum ratios of the azithromycin AUC0-24, as well as similar AUC0-120 ratios (Table 2). The averages of AUC0-24 and AUC0-120 in MNL were approximately 250-fold to 300-fold higher than those in serum. The average AUC0-24 in PMNL was approximately 250-fold higher than that in serum, while the average AUC0-120 in PMNL was approximately 600-fold higher than that in serum.
Safety.
No deaths, serious AEs, or withdrawals due to AEs were reported for this study. No notable mean changes from the baseline (day 0, before the start of dosing) were observed for sitting systolic and diastolic BP or for sitting HR. In the group receiving the single-dose ER regimen, 4 out of 12 subjects reported 8 AEs including abdominal pain (n = 1), headache (n = 1), diarrhea (n = 3), asthenia (n = 1), taste perversion (n = 1), and syncope (n = 1). Except for the incidence of syncope, all the AEs were considered treatment related and mild in severity. A subject had a severe episode of syncope (transient collapse attributed to a vagovasal reaction after venipuncture for blood withdrawal) at 24 h after receiving the ER formulation, which was not considered to be related to the study drug. Other AEs reported by this subject were abdominal pain and diarrhea on day 1. In the group receiving the 3-day IR regimen, 7 out of 12 subjects reported 9 AEs including abdominal pain (n = 2), headache (n = 2), nausea (n = 3), dyspepsia (n = 1), and vomiting (n = 1). All the AEs were considered treatment related. Four events were mild, and five events were moderate. Both the ER and IR regimens were generally safe and well tolerated with respect to GI symptoms.
DISCUSSION
This study demonstrates the advantage of the single-dose azithromycin ER regimen over the 3-day IR regimen in that the former achieves higher exposures in serum and WBC during the first 24 h after the start of therapy while maintaining similar total exposures. “Front-loading” with the single-dose ER regimen achieved approximately threefold-higher azithromycin exposure in serum and WBC during the first 24 h after the start of dosing, which would subsequently result in higher initial exposure at the infection site, than the 3-day IR regimen. Therefore, a single-dose ER regimen is expected to provide additional therapeutic benefit by maximizing drug exposure at the infection site at a time when the bacterial burden is likely to be the highest. However, it is difficult to differentiate between the ER and IR regimens based on clinical efficacy, since the clinical success rates of these regimens are very high (i.e., 90 to 95%). A pharmacokinetic advantage of the ER regimen with respect to clinical outcome might be demonstrated if more resistant pathogens were present. This would have to be confirmed with relevant clinical studies. Compared to the 3-day IR regimen, the single-dose ER regimen is expected to achieve improved patient compliance. Although there is no strong evidence to prove that the high-dose, short-course therapy can reduce resistance, the low-dose, long-duration therapy has been shown to accelerate the emergence of resistance (8, 14, 18).
This study also evaluated whether saturation of uptake by phagocytes would occur at high doses of azithromycin. The similarities of the MNL/serum AUC ratios for the two regimens, as well as the PMNL/serum AUC ratios, confirmed that the distribution of azithromycin to WBC is approximately dose proportional and does not saturate at high doses up to 2.0 g. For both regimens, the MNL/serum AUC0-24 ratios were similar to the PMNL/serum AUC0-24 ratios (∼ 250-fold), which may suggest that azithromycin distribution has no preference for PMNL or MNL. The twofold difference in AUC0-120 between PMNL and MNL may indicate a longer azithromycin residence time in PMNL than in MNL. The Cmax values in MNL and PMNL across the entire dosing period for the 3-day IR regimen mostly presented on day 3 and were much higher than those on day 1, also suggesting a significant accumulation of azithromycin in WBC, especially in PMNL.
In this study, both regimens were generally safe and well tolerated. This study was not powered to compare the incidence of AEs in the two regimens. Based on the data from well-controlled phase-3 studies, the single-dose ER regimen has a tolerability profile comparable to those of the 3- or 5-day IR regimens except for the moderately higher rate of diarrhea/loose stools (Zithromax and Zmax package inserts; Pfizer Inc., New York, NY). Specifically, the most commonly reported AEs (≥1%) for the single-dose ER regimen are diarrhea/loose stools (11.6%), nausea (3.9%), abdominal pain (2.7%), and vomiting (1.1%), and those for the multiple-dose IR regimens are diarrhea/loose stools (5%), nausea (3%), and abdominal pain (3%) (Zithromax and Zmax package inserts).
The results of this study regarding the higher azithromycin exposure in WBC than in serum are consistent with those from several previous studies evaluating the WBC profiles of azithromycin IR regimens (2, 3, 21). Wildfeuer et al. have reported that the 3-day IR regimen provided detectable azithromycin concentrations in PMNL and MNL even at day 14 after the start of dosing, and the phagocytosis tests with PMNL confirmed their enhanced intracellular activity (21). Amsden et al. have demonstrated that the azithromycin exposures in serum, MNL, and PMNL with the 3-day IR regimen are similar to those with the 5-day IR regimen (3). In addition, a comparison of the serum and WBC azithromycin profiles of a 3-day IR regimen and a 1.5-g single dose of an azithromycin IR formulation showed no significant difference in the total exposure in serum and WBC between these two regimens (2). The previous studies also demonstrated that the 3-day IR regimen provides azithromycin concentrations in WBC well above the MIC for most community-acquired respiratory pathogens for at least 10 days, which provided evidence of prolonged exposure to azithromycin in the body. Though samples were collected for only 5 days in this study, it is reasonable to project that a 2.0-g single-dose ER regimen could provide azithromycin levels in WBC after 5 days postdosing, like the 3-day IR regimen, since the two regimens provided similar concentrations in WBC at 120 h after the start of dosing. It should be noted that while the extensive distribution of azithromycin to WBC may be relevant to clinical activity, high concentrations in WBC should not be interpreted as being quantitatively related to clinical efficacy.
In summary, the 2.0-g single-dose ER regimen was well tolerated and provided total exposures in serum and WBC similar to those for the 3-day IR regimen, with an additional therapeutic benefit due to “front-loading” of the dose, which achieved significantly higher exposures in serum and WBC during the first 24 h after the start of therapy.
Acknowledgments
We sincerely thank the staff of PHAROS GmbH, Ulm, Germany, who were involved in conducting this study. We thank our assay specialist, Penelope Crownover, and BAS Analytics (West Lafayette, IN) for the analytical assay support.
All the authors are employees of Pfizer except for Arvid Jungnik, who was the principal investigator for this clinical study, and Kem Phillips, who was an independent consultant. This study was sponsored by Pfizer.
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Amsden, G. W. 1996. Erythromycin, clarithromycin, and azithromycin: are the differences real? Clin. Ther. 18:55-72. [DOI] [PubMed] [Google Scholar]
- 2.Amsden, G. W., and C. L. Gray. 2001. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single dose or over a 3 day period in healthy volunteers. J. Antimicrob. Chemother. 47:61-66. [DOI] [PubMed] [Google Scholar]
- 3.Amsden, G. W., A. N. Nafziger, and G. Foulds. 1999. Pharmacokinetics in serum and leukocyte exposures of oral azithromycin, 1,500 milligrams, given over a 3- or 5-day period in healthy subjects. Antimicrob. Agents Chemother. 43:163-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beal, S. L., and L. B. Sheiner. 1988-1998. NONMEM users guides, parts I-VIII. NONMEM Project Group C255. University of California at San Francisco, San Francisco.
- 5.Chandra, R., P. Liu, J. D. Breen, J. Fisher, C. Xie, R. LaBadie, R. J. Benner, L. J. Benincosa, and A. Sharma. Clinical pharmacokinetics and gastrointestinal tolerability of a novel extended release microsphere formulation of azithromycin. Clin. Pharmacokinet., in press. [DOI] [PubMed]
- 6.Drehobl, M. A., M. C. De Salvo, D. E. Lewis, and J. D. Breen. 2005. Single-dose azithromycin microspheres vs clarithromycin extended release for the treatment of mild-to-moderate community-acquired pneumonia in adults. Chest 128:2230-2237. [DOI] [PubMed] [Google Scholar]
- 7.Drusano, G. L., and W. A. Craig. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J. Chemother. 9(Suppl. 3):38-44. [PubMed] [Google Scholar]
- 8.File, T. M., Jr. 2004. Clinical efficacy of newer agents in short-duration therapy for community-acquired pneumonia. Clin. Infect. Dis. 39(Suppl. 3):S159-S164. [DOI] [PubMed] [Google Scholar]
- 9.Foulds, G., and R. B. Johnson. 1993. Selection of dose regimens of azithromycin. J. Antimicrob. Chemother. 31(Suppl. E):39-50. [DOI] [PubMed] [Google Scholar]
- 10.Girard, D., J. M. Bergeron, W. B. Milisen, and J. A. Retsema. 1993. Comparison of azithromycin, roxithromycin, and cephalexin penetration kinetics in early and mature abscesses. J. Antimicrob. Chemother. 31(Suppl. E):17-28. [DOI] [PubMed] [Google Scholar]
- 11.Girard, D., S. M. Finegan, M. W. Dunne, and M. E. Lame. 2005. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J. Antimicrob. Chemother. 56:365-371. [DOI] [PubMed] [Google Scholar]
- 12.Gladue, R. P., G. M. Bright, R. E. Isaacson, and M. F. Newborg. 1989. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 33:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladue, R. P., and M. E. Snider. 1990. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob. Agents Chemother. 34:1056-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillemot, D., C. Carbon, B. Balkau, P. Geslin, H. Lecoeur, F. Vauzelle-Kervroedan, G. Bouvenot, and E. Eschwege. 1998. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 279:365-370. [DOI] [PubMed] [Google Scholar]
- 15.Kupper, L., and K. Hafner. 1989. How appropriate are popular sample size formulas? Am. Statistician 43:101-105. [Google Scholar]
- 16.Nibbering, P. H., T. P. Zomerdijk, A. J. Corsel-Van Tilburg, and R. Van Furth. 1990. Mean cell volume of human blood leucocytes and resident and activated murine macrophages. J. Immunol. Methods 129:143-145. [DOI] [PubMed] [Google Scholar]
- 17.Periti, P., T. Mazzei, E. Mini, and A. Novelli. 1993. Adverse effects of macrolide antibacterials. Drug Saf. 9:346-364. [DOI] [PubMed] [Google Scholar]
- 18.Segreti, J., H. R. House, and R. E. Siegel. 2005. Principles of antibiotic treatment of community-acquired pneumonia in the outpatient setting. Am. J. Med. 118(Suppl. 7A):21S-28S. [DOI] [PubMed] [Google Scholar]
- 19.Takeshita, E., B. Matsuura, M. Dong, L. J. Miller, H. Matsui, and M. Onji. 2006. Molecular characterization and distribution of motilin family receptors in the human gastrointestinal tract. J. Gastroenterol. 41:223-230. [DOI] [PubMed] [Google Scholar]
- 20.Weber, F. H., Jr., R. D. Richards, and R. W. McCallum. 1993. Erythromycin: a motilin agonist and gastrointestinal prokinetic agent. Am. J. Gastroenterol. 88:485-490. [PubMed] [Google Scholar]
- 21.Wildfeuer, A., H. Laufen, and T. Zimmermann. 1996. Uptake of azithromycin by various cells and its intracellular activity under in vivo conditions. Antimicrob. Agents Chemother. 40:75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]