Abstract
Current assays for screening new antimalarials need initiators of β-hematin formation that require laborious preparation, special devices, and substrates. In this study, based on reduction of heme absorption in β-hematin formation, we developed a simple colorimetric assay using Tween 20 as an initiator and a microplate reader for high-throughput screening of inhibitors of β-hematin formation.
Plasmodium spp., which are malarial parasites, degrade hemoglobin in host erythrocytes to use the catabolic products as a source of amino acids during development and proliferation. This is accompanied by a release of free heme. The free heme is oxidatively active and toxic to both the host cell and the malarial parasite, and it causes parasite death. Due to the absence of heme oxygenase, the parasite is unable to cleave heme into an open-chain tetrapyrrole, which is necessary for cellular excretion (8). To protect itself, the parasite detoxifies free heme via neutralization with histidine-rich protein 2 (14, 23), degradation with reduced glutathione (2, 13), or crystallization into hemozoin (HZ), which is a water-insoluble malarial pigment produced in the food vacuole (12, 23). Chloroquine (CQ) and other antimalarial drugs have been demonstrated to inhibit the formation of a synthetic heme crystal, β-hematin (BH), which is structurally identical to hemozoin (21, 24), and are believed to inhibit hemozoin formation in the food vacuole of the malarial parasite. Current reports indicate that blocking the BH formation is an ideal target for antimalarial screening (1, 19, 22). Several factors such as temperature (9), histidine-rich protein (14, 23), lipids (6, 26), and preformed BH (22) have been reportedly responsible for promoting BH formation. However, the thermal BH crystallization is not natural and is a nonphysiological process. Some strains of Plasmodium spp. lack histidine-rich proteins but still form HZ (22), suggesting that histidine-rich proteins are sufficient but not completely necessary for HZ formation. Therefore, the two most important and natural initiators of BH formation are probably lipids and preformed BH. Several studies have used preformed BH (4, 6, 7) and lipids extracted from parasites (11), infected plasma (26), or commercial lecithin (25) as initiators of BH formation in assays. However, the preformed BH-based assay has a high background absorbance in the colorimetric assay (26) and requires fresh purification of BH, while using lipids requires laborious preparation and strict storage. In this study, we demonstrated that a surfactant (Tween 20), considered a good model for the biological membrane (3), can be used as an initiator for BH formation in assays in vitro.
Recently, high-throughput screening for BH inhibition of new antimalarial compounds has been suggested as a useful tool in the discovery of new antimalarial compounds (17, 18). Several methods have been described for the measurement of BH crystallization in high-throughput screening of antimalarials; however, they involve special filtration microplates (5, 27), expensive 3H-labeled heme (17), a laborious transfer of samples to another 96-well plate (18), or the use of toxic substances such as pyridine and 3H-labeled heme. Here, based on reduction of heme absorption in BH formation (16), we have developed a simple, reproducible, and fast colorimetric assay using Tween 20 as an initiator of BH for high-throughput screening of antimalarial candidates.
Hemin chloride (16.3 mg; Sigma) was dissolved in 1 ml of dimethyl sulfoxide. The solution was passed through a 0.2-μm-pore membrane filter to remove insoluble particles. The solution can be kept at 4°C up to 1 month as a stock solution (25) and diluted to 111.1 μM of heme with 1 M acetate buffer, pH 4.8, just before being used. In order to determine heme concentration in stock solution, the absorbance at 400 nm was measured after the solution was diluted with 2.5% sodium dodecyl sulfate in 0.1 M NaOH. The heme concentration was calculated with an extinction coefficient of 105 at 400 nm as described previously (13).
CQ (Sigma), amodiaquine (from MP Biomedical Inc.), and quinacrine (from Calbiochem, Canada) were dissolved in distilled water. Quinine sulfate (Q; Sigma), quinidine (from Wako, Osaka, Japan), and 8-hydroxyquinoline (Sigma) were dissolved in 20 mM sulfuric acid. Mefloquine (from Sigma) was prepared in methanol. pH was controlled at 4.8 in all assays. Tween 20 (1 g/100 ml) was dissolved in distilled water.
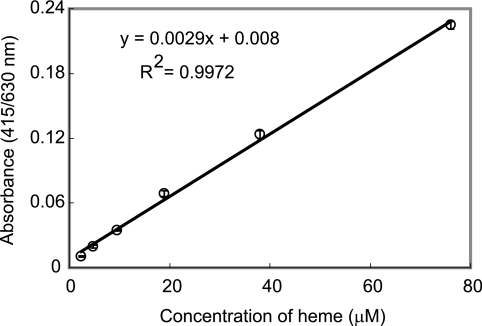
Two hundred microliters of heme buffered by 1 M acetate buffer, pH 4.8 (final concentrations ranged from 2.4 to 76 μM in serial 1:2 dilutions), was dispensed in a 96-well plate. The plate was scanned at 415/630 nm (absorbance at 630 nm was subtracted from absorbance at 415 nm) using an MTP-120 microplate reader (Corona Electric Co., Ibaragi, Japan). The heme absorbance at 415 nm must be corrected by subtraction of turbidity of BH. The recorded absorbance at 415/630 nm was found linearly over the heme concentration ranges of 2.4 to 76 μM (Fig. 1). Thus, we used 50 μM of heme in all assays to evaluate the effect of drugs on BH formation.
FIG. 1.
Linear relationship between absorbance at 415/630 nm and heme concentration.
To establish effective conditions for BH formation, we studied the efficiency of BH formation induced by various concentrations of Tween 20 in vitro at 37°C. One hundred microliters of Tween 20 (various concentrations in serial 1:2 dilutions by distilled water) was added in each well of a 96-well plate. Next, 100 μl of heme (50 μM, buffered by 1 M acetate buffer, pH 4.8) was added to each well and incubated at 37°C for 250 min. The plate was read at 415/630 nm as described above. Based on our previous study (16), the yield of heme converted to BH was calculated by the following equation:
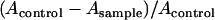 |
(1) |
where Acontrol is the absorbance of the heme without Tween 20 at 415/630 nm, while Asample represents the absorbance of the heme in the presence of Tween 20.
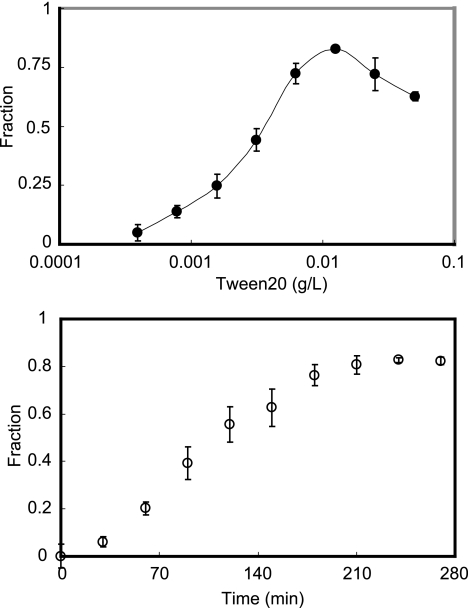
The results are shown in Fig. 2. The yield of BH reached maximum, approximately 80%, at 0.012 g/liter of Tween 20 (Fig. 2, top) and at 250 min (Fig. 2, bottom), indicating a relatively high efficiency in converting heme into BH in the presence of Tween 20. The crystal growth curves showed a sigmoidal pattern (Fig. 2, bottom), in good agreement with previous reports, describing the transition from a metastable state to a stable equilibrium state, suggesting that Tween 20 plays a role in stabilizing nuclei of BH (25). To our surprise, the rate of Tween 20-induced BH formation is higher than that of other assays using initiators such as lipids and preformed BH.
FIG. 2.
Catalysis of BH formation induced by Tween 20. (Top) Dose response of Tween 20-induced BH formation. (Bottom) Time course of BH crystallization induced by Tween 20 (0.012 g/liter). BH crystallization was expressed as percent heme converted into BH.
The characteristics of purified BH were further confirmed by infrared spectroscopy as described previously (15), demonstrating the expected peaks at 1,210 and 1,664 cm−1 (data not shown). Based on the above results, heme in all experiments for high-throughput screening antimalarial drugs was incubated in the presence of 0.012 g/liter of Tween 20 for 250 min.
To evaluate the availability of the BH formation induced by Tween 20 for high-throughput screening of new antimalarial drugs, the inhibitory effects of quinoline antimalarial drugs on BH formation were investigated using Tween 20 (Fig. 3) as an inducer. The procedure for high-throughput screening of new antimalarial drugs was performed in the following order of steps. Firstly, drugs were prepared at various concentrations using distilled water and sulfuric acid (20 mM). Secondly, 90 μl of heme solution (50 μM), freshly buffered by 1 M acetate buffer (pH 4.8) from dimethyl sulfoxide stock, was added into the plate. Finally, Tween 20 was added into each well at 0.012 g/liter. After incubation at 37°C for 250 min, samples were mixed by being pipetted three times, and then the plate was read at 415/630 nm. The fraction (f) of heme converted to BH was calculated as in a previous study (16):
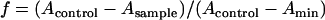 |
(2) |
where Acontrol is the absorbance of the heme without Tween 20 or an antimalarial at 415/630 nm, while Asample represents the absorbance of the heme in the presence of both Tween 20 and drugs and Amin is the absorbance of the heme with Tween 20 in the absence of an antimalarial at 415/630 nm.
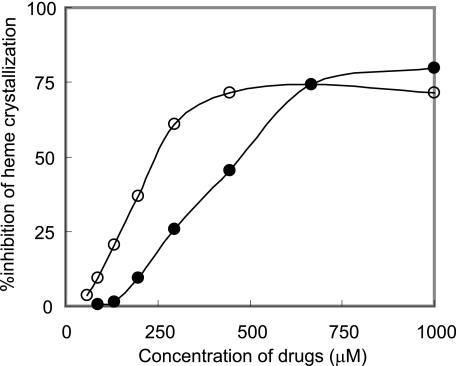
FIG. 3.
Inhibition of heme crystallization by antimalarials. After incubation of heme with various concentrations of CQ (open circles) and Q (closed circles) in duplicate, the absorbance at 415/630 nm was recorded and the fraction (f) of heme converted to BH was calculated as described in the text. The results are representative data from three independent experiments.
Percentage of inhibition of BH by drugs was then described by the following equation:
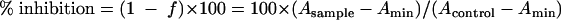 |
(3) |
The values obtained from duplicate assays were plotted, and the IC50 values, the concentrations inhibiting 50% of heme crystallization, were calculated graphically. The results, obtained from four independent duplicate experiments, showed that all quinoline antimalarials used in this study can inhibit Tween 20-induced BH formation (IC50 values of 252 ± 49 μM, 365 ± 103 μM, 315 ± 123 μM, 167 ± 59 μM, 325 ± 87 μM, and 292 ± 99 μM for CQ, Q, mefloquine, amodiaquine, quinidine, and quinacrine, respectively). The mechanisms of the drugs are believed to relate to heme crystallization; they bind heme and inhibit hemozoin formation in the lysosomal digestive vacuole of the parasite (20). The IC50 values for these drugs were higher than those of assays based on preformed BH-based and lecithin (25). The reason is obscure, but the slight difference in the abilities of antimalarial drugs to bind to heme in the presence of inducers and the difference in the mechanism of initiation of heme crystallization between Tween 20 and other inducers might affect the values. In addition, the IC50 value is widely varied depending on the incubation time of the assay (10). However, these values were within a threefold range, in good agreement with previous reports (4, 7, 26), suggesting that the IC50 values in our assay of inhibition of Tween 20-induced BH formation are acceptable for high-throughput screening of new antimalarial drugs.
Our results showed that using Tween 20 has several advantages over using current initiators: for instance, (i) the assay is fast and based on a simple technique using a microplate reader, which is available in most labs; (ii) the Tween 20-based assay is not expensive for large-scale screening; (iii) the method is a convenient method that requires no fresh preparation of inducers; (iv) Tween 20 can be prepared in stock solution and requires no strict conditions for storage; (v) the assay does not use pyridine or mutagenic and carcinogenic agents; (vi) the assay uses a simple 96-well plate, which requires no purification or transfer steps, promising an ideal high-throughput screening of antimalarial candidates.
In conclusion, our assay of inhibition of BH formation using Tween 20 is cheap, reproducible, nontoxic, and more convenient than other assays for large-scale screening of novel antimalarials.
Footnotes
Published ahead of print on 6 November 2006.
REFERENCES
- 1.Adams, P. A., P. A. Berman, T. J. Egan, P. J. Marsh, and J. Silver. 1996. The iron environment in heme and heme-antimalarial complexes of pharmacological interest. J. Inorg. Biochem. 63:69-77. [DOI] [PubMed] [Google Scholar]
- 2.Atamna, H., and H. Ginsburg. 1995. Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J. Biol. Chem. 270:24876-24883. [DOI] [PubMed] [Google Scholar]
- 3.Barber, J. 1989. Membrane proteins. Detergent ringing true as a model for membranes. Nature 340:601. [DOI] [PubMed] [Google Scholar]
- 4.Chong, C. R., and D. J. Sullivan, Jr. 2003. Inhibition of heme crystal growth by antimalarials and other compounds: implications for drug discovery. Biochem. Pharmacol. 66:2201-2212. [DOI] [PubMed] [Google Scholar]
- 5.Deharo, E., R. N. Garcia, P. Oporto, A. Gimenez, M. Sauvain, V. Jullian, and H. Ginsburg. 2002. A non-radiolabelled ferriprotoporphyrin IX biomineralisation inhibition test for the high throughput screening of antimalarial compounds. Exp. Parasitol. 100:252-256. [DOI] [PubMed] [Google Scholar]
- 6.Dorn, A., R. Stoffel, H. Matile, A. Bubendorf, and R. G. Ridley. 1995. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature 374:269-271. [DOI] [PubMed] [Google Scholar]
- 7.Dorn, A., S. R. Vippagunta, H. Matile, A. Bubendorf, J. L. Vennerstrom, and R. G. Ridley. 1998. A comparison and analysis of several ways to promote haematin (haem) polymerisation and an assessment of its initiation in vitro. Biochem. Pharmacol. 55:737-747. [DOI] [PubMed] [Google Scholar]
- 8.Eckman, J. R., S. Modler, J. W. Eaton, E. Berger, and R. R. Engel. 1977. Host heme catabolism in drug-sensitive and drug-resistant malaria. J. Lab. Clin. Med. 90:767-770. [PubMed] [Google Scholar]
- 9.Egan, T. J., E. Hempelmann, and W. W. Mavuso. 1999. Characterisation of synthetic beta-haematin and effects of the antimalarial drugs quinidine, halofantrine, desbutylhalofantrine and mefloquine on its formation. J. Inorg Biochem. 73:101-107. [DOI] [PubMed] [Google Scholar]
- 10.Egan, T. J., and K. K. Ncokazi. 2005. Quinoline antimalarials decrease the rate of beta-hematin formation. J. Inorg. Biochem. 99:1532-1539. [DOI] [PubMed] [Google Scholar]
- 11.Fitch, C. D., G. Z. Cai, Y. F. Chen, and J. D. Shoemaker. 1999. Involvement of lipids in ferriprotoporphyrin IX polymerization in malaria. Biochim. Biophys. Acta 1454:31-37. [DOI] [PubMed] [Google Scholar]
- 12.Francis, S. E., D. J. Sullivan, Jr., and D. E. Goldberg. 1997. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 51:97-123. [DOI] [PubMed] [Google Scholar]
- 13.Huy, N. T., K. Kamei, T. Yamamoto, Y. Kondo, K. Kanaori, R. Takano, K. Tajima, and S. Hara. 2002. Clotrimazole binds to heme and enhances heme-dependent hemolysis: proposed antimalarial mechanism of clotrimazole. J. Biol. Chem. 277:4152-4158. [DOI] [PubMed] [Google Scholar]
- 14.Huy, N. T., S. Serada, D. T. Trang, R. Takano, Y. Kondo, K. Kanaori, K. Tajima, S. Hara, and K. Kamei. 2003. Neutralization of toxic heme by Plasmodium falciparum histidine-rich protein 2. J. Biochem. (Tokyo) 133:693-698. [DOI] [PubMed] [Google Scholar]
- 15.Huy, N. T., D. T. Trang, T. Kariu, M. Sasai, K. Saida, S. Harada, and K. Kamei. 2006. Leukocyte activation by malarial pigment. Parasitol. Int. 55:75-81. [DOI] [PubMed] [Google Scholar]
- 16.Huy, N. T., D. T. Uyen, M. Sasai, D. T. Trang, T. Shiono, S. Harada, and K. Kamei. 2006. A simple and rapid colorimetric method to measure hemozoin crystal growth in vitro. Anal. Biochem. 354:305-307. [DOI] [PubMed] [Google Scholar]
- 17.Kurosawa, Y., A. Dorn, M. Kitsuji-Shirane, H. Shimada, T. Satoh, H. Matile, W. Hofheinz, R. Masciadri, M. Kansy, and R. G. Ridley. 2000. Hematin polymerization assay as a high-throughput screen for identification of new antimalarial pharmacophores. Antimicrob. Agents Chemother. 44:2638-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ncokazi, K. K., and T. J. Egan. 2005. A colorimetric high-throughput beta-hematin inhibition screening assay for use in the search for antimalarial compounds. Anal. Biochem. 338:306-319. [DOI] [PubMed] [Google Scholar]
- 19.Ridley, R. G., A. Dorn, S. R. Vippagunta, and J. L. Vennerstrom. 1997. Haematin (haem) polymerization and its inhibition by quinoline antimalarials. Ann. Trop. Med. Parasitol. 91:559-566. [DOI] [PubMed] [Google Scholar]
- 20.Slater, A. F. 1993. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol. Ther. 57:203-235. [DOI] [PubMed] [Google Scholar]
- 21.Slater, A. F., and A. Cerami. 1992. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature 355:167-169. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan, D. J. 2002. Theories on malarial pigment formation and quinoline action. Int. J. Parasitol. 32:1645-1653. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan, D. J., Jr., I. Y. Gluzman, and D. E. Goldberg. 1996. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 271:219-222. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan, D. J., Jr., I. Y. Gluzman, D. G. Russell, and D. E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trang, D. T., N. T. Huy, D. T. Uyen, M. Sasai, T. Shiono, S. Harada, and K. Kamei. 2006. Inhibition assay of beta-hematin formation initiated by lecithin for screening new antimalarial drugs. Anal. Biochem. 349:292-296. [DOI] [PubMed] [Google Scholar]
- 26.Tripathi, A. K., A. Gupta, S. K. Garg, and B. L. Tekwani. 2001. In vitro beta-hematin formation assays with plasma of mice infected with Plasmodium yoelii and other parasite preparations: comparative inhibition with quinoline and endoperoxide antimalarials. Life Sci. 69:2725-2733. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi, A. K., S. I. Khan, L. A. Walker, and B. L. Tekwani. 2004. Spectrophotometric determination of de novo hemozoin/beta-hematin formation in an in vitro assay. Anal. Biochem. 325:85-91. [DOI] [PubMed] [Google Scholar]