Abstract
In a mouse model, ertapenem inhibited the anaerobic intestinal microflora and promoted overgrowth of enterococci, whereas imipenem-cilastatin had no effect on the indigenous microflora. Ertapenem, but not imipenem-cilastatin, promoted modest overgrowth of vancomycin-resistant enterococci when exposure occurred during treatment. Neither agent promoted colonization with extended-spectrum beta-lactamase-producing Klebsiella pneumoniae.
The indigenous microflora of the colon provides an important host defense by inhibiting colonization by pathogenic microorganisms (4). Antibiotics that are excreted in high concentrations into bile may cause marked disruption of the indigenous microflora, thereby promoting colonization by a variety of pathogens, including vancomycin-resistant enterococci (VRE) and antibiotic-resistant gram-negative bacilli (4). Disruption of the anaerobic component of the microflora may play a particularly important role in facilitating colonization by pathogens (3-5). Although carbapenems have potent activity against anaerobic bacteria, several studies suggest that agents such as imipenem-cilastatin and meropenem may cause only mild disruption of intestinal anaerobes in healthy volunteers or patients (1, 6, 9). This observation has been attributed to the fact that these carbapenems are excreted in relatively low concentrations in bile (1, 6, 9). In contrast, a recent study suggested that ertapenem is excreted in higher concentrations in bile, resulting in inhibition of anaerobes and overgrowth of enterococci (7). In this study, we used a mouse model to examine the effects of the carbapenem antibiotics imipenem-cilastatin and ertapenem on the intestinal microflora and on establishment of colonization by VRE and Klebsiella pneumoniae. For comparison, we studied three agents that we have previously shown to disrupt the indigenous microflora and promote VRE and K. pneumoniae colonization (i.e., piperacillin-tazobactam and ceftriaxone) (3-5).
VRE strain C68 is a clinical Van-B isolate that we have used for previous mouse colonization studies (3). K. pneumoniae strain P62 produces an SHV extended-spectrum beta-lactamase (ESBL) and has been used in previous mouse studies (5). The broth dilution MICs of drugs for strains C68 and P62, respectively, were as follows: imipenem-cilastatin, 512 and 1 μg/ml; ertapenem, >1,024 and <0.25 μg/ml; piperacillin-tazobactam, 1,250 and 4 μg/ml; and ceftriaxone, >10,000 and 4 μg/ml.
The experimental protocol was approved by the Cleveland Veterans Affairs Medical Center's Animal Care Committee. Female CF1 mice (Harlan Sprague-Dawley, Indianapolis, IN) weighing 25 to 30 g were housed individually. Mice received treatment with normal saline or the study antibiotics for 5 days. The dose of the antibiotics was based on the daily dose recommended for human adults (in milligrams per kilogram of body weight). All antibiotics were administered subcutaneously once each day in 0.2 ml of phosphate-buffered saline.
Stool samples were collected on day 3 of treatment to assess the effects of the antibiotics on the stool microflora and to measure antibiotic levels in stool. To assess the effects on the microflora, fresh stool samples were diluted in normal saline and plated onto Enterococcosel agar, MacConkey agar, brucella agar, and Bacteroides bile-esculin agar (Becton Dickinson) to measure concentrations of enterococci, total and facultative gram-negative bacilli, total anaerobes, and Bacteroides spp., respectively. Cultures of total anaerobes and Bacteroides spp. were performed inside an anaerobic chamber (Coy Laboratories). The concentrations of antibiotics in stool samples were determined by an agar diffusion assay with Escherichia coli as the indicator strain (8). Two days after discontinuation of antibiotic treatment, mice received 10,000 CFU of VRE or K. pneumoniae by orogastric gavage. Separate experiments were conducted with VRE and K. pneumoniae. The organisms were suspended in 0.5 ml of phosphate-buffered saline and administered using a stainless steel feeding tube (Perfektum; Popper & Sons, New Hyde Park, NY). The densities of VRE or ESBL-producing K. pneumoniae in stool samples were measured at baseline and on days 1, 3, and 6 after orogastric gavage as previously described (3, 5). All experiments were performed twice with a total of eight mice per treatment group.
An additional set of experiments was performed in which the pathogens were administered during antibiotic treatment (day 3 of 6 of antibiotic treatment). This set of experiments was included because antibiotics may have differing effects on establishment of pathogen colonization if pathogens are administered during versus after completion of treatment (i.e., during treatment, antibiotics may inhibit the establishment of colonization, whereas the same agent may promote colonization if pathogens are administered after completion of treatment during the period of recovery of the indigenous microflora) (4).
Because the initial experimental results indicated that ertapenem was not detected in stool samples after 3 days of treatment despite the fact that it caused significant changes in the microflora, we measured the concentrations of ertapenem and imipenem-cilastatin in stool samples from additional mice (eight per group) after 5 days of treatment with these antibiotics. The antibiotics were administered daily in the dosages noted above.
Data analyses were performed with the use of Stata software (version 6.0, Stata, College Station, TX). A one-way analysis of variance was performed to compare the groups with P values adjusted for multiple comparisons using the Scheffe correction.
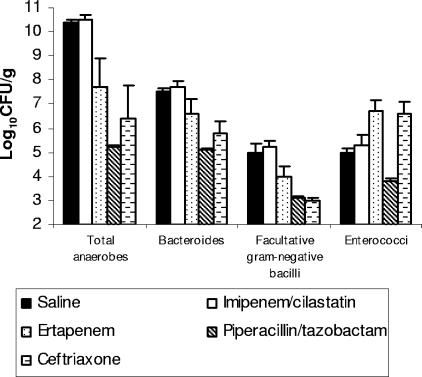
The effect of antibiotic treatment on the stool microflora is shown in Fig. 1. Imipenem-cilastatin treatment did not result in significant changes in any of the measured components of the microflora in comparison to saline controls (P ≥ 0.5). Ertapenem, piperacillin-tazobactam, and ceftriaxone treatment resulted in significant reductions in the densities of total anaerobes, Bacteroides spp., and facultative gram-negative bacilli in comparison to saline controls (P < 0.03). Ertapenem inhibited total anaerobes and Bacteroides spp. to a lesser degree than piperacillin-tazobactam did (P < 0.03), but there was no significant difference in suppression of these groups between the ertapenem and ceftriaxone groups (P > 0.22). Piperacillin-tazobactam treatment resulted in suppression of enterococci (P = 0.001), whereas ertapenem and ceftriaxone promoted overgrowth of enterococci (P < 0.02). The mean concentrations of piperacillin-tazobactam and ceftriaxone in stool samples on day 3 of treatment were 37.5 μg/g (range, 0 to 100 μg/g) and 93.8 μg/g (range, 62 to 125 μg/g), respectively. Imipenem-cilastatin and ertapenem were not detectable in stool samples on day 3 of treatment (limit of detection, 5 μg/g). For the additional groups of mice that received the carbapenem antibiotics for 5 days prior to assessment of drug levels in stool samples, imipenem-cilastatin remained undetectable, whereas ertapenem was detected in the stool samples from four of eight mice (mean concentration, 10.5 μg/g [range, 0 to 56 μg/g]).
FIG. 1.
Effect of subcutaneous antibiotic treatment on the density of total anaerobes, Bacteroides spp., facultative gram-negative bacilli, and Enterococcus spp. in stool samples from mice. Mice received antibiotic treatment once daily for 3 days. Stool samples were collected and plated onto selective medium to determine bacterial densities. If organisms were not detected in stool samples, the lower limit of detection (∼2 log10 CFU/g) was assigned.
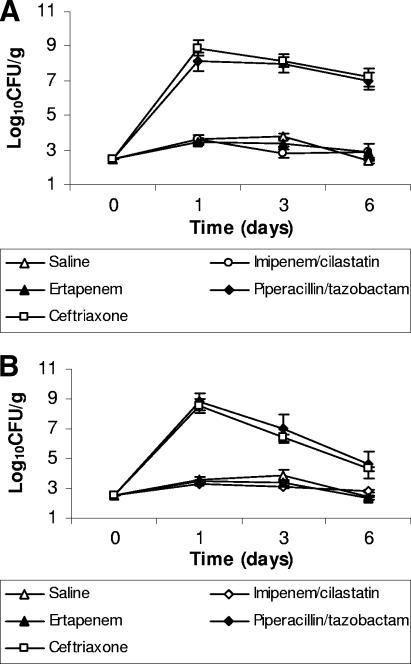
The effect of antibiotic treatment on establishment of colonization when the pathogens were inoculated 2 days after discontinuation of treatment is shown in Fig. 2. At baseline, the mice did not have detectable VRE or ceftazidime-resistant gram-negative bacilli in stool samples (level of detection, ∼2.5 log10 CFU/g). Piperacillin-tazobactam and ceftriaxone promoted overgrowth of VRE and K. pneumoniae in comparison to saline controls (P < 0.001), whereas imipenem-cilastatin and ertapenem did not (P > 0.95).
FIG. 2.
Effect of subcutaneous antibiotic treatment on the establishment of colonization with extended-spectrum β-lactamase-producing Klebsiella pneumoniae (A) and vancomycin-resistant enterococci (B) in mice exposed to the pathogens 2 days after completion of antibiotic treatment. Mice received antibiotic treatment once daily for 5 days, and 2 days after completion of antibiotics (day 0), 104 CFU of one of the pathogens was administered by orogastric gavage. If the pathogens were not detected in stool samples, the lower limit of detection (∼2 log10 CFU/g) was assigned.
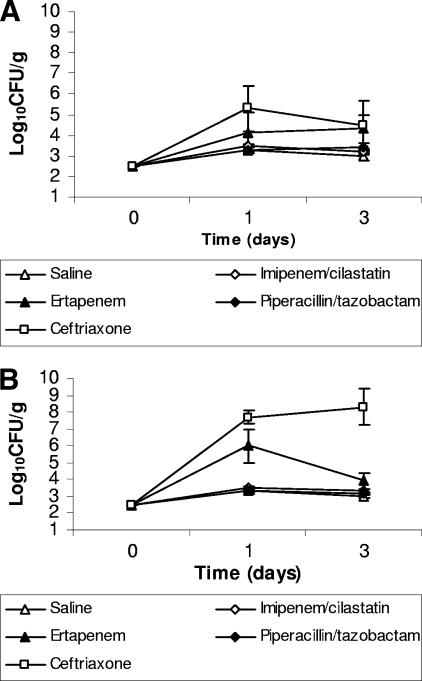
The effects of the antibiotics on establishment of colonization when the pathogens were inoculated during treatment is shown in Fig. 3. None of the antibiotics promoted significant overgrowth of K. pneumoniae in comparison to saline controls (P > 0.45) (Fig. 3A). Ceftriaxone promoted significant overgrowth of VRE (P < 0.001), whereas piperacillin-tazobactam and imipenem-cilastatin did not (P = 1) (Fig. 3B). Ertapenem treatment resulted in significant overgrowth of VRE at day 1 in comparison to saline controls (P = 0.02); however, there was no significant difference between these groups at day 3, and there was no overall difference when both days were included in the analysis (Fig. 3B).
FIG. 3.
Effect of subcutaneous antibiotic treatment on the establishment of colonization with extended-spectrum β-lactamase-producing Klebsiella pneumoniae (A) and vancomycin-resistant enterococci (B) in mice exposed to the pathogens during treatment. Mice received antibiotic treatment once daily for 6 days and were administered 104 CFU of one of the pathogens by orogastric gavage on day 3 of antibiotic treatment (day 0 in the figure). If the pathogens were not detected in stool samples, the lower limit of detection (∼2 log10 CFU/g) was assigned.
In summary, we found that imipenem-cilastatin treatment did not disrupt the indigenous stool microflora of mice, whereas ertapenem treatment resulted in suppression of total anaerobes, Bacteroides spp., and facultative gram-negative bacilli and promoted overgrowth of indigenous enterococci. These findings are consistent with previous studies of the effects of these agents on the microflora of human volunteers and probably reflect the fact that imipenem-cilastatin is excreted in minimal concentrations in bile whereas ertapenem achieves higher concentrations (1, 6, 7, 9). The stool concentrations of these agents in mice were similar to levels previously reported in human volunteers (7, 8). As would be anticipated on the basis of the absence of an effect on the anaerobic microflora (4), imipenem-cilastatin did not promote establishment of intestinal colonization with VRE or K. pneumoniae. Ertapenem promoted transient overgrowth of VRE but not K. pneumoniae when the exposure occurred during treatment, but neither pathogen was promoted when exposure occurred 2 days after discontinuation of treatment. In contrast, piperacillin-tazobactam and ceftriaxone promoted marked overgrowth of both pathogens when exposure occurred 2 days after discontinuation of treatment. We hypothesize that this difference could possibly be due to relatively rapid recovery of the microflora of ertapenem-treated mice once treatment is discontinued. In addition, ertapenem caused less inhibition of total anaerobes and Bacteroides spp. than piperacillin-tazobactam did.
In addition to promoting colonization of pathogens by disturbing the indigenous microflora, antibiotics that are excreted into the intestinal tract may inhibit growth of pathogens during treatment (4). The biphasic effect of piperacillin-tazobactam on establishment of colonization by VRE and K. pneumoniae is consistent with the results of our previous studies and illustrates this principle (i.e., both pathogens were inhibited when exposure occurred during treatment, but colonization was promoted when exposure occurred after treatment but before recovery of the indigenous microflora) (4). Similarly, ceftriaxone presumably had sufficient inhibitory activity to inhibit colonization by the K. pneumoniae strain during treatment but not 2 days after completion of treatment. A recent study found that intestinal colonization with resistant members of the family Enterobacteriaceae increased significantly in patients receiving piperacillin-tazobactam or ceftriaxone plus metronidazole, but not in patients receiving ertapenem (2). Our data are consistent with these clinical observations. In contrast to piperacillin-tazobactam and ceftriaxone, ertapenem did not promote colonization with ESBL-producing K. pneumoniae when exposure occurred after completion of treatment. In addition, ertapenem did not promote colonization with the K. pneumoniae strain when exposure occurred during treatment, possibly due in part to inhibitory activity against the strain.
Acknowledgments
This work was supported by a grant from Merck Pharmaceuticals and an Advanced Research Career Development Award from the Department of Veterans Affairs to C.J.D.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Bergan, T., C. E. Nord, and S. B. Thorsteinsson. 1991. Effect of meropenem on the intestinal microflora. Eur. J. Clin. Microbiol. Infect. Dis. 10:524-527. [DOI] [PubMed] [Google Scholar]
- 2.Dinubile, M. J., I. Friedland, C. Y. Chan, M. R. Motyl, H. Giezek, M. Shivaprakash, R. A. Weinstein, and J. P. Quinn. 2005. Bowel colonization with resistant gram-negative bacilli after antimicrobial therapy of intra-abdominal infections: observations from two randomized comparative clinical trials of ertapenem therapy. Eur. J. Clin. Microbiol. Infect. Dis. 24:443-449. [DOI] [PubMed] [Google Scholar]
- 3.Donskey, C. J., J. A. Hanrahan, R. A. Hutton, and L. B. Rice. 1999. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J. Infect. Dis. 180:384-390. [DOI] [PubMed] [Google Scholar]
- 4.Donskey, C. J. 2004. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39:219-226. [DOI] [PubMed] [Google Scholar]
- 5.Hoyen, C. K., N. J. Pultz, D. L. Paterson, D. C. Aron, and C. J. Donskey. 2003. Effect of parenteral antibiotic administration on establishment of intestinal colonization in mice by Klebsiella pneumoniae strains producing extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 47:3610-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nord, C. E., L. Kager, A. Philipson, and G. Stiernstedt. 1985. Effect of imipenem/cilastatin on the colonic microflora. Rev. Infect. Dis. 7(Suppl. 3):S432-S434. [DOI] [PubMed] [Google Scholar]
- 7.Pletz, M. W. R., M. Rau, J. Bulitta, A. De Roux, O. Burkhardt, G. Kruse, M. Kurowski, C. E. Nord, and H. Lode. 2004. Ertapenem pharmacokinetics and impact on intestinal microflora, in comparison to those of ceftriaxone, after multiple dosing in male and female volunteers. Antimicrob. Agents Chemother. 48:3765-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microbiota. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 9.Wexler, H. M., and S. M. Finegold. 1985. Impact of imipenem/cilastatin therapy on normal fecal flora. Am. J. Med. 78(6A):41-46. [DOI] [PubMed] [Google Scholar]