Abstract
Quinolone activity against Escherichia coli was examined during aerobic growth, aerobic treatment with chloramphenicol, and anaerobic growth. Nalidixic acid, norfloxacin, ciprofloxacin, and PD161144 were lethal for cultures growing aerobically, and the bacteriostatic activity of each quinolone was unaffected by anaerobic growth. However, lethal activity was distinct for each quinolone with cells treated aerobically with chloramphenicol or grown anaerobically. Nalidixic acid failed to kill cells under both conditions; norfloxacin killed cells when they were grown anaerobically but not when they were treated with chloramphenicol; ciprofloxacin killed cells under both conditions but required higher concentrations than those required with cells grown aerobically; and PD161144, a C-8-methoxy fluoroquinolone, was equally lethal under all conditions. Following pretreatment with nalidixic acid, a shift to anaerobic conditions or the addition of chloramphenicol rapidly blocked further cell death. Formation of quinolone-gyrase-DNA complexes, observed as a sodium dodecyl sulfate (SDS)-dependent drop in cell lysate viscosity, occurred during aerobic and anaerobic growth and in the presence and in the absence of chloramphenicol. However, lethal chromosome fragmentation, detected as a drop in viscosity in the absence of SDS, occurred with nalidixic acid treatment only under aerobic conditions in the absence of chloramphenicol. With PD161144, chromosome fragmentation was detected when the cells were grown aerobically and anaerobically and in the presence and in the absence of chloramphenicol. Thus, all quinolones tested appear to form reversible bacteriostatic complexes containing broken DNA during aerobic growth, during anaerobic growth, and when protein synthesis is blocked; however, the ability to fragment chromosomes and to rapidly kill cells under these conditions depends on quinolone structure.
When DNA topoisomerases bind to bacterial DNA, they form complexes that are trapped by the quinolone antibacterials (for a review, see reference 6). These ternary drug-enzyme-DNA complexes block DNA replication (5, 27, 29, 32), RNA synthesis (22, 33), and cell growth (2, 12). A key feature of the complexes is that the trapped DNA moiety is broken, with its ends constrained through covalent linkage to the GyrA protein (24, 25). As a result, quinolone concentrations sufficient to block replication do not relax chromosomal DNA supercoiling (29). When the quinolone is removed, the breaks are readily resealed (11, 31) and the inhibitory effects of the compounds are reversed (13, 21, 27). Irreversible events that lead to rapid cell death occur at higher quinolone concentrations (1, 20). We have proposed that rapid cell death arises from chromosome fragmentation that occurs when double-strand DNA breaks are released from the protein-mediated constraint present in ternary complexes. At rapidly lethal quinolone concentrations, supercoils cannot be maintained (1) and fragmented DNA is obtained under conditions that allow resealing at bacteriostatic drug concentrations (21).
Rapid quinolone-mediated cell death, which does not appear to require interruption of active DNA replication fork movement (35), occurs in two ways: one that requires ongoing protein synthesis and one that does not (15). The relative contribution of each of these two lethal pathways depends on quinolone structure. For example, the lethal actions of nalidixic and oxolinic acids are blocked by chloramphenicol, an inhibitor of protein synthesis (1, 17). In contrast, fluoroquinolones, such as ciprofloxacin, do not require ongoing protein or RNA synthesis to kill cells (1, 17). Whether other perturbations of bacterial metabolism distinguish the two pathways is unknown.
Anaerobiosis is potentially useful for the study of quinolone lethality, since it affects gyrase and supercoiling (16) and it allows quinolones (oxolinic acid) to form ternary complexes (7). Moreover, fluoroquinolones kill Escherichia coli under some anaerobic conditions (3). To date, structure-activity relationships have not been established for anaerobic activity.
In the present study we examined quinolone-mediated lethality after passing an anaerobic gas mixture through a growing culture of E. coli. The gas mixture had no effect on the ability of the quinolones to block growth. However, it allowed only fluoroquinolones to kill the cells. The effects of anaerobiosis and inhibition of protein synthesis on quinolone lethality provided a way to classify the quinolones into groups, to distinguish bacteriostatic action from bactericidal action, and to correlate chromosome fragmentation with rapid cell death.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and quinolone susceptibility.
The E. coli strains used in the study were DM4100 (30) and the following derivatives of DM4100 constructed by P1-mediated transduction: KD1373 (ParC Ser-80 to Leu), KD2750 (GyrA Ser-83 to Leu and Asp-87 to Tyr), and KD2329 (GyrA Ser-83 to Leu, Asp-87 to Tyr, and ParC Ser-80 to Leu). Introduction of the mutations was confirmed by nucleotide sequence determination of the quinolone resistance-determining regions for gyrA and parC (10, 34). Strains were grown on LB agar (23) or in LB liquid medium containing 1% glucose and 100 mM equimolar sodium phosphate buffer (pH 6.8). Exponentially growing cultures were shifted to anaerobic conditions by passing a mixture of 85% N2, 10% H2, and 5% CO2 through the culture (the gas mixture was scrubbed by passage through a powdered zinc suspension in 20 μM phenazine methosulfate [pH 4] and then bubbled through the culture) (16).
The MICs were measured by incubation of 104 to 105 cells/ml in liquid medium containing serial twofold dilutions of a quinolone at 37°C either with shaking (2-ml cultures in 2.5-cm diameter tubes) for aerobic cultures or in sealed Vacutainer tubes (1.5-cm diameter tubes) following 20 min of treatment with the anaerobic gas mixture. To measure lethal action, cells were grown aerobically with shaking at 37°C in liquid medium to mid-log phase. The cells were split into 2-ml portions, with one portion grown under aerobic conditions with shaking and the other portion grown in a sealed tube treated with the anaerobic gas mixture. After 20 min, aerobic or anaerobic solutions of a quinolone were added, the cultures were mixed with a Vortex mixer, and incubation was continued for 2 h with (anaerobic) or without (aerobic) passage of the anaerobic gas mixture. Cells were diluted in ice-cold LB medium, applied to LB agar plates lacking drug, and incubated aerobically overnight at 37°C on agar plates to determine the number of CFU, which was expressed relative to the number of CFU at the time of quinolone addition. For measurement of killing in the absence of protein synthesis, chloramphenicol was added to 20 μg/ml 10 min prior to addition of a quinolone. Cultures that were aerated by shaking or by passage of atmospheric air (bubbling) exhibited the same sensitivity to killing by nalidixic acid (not shown).
Antimicrobial agents.
Nalidixic acid, norfloxacin, and chloramphenicol were obtained from Sigma-Aldrich (St Louis, MO); ciprofloxacin was obtained from Bayer Corp. (West Haven, CT); and PD161144 was obtained from John Domagala, Parke-Davis Pharmaceutical Division of Pfizer, Ann Arbor, MI. The quinolones were dissolved in 0.1 ml of 1 N NaOH at 1/10 of the final volume, followed by the addition of sterile water to give a final concentration of 10 mg/ml. Stock solution aliquots were kept at −20°C for several weeks during the experiments. Aliquots were used only once; after the aliquots were thawed, dilutions were prepared with sterile distilled water.
Detection of chromosome fragmentation by viscosity.
Bacterial cells were gently lysed by incubation with lysozyme and nonionic detergents at 20°C for 2 to 3 min, as described previously (29). Cells grown anaerobically were lysed under anaerobic conditions. Serial, twofold dilutions of cell lysates (0.2 ml) were transferred to glass tubes (10 by 75 mm) with minimal shearing. Pancreatic RNase was added to 20 μg/ml; the samples were then treated at 80°C for 2 min to unfold the chromosomal DNA, chilled on ice, and brought to 20°C in a water bath. A 0.025-ml glass microcapillary pipette (catalog no. 71900-25; Kimble Glass Co.) was placed in the lysate samples, and the time to fill the capillary, after subtraction of the time for buffer alone to fill the capillary, was taken as an empirical measure of viscosity (η*). Use of viscosity to detect chromosome fragmentation was previously validated by sedimentation measurements (21). In addition, a dramatic decrease in viscosity was observed after addition of DNase I (1 μg/ml for 10 min) to lysates grown either aerobically or anaerobically (not shown).
RESULTS
Effect of anaerobic growth on bacteriostatic activity of quinolones.
When an anaerobic gas mixture was passed through an exponentially growing culture of wild-type E. coli (strain DM4100), growth slowed modestly (Fig. 1). The bacteriostatic actions (MICs) of nalidixic acid, norfloxacin, ciprofloxacin, and PD161144 (see Fig. 2 for the compound structures) were unaffected by the shift to anaerobic conditions (Table 1). Since the MIC is likely to reflect the formation of quinolone-gyrase-DNA complexes (2, 29), the data in Table 1 suggest that anaerobic growth has little effect on ternary complex formation.
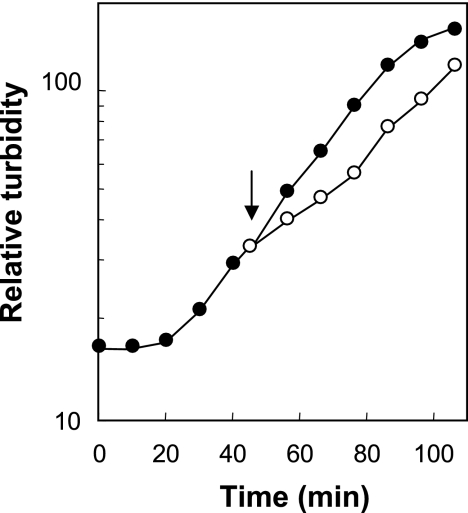
FIG. 1.
Effect of anaerobic conditions on growth of E. coli. Wild-type strain DM4100 was grown under aerobic conditions (filled circles), and at the time indicated by the arrow, part of the culture was shifted to anaerobic conditions (open circles) by passing an anaerobic gas mixture (85% N2, 10% H2, and 5% CO2) through the culture. Culture turbidity was measured with a Klett-Summerson colorimeter.
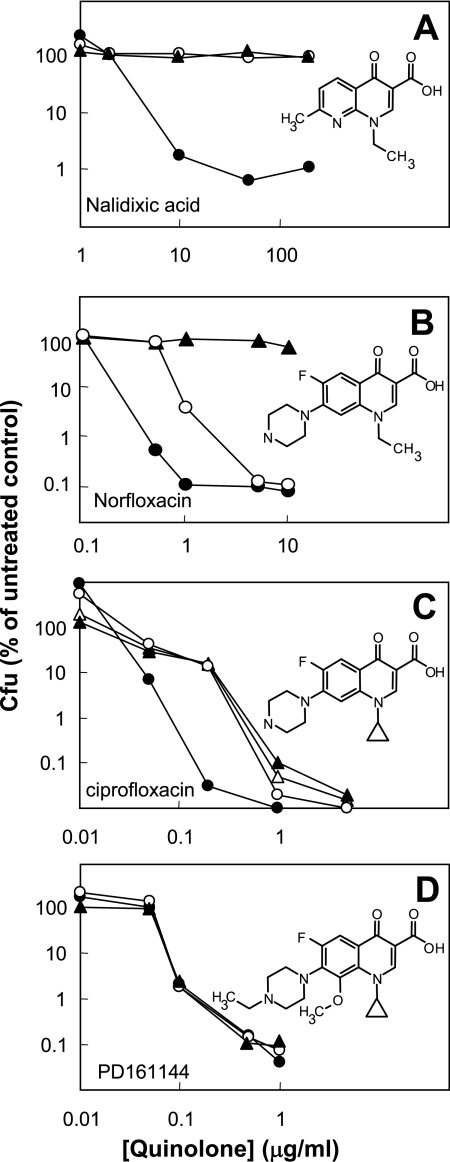
FIG. 2.
Effect of anaerobic conditions on quinolone lethality. Exponentially growing E. coli (strain DM4100) was treated with nalidixic acid (A), norfloxacin (B), ciprofloxacin (C), or PD161144 (D) for 2 h under aerobic conditions (filled circles), anaerobic conditions (open circles) initiated 20 min before drug addition, or aerobic conditions that included treatment with 20 μg/ml chloramphenicol (filled triangles) added 10 min before the quinolone. For panel C, cells growing anaerobically were treated with chloramphenicol for 10 min, followed by treatment with ciprofloxacin (open triangles). At the end of the incubations, the cells were diluted aerobically, applied to drug-free agar, and incubated to determine the fraction of CFU relative to the number of CFU at the time of quinolone addition. Replicate experiments with each compound produced similar results.
TABLE 1.
Effect of anaerobic growth on bacteriostatic and bactericidal activities of quinolones
| Quinolone | Bacteriostatic activity (MIC [μg/ml])a
|
Bactericidal activityb
|
||||
|---|---|---|---|---|---|---|
| Aerobic | Anaerobic | Aerobicc | Salined | Anaerobicc | Cmc | |
| Nalidixic acid | 5 | 5 | + | − | − | − |
| Norfloxacin | 0.075 | 0.075 | + | + | +e | − |
| Ciprofloxacin | 0.01 | 0.01 | + | + | +e | +e |
| PD161144 | 0.08 | 0.08 | + | ND | + | + |
Data were obtained with E. coli strain DM4100.
Symbols and abbreviations: Cm, chloramphenicol; ND, not determined; −, no activity; +, lethal activity.
Data are summarized from Fig. 2.
Summarized elsewhere (15).
A quinolone concentration higher than that observed under aerobic conditions is required to kill cells (for data, see Fig. 2).
Effect of anaerobic growth or chloramphenicol on quinolone-mediated lethality.
To compare the abilities of the quinolones to rapidly kill E. coli under anaerobic conditions, the anaerobic gas mixture was passed through cultures of exponentially growing cells for 20 min. Then, nalidixic acid, norfloxacin, ciprofloxacin, or PD161144 was added anaerobically at various concentrations, followed by passage of the anaerobic gas mixture for 2 h. The cells were diluted and plated on drug-free agar to determine the number of viable cells present in the culture. A portion of each aerobically growing culture was also treated with chloramphenicol for 10 min before addition of a quinolone to measure the effect of inhibition of protein synthesis on quinolone lethality. Both anaerobic growth and treatment with chloramphenicol blocked the killing of E. coli by nalidixic acid (Fig. 2A). The lethal activity of norfloxacin was blocked by chloramphenicol but not by anaerobic growth, although a higher norfloxacin concentration was required for anaerobic lethality (Fig. 2B). Ciprofloxacin killed the E. coli cells anaerobically and in the presence of chloramphenicol, but increased ciprofloxacin concentrations were required (Fig. 2C). With ciprofloxacin the two perturbations appeared to act on the same process, since a combined treatment (Fig. 2C) had the same effect as either treatment alone. Neither anaerobic growth nor chloramphenicol treatment affected the activity of PD161144, a C-8-methoxy fluoroquinolone (Fig. 2D). These data indicate that the quinolones fall into four categories with respect to their ability to kill cells shifted to anaerobic conditions or treated with chloramphenicol (summarized in Table 1).
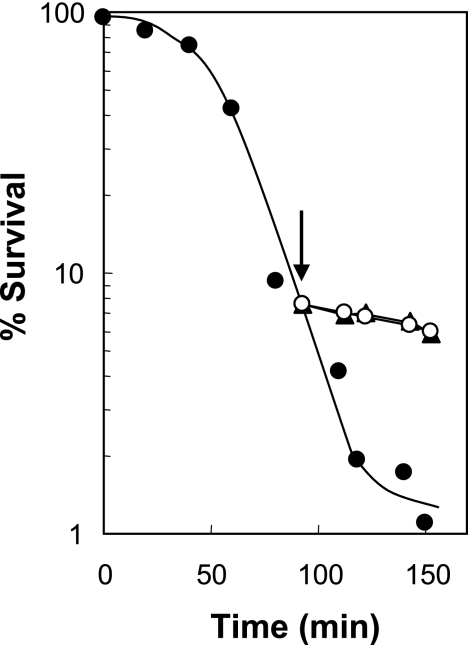
The rate at which a shift to low oxygen tension blocked lethality was measured by growing the cells aerobically, treating them with nalidixic acid for 90 min, and then passing an anaerobic gas mixture through the culture. The lethal activity of the quinolone was quickly inhibited (Fig. 3). Addition of chloramphenicol also quickly blocked lethal activity (Fig. 3), consistent with findings presented in previous work (4). Thus, lethal factors synthesized under aerobic conditions appear to be unstable and/or are not synthesized under anaerobic conditions.
FIG. 3.
Concordance of chloramphenicol and anaerobic effects on quinolone-mediated lethality. Exponentially growing cells (strain DM4100) were treated with 50 μg/ml nalidixic acid (10 times the MIC; filled circles), and after 90 min (arrow) a portion of the culture was shifted to anaerobic conditions (open circles) or was treated with chloramphenicol at 20 μg/ml (filled triangles). At the indicated times, aliquots were processed as described in the legend to Fig. 2. Replicate experiments produced similar results.
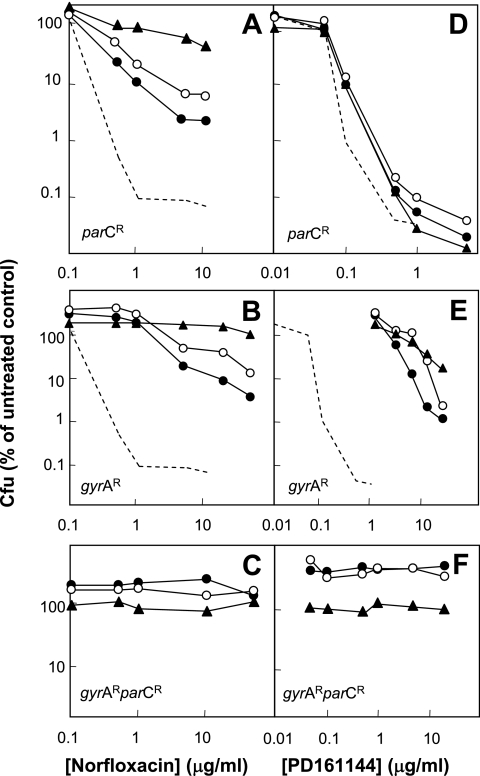
Concentrations of norfloxacin that inhibit gyrase may also affect topoisomerase IV (9); consequently, we examined the effect of resistance mutations to determine whether target preference was affected by low oxygen tension. Norfloxacin appears to interact with both enzymes within the concentration range examined, since resistance mutations in gyrA or parC conferred significant levels of protection (Fig. 4A and B). With both mutants, anaerobic conditions only partially blocked the lethal activity of norfloxacin, while chloramphenicol was more inhibitory. A gyrA parC double mutant was insensitive to norfloxacin (Fig. 4C), confirming that gyrase and topoisomerase IV were the major targets of this quinolone.
FIG. 4.
Effects of gyrA and parC resistance mutations on lethal action of norfloxacin and PD161144. Exponentially growing E. coli mutants were treated with the indicated concentrations of norfloxacin (A to C) or PD161144 (D and E) for 2 h under aerobic conditions (filled circles), anaerobic conditions (open circles), or aerobic conditions following treatment for 10 min with 20 μg/ml chloramphenicol (filled triangles). Following incubation, aliquots were processed as described in the legend to Fig. 2. (A and D) mutant with parC-mediated resistance (strain KD1373); (B and E) mutant with gyrA-mediated resistance (strain KD2750); (C and F) mutant with gyrA- and parC-mediated resistance (strain KD2329). The dotted lines in panels A, B, D, and E represent the results for wild-type cells treated with the quinolone (data from Fig. 2). Replicate experiments with each drug and each strain produced similar results.
When PD161144 was tested with strain KD1373, a mutant of DM4100 with parC-encoded resistance, little difference from the results obtained with the wild type was observed: the compound was equally lethal during aerobic growth, during anaerobic growth, and in the presence of chloramphenicol (Fig. 4D). When gyrA encoded resistance, the lethal activity of PD161144 was reduced substantially, with anaerobic conditions and chloramphenicol having little additional effect (Fig. 4E). As expected, when both gyrA and parC encoded resistance, the lethal activity was eliminated (Fig. 4F). These are the results expected if gyrase is the major target.
Effects of anaerobic growth and chloramphenicol treatment on quinolone-mediated chromosome fragmentation.
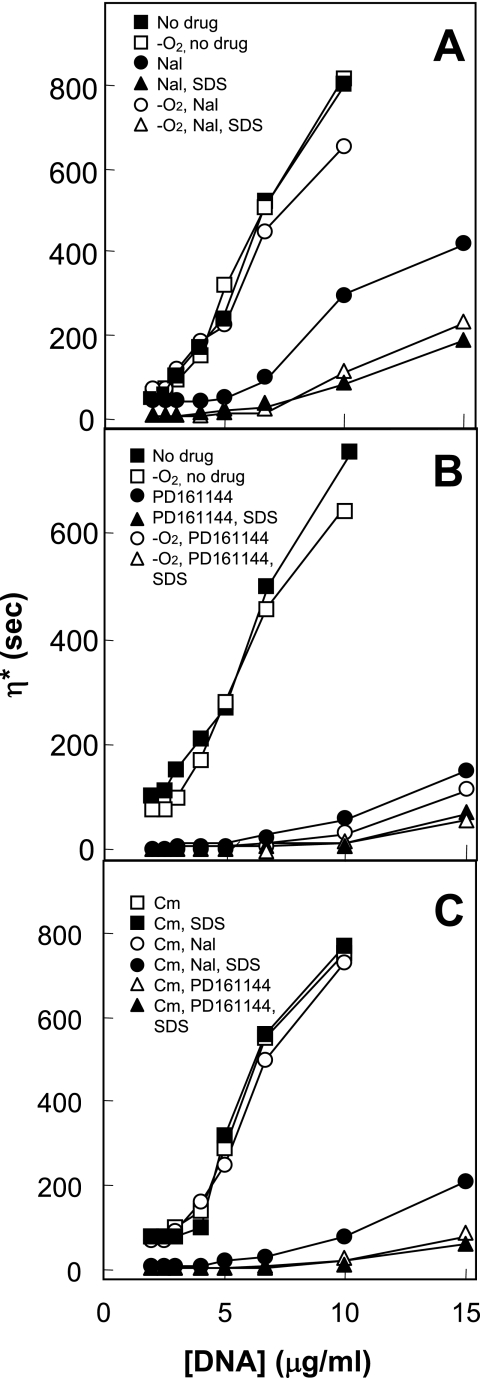
When E. coli cells are gently lysed in the absence of proteases or ionic detergents, the nucleoid retains its structure and the lysates have very low viscosities (8). Disruption of the nucleoid structure by treatment with RNase and/or mild thermal treatment causes the solutions to become very viscous (8). Exposure of cells to nalidixic acid for 2 h under lethal, aerobic conditions lowered the lysate viscosity when it was measured by capillary viscometry (Fig. 5A), consistent with the occurrence of intracellular chromosome fragmentation. In contrast, treatment with nalidixic acid under anaerobic conditions for 2 h, which was not lethal, resulted in high viscosity (Fig. 5A), similar to values measured with lysates from untreated cells grown under anaerobic conditions (Fig. 5A) or aerobic conditions (Fig. 5A). Since lysates from cells growing anaerobically and exposed to nalidixic acid exhibited low viscosities after treatment with sodium dodecyl sulfate (SDS) to release the DNA breaks from protein constraint (Fig. 5A), drug-gyrase-DNA ternary complexes form anaerobically, a result that is consistent with growth inhibition (Table 1). These results confirmed that nalidixic acid formed ternary complexes under anaerobic conditions, but the complexes were not processed to release the DNA breaks that fragmented the chromosomes. The fluoroquinolone PD161144 was expected to behave differently from nalidixic acid, since PD161144 kills E. coli under anaerobic conditions. Lysates prepared from cells treated with PD161144 for 2 h either aerobically or anaerobically exhibited very low viscosities in the absence of SDS (Fig. 5B), similar to those observed when SDS released the breaks from the ternary complexes (Fig. 5B).
FIG. 5.
Quinolone-mediated chromosome fragmentation detected by lysate viscosity. Wild-type E. coli (strain DM4100) was grown exponentially under aerobic or anaerobic conditions and then treated for 2 h with 50 μg/ml nalidixic acid (Nal; 10 times the MIC) (A) or with 0.8 μg/ml PD161144 (10 times the MIC) (B). Cells were also treated with no drug (squares). Cells were gently lysed under aerobic or anerobic conditions, and lysate viscosity was measured as described in Materials and Methods. Open symbols, cells grown and lysed under anaerobic conditions in the absence (circles) or the presence of SDS (triangles); filled symbols, cells grown and lysed under aerobic conditions in the absence (circles) or the presence of SDS (triangles). (C) Effect of 20 μg/ml chloramphenicol (Cm) alone (squares) and in combination with either nalidixic acid (circles) or PD161144 (triangles). SDS was added to (filled symbols) or omitted from (open symbols) the cell lysates. Replicate experiments produced similar results.
We also examined the effect of chloramphenicol on quinolone-mediated changes in lysate viscosity. Lysates from cells treated with both chloramphenicol and nalidixic acid were viscous unless they were treated with SDS (Fig. 5C), while those from cells treated with chloramphenicol and PD161144 were nonviscous in the presence or absence of SDS (Fig. 5C). As expected, chloramphenicol alone had no effect on lysate viscosity with or without SDS treatment (Fig. 5C). Thus, only treatments that rapidly kill E. coli correlated with a loss of lysate viscosity.
DISCUSSION
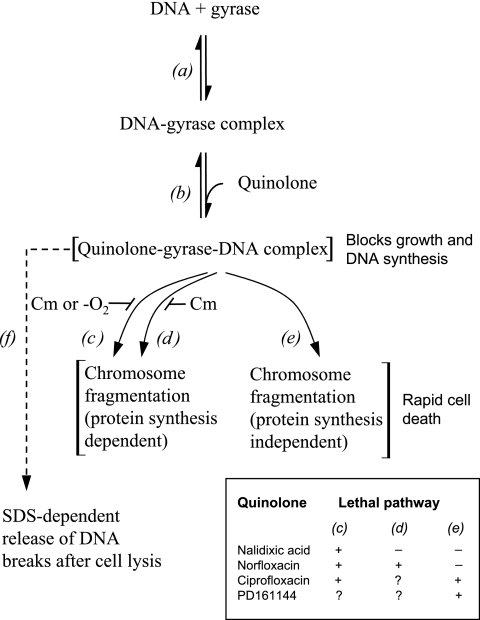
Rapid killing of bacterial cells by the quinolones can be described as a two-step process (Fig. 6) (1, 21). The first step is the formation of reversible, bacteriostatic drug-gyrase-DNA complexes containing DNA breaks constrained by protein (Fig. 6, step b). The second step is the release of DNA breaks from the complexes, which results in chromosome fragmentation and cell death (Fig. 6, steps c to e). In the present work, anaerobic growth allowed nalidixic acid, the prototype quinolone, to block growth (Table 1) and to trap gyrase on DNA, as indicated by an SDS-dependent drop in cell lysate viscosity (Fig. 5A; depicted in Fig. 6 as step f). However, lethal action (Fig. 2A) and chromosome fragmentation (decrease in lysate viscosity in the absence of SDS; Fig. 5A) were blocked by anaerobiosis and by chloramphenicol treatment. These data distinguished steps b and c in Fig. 6. Norfloxacin killed E. coli under anaerobic conditions, but not in the presence of chloramphenicol (Fig. 2B; depicted as step d in Fig. 6 and discussed below). Ciprofloxacin and PD161144 were lethal anaerobically and in the presence of chloramphenicol (Fig. 2C and D), thereby defining an additional lethal pathway (Fig. 6, step e). The concentration of ciprofloxacin needed to kill cells was increased by anaerobic and chloramphenicol treatments, with the two treatments having no additional effect when they were combined (Fig. 2C). These data suggest that ciprofloxacin acts by pathways c and e of Fig. 6 (activity through step d would be seen as chloramphenicol inhibiting ciprofloxacin activity more than anaerobiosis did, which was not observed). The lethal action of the C-8-methoxy compound PD161144 was unaffected by anaerobic growth or chloramphenicol (Fig. 2D), suggesting that this compound acts largely by step e in Fig. 6 (activity through steps c and d would be manifested as a reduction in PD161144-dependent killing by chloramphenicol or anaerobiosis, which was not observed). Thus, anaerobic experiments support the two-step scheme (21), and they divide protein-synthesis-dependent killing into two pathways (Fig. 6, steps c and d).
FIG. 6.
Schematic representation of quinolone action. (a) Binding of gyrase to DNA; (b) formation of quinolone-gyrase-DNA complexes; (c) lethal chromosome fragmentation that requires ongoing protein synthesis under aerobic conditions; (d) Lethal chromosome fragmentation that requires ongoing protein synthesis but not aerobic conditions; (e) lethal chromosome fragmentation that does not require ongoing protein synthesis or aerobic conditions; (f) DNA breakage detected after treatment of cell lysate with an ionic detergent, such as SDS. (Inset) The use of lethal pathways by each quinolone is indicated; question marks indicate a lack of evidence for particular pathways (see Discussion).
While the experiments described above indicate that fluoroquinolones can kill E. coli cells exposed to an anaerobic gas mixture, they do not resolve an earlier controversy over whether the compounds are lethal when a different method for achieving anaerobiosis is used (3, 18, 26). The anaerobic lethalities of fluoroquinolones may be sensitive to experimental conditions.
The concordant effects of anaerobiosis on quinolone lethality and cell lysate viscosity (Fig. 2A and D and Fig. 5) support the conclusion that chromosome fragmentation is responsible for rapid quinolone-mediated cell death. In earlier work we showed (i) that nucleoids can maintain supercoils when they are isolated from E. coli cells treated with quinolones under bacteriostatic conditions but not bactericidal conditions (1); (ii) that chromosome fragmentation, measured by sedimentation analyses, occurs during the same time frame as cell death (21); and (iii) that chromosome fragmentation fails to occur when cell death is blocked by chloramphenicol (21). We emphasize that these data do not address the contributions of other processes, such as lethal filamentation (12) and induction of toxin-antitoxin systems (14), to cell death.
Nalidixic acid requires aerobic growth and ongoing protein synthesis to kill E. coli, suggesting the involvement of an induced lethal protein factor. Such a factor may be unstable and may not be synthesized under anaerobic conditions, since the loss of lethality occurred quickly upon treatment with chloramphenicol or anaerobic conditions (Fig. 3A) (4). Anaerobiosis provides a way to narrow genomic RNA profiling searches for the gene(s) encoding the lethal factor.
Examination of norfloxacin lethality distinguished anaerobic effects from inhibition of protein synthesis by chloramphenicol (Fig. 2C), thereby establishing a distinct lethal pathway (Fig. 6, step d). In earlier work norfloxacin was distinguished by its ability to kill E. coli cells that had been resuspended in cold saline, a feature not observed with nalidixic acid (15). These effects of norfloxacin are not readily explained by the propensity of the compound to target both topoisomerase IV and gyrase (both parC and gyrA resistance alleles lower norfloxacin lethality [9]); Fig. 4A and B). The inhibitory effect of anaerobiosis on norfloxacin lethality was similar with topoisomerase IV as the target (gyrA resistance mutant) and gyrase as the target (parC resistance mutant) (Fig. 4A and B). Additional work is required to explain the ability of norfloxacin to kill cells when they are growing anaerobically or when they are suspended in cold saline but not when they are treated with chloramphenicol.
The abilities of some fluoroquinolones to kill bacteria under anaerobic conditions may be relevant for efforts to identify new derivatives that kill nongrowing Mycobacterium tuberculosis. Gene expression studies with a murine model of infection indicate that M. tuberculosis shifts from aerobic to anaerobic respiration during the transition to growth arrest (28). Thus, C-8-methoxy fluoroquinolones that are superior at killing under anaerobic conditions and in the absence of protein synthesis (Fig. 2) may be good candidates as new antituberculosis agents. We note that moxifloxacin, a C-8-methoxy fluoroquinolone, is particularly effective at killing M. tuberculosis when protein synthesis is blocked (19).
Acknowledgments
We thank the following for critical comments on the manuscript: Marila Gennaro, Richard Pine, and Xilin Zhao.
The work was supported by NIH grants AI35277 and AI063431.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Chen, C. R., M. Malik, M. Snyder, and K. Drlica. 1996. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J. Mol. Biol. 258:627-637. [DOI] [PubMed] [Google Scholar]
- 2.Chow, R., T. Dougherty, H. Fraimow, E. Bellin, and M. Miller. 1988. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob. Agents Chemother. 32:1113-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper, M. A., J. M. Andrews, and R. Wise. 1991. Bactericidal activity of sparfloxacin and ciprofloxacin under anaerobic conditions. J. Antimicrob. Chemother. 28:399-405. [DOI] [PubMed] [Google Scholar]
- 4.Crumplin, G. C., and J. T. Smith. 1975. Nalidixic acid: an antibacterial paradox. Antimicrob. Agents Chemother. 8:251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deitz, W. H., T. M. Cook, and W. A. Goss. 1966. Mechanism of action of nalidixic acid on Escherichia coli. III. Conditions required for lethality. J. Bacteriol. 91:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drlica, K., and M. Malik. 2003. Fluoroquinolones: action and resistance. Curr. Top. Med. Chem. 3:1349-1364. [DOI] [PubMed] [Google Scholar]
- 7.Drlica, K., G. Pruss, R. Burger, R. Franco, L.-S. Hsieh, and B. Berger. 1990. Roles of DNA topoisomerases in bacterial chromosome structure and function, p. 195-204. In K. Drlica and M. Riley (ed.), The bacterial chromosome. American Society for Microbiology, Washington, DC.
- 8.Drlica, K., and A. Worcel. 1975. Conformational transitions in the Escherichia coli chromosome: analysis by viscometry and sedimentation. J. Mol. Biol. 98:393-411. [DOI] [PubMed] [Google Scholar]
- 9.Fournier, B., X. Zhao, T. Lu, K. Drlica, and D. Hooper. 2000. Selective targeting of topoisomerase IV and DNA gyrase in Staphylococcus aureus: different patterns of quinolone-induced inhibition of DNA synthesis. Antimicrob. Agents Chemother. 44:2160-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman, S. M., T. Lu, and K. Drlica. 2001. A mutation in the DNA gyrase A gene of Escherichia coli that expands the quinolone-resistance-determining region. Antimicrob. Agents Chemother. 45:2378-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gellert, M., K. Mizuuchi, M. H. O'Dea, T. Itoh, and J.-L. Tomizawa. 1977. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc. Natl. Acad. Sci. USA 74:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goss, W., W. Deitz, and T. Cook. 1964. Mechanism of action of nalidixic acid on Escherichia coli. J. Bacteriol. 88:1112-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goss, W., W. Deitz, and T. Cook. 1965. Mechanism of action of nalidixic acid on Escherichia coli. II. Inhibition of deoxyribonucleic acid synthesis. J. Bacteriol. 89:1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazan, R., B. Sat, and H. Engelberg-Kulka. 2004. Escherichia coli mazEF-mediated cell death is triggered by various stressful conditions. J. Bacteriol. 186:3663-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard, B. M., R. J. Pinney, and J. T. Smith. 1993. 4-Quinolone bactericidal mechanisms. Arzneimittelforsch./Drug Res. 43:1125-1129. [PubMed] [Google Scholar]
- 16.Hsieh, L.-S., R. M. Burger, and K. Drlica. 1991. Bacterial DNA supercoiling and [ATP]/[ADP]: changes associated with a transition to anaerobic growth. J. Mol. Biol. 219:443-450. [DOI] [PubMed] [Google Scholar]
- 17.Lewin, C., B. Howard, and J. Smith. 1991. Protein- and RNA-synthesis independent bactericidal activity of ciprofloxacin that involves the A subunit of DNA gyrase. J. Med. Microbiol. 34:19-22. [DOI] [PubMed] [Google Scholar]
- 18.Lewin, C., I. Morrissey, and J. Smith. 1991. The mode of action of quinolones: the paradox in activity of low and high concentrations and activity in the anaerobic environment. Eur. J. Clin. Microbiol. Infect. Dis. 10:240-248. [DOI] [PubMed] [Google Scholar]
- 19.Malik, M., and K. Drlica. 2006. Moxifloxacin lethality with Mycobacterium tuberculosis in the presence and absence of chloramphenicol. Antimicrob. Agents Chemother. 50:2842-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik, M., T. Lu, X. Zhao, A. Singh, C. Hattan, J. Domagala, R. Kerns, and K. Drlica. 2005. Lethality of quinolones agains Mycobacterium smegmatis in the presence or absence of chloramphenicol. Antimicrob. Agents Chemother. 49:2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik, M., X. Zhao, and K. Drlica. 2006. Lethal fragmentation of bacterial chromosomes mediated by DNA gyrase and quinolones. Mol. Microbiol. 61:810-825. [DOI] [PubMed] [Google Scholar]
- 22.Manes, S. H., G. J. Pruss, and K. Drlica. 1983. Inhibition of RNA synthesis by oxolinic acid is unrelated to average DNA supercoiling. J. Bacteriol. 155:420-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Mizuuchi, K., L. M. Fisher, M. O'Dea, and M. Gellert. 1980. DNA gyrase action involves the introduction of transient double-strand breaks into DNA. Proc. Natl. Acad. Sci. USA 77:1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison, A., N. P. Higgins, and N. R. Cozzarelli. 1980. Interaction between DNA gyrase and its cleavage site on DNA. J. Biol. Chem. 255:2211-2219. [PubMed] [Google Scholar]
- 26.Morrissey, L., and J. T. Smith. 1992. Absence of bactericidal activity of sparfloxacin and ciprofloxacin under anaerobic conditions. J. Antimicrob. Chemother. 29:589. [DOI] [PubMed] [Google Scholar]
- 27.Pohlhaus, J., and K. N. Kreuzer. 2005. Norfloxacin-induced DNA gyrase cleavage complexes block Escherichia coli replication forks, causing double-stranded breaks in vivo. Mol. Microbiol. 56:1416-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi, L., C. D. Sohaskey, B. Kana, S. Dawes, R. J. North, V. Mizrahi, and M. Gennaro. 2005. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc. Natl. Acad. Sci. USA 102:15629-15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snyder, M., and K. Drlica. 1979. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J. Mol. Biol. 131:287-302. [DOI] [PubMed] [Google Scholar]
- 30.Sternglanz, R., S. DiNardo, K. A. Voelkel, Y. Nishimura, Y. Hirota, A. K. Becherer, L. Zumstein, and J. C. Wang. 1981. Mutations in the gene coding for Escherichia coli DNA topoisomerase I affecting transcription and transposition. Proc. Natl. Acad. Sci. USA 78:2747-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugino, A., C. Peebles, K. Kruezer, and N. Cozzarelli. 1977. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc. Natl. Acad. Sci. USA 74:4767-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wentzell, L., and A. Maxwell. 2000. The complex of DNA gyrase and quinolone drugs on DNA forms a barrier to the T7 DNA polymerase replication complex. J. Mol. Biol. 304:779-791. [DOI] [PubMed] [Google Scholar]
- 33.Willmott, C. J. R., S. E. Critchlow, I. C. Eperon, and A. Maxwell. 1994. The complex of DNA gyrase and quinolone drugs with DNA forms a barrier to transcription by RNA polymerase. J. Mol. Biol. 242:351-363. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida, H., M. Bogaki, M. Nakamura, and S. Nakamura. 1990. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob. Agents Chemother. 34:1271-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao, X., M. Malik, N. Chan, A. Drlica-Wagner, J.-Y. Wang, X. Li, and K. Drlica. 2006. Lethal action of quinolones with a temperature-sensitive dnaB replication mutant of Escherichia coli. Antimicrob. Agents Chemother. 50:362-364. [DOI] [PMC free article] [PubMed] [Google Scholar]