Abstract
The combination of flucytosine and amphotericin B was tested against 10 flucytosine-resistant isolates of Cryptococcus neoformans by checkerboard, killing curves, and Etest. Although differences were observed depending on the technique used, antagonism was never observed. The synergistic interaction was related to the mechanism of flucytosine resistance of the isolates.
The combination of amphotericin B (AMB) and flucytosine (FC) is the treatment of choice for cryptococcal meningitis (21). The higher efficacy of the combination compared to that of AMB alone has been demonstrated in human immunodeficiency virus-negative (2) and human immunodeficiency virus-positive (6, 27) patients. For infections with FC-resistant isolates, the combination might not add any effect (15) or could be deleterious. However, data to support this are scarce (12).
As several mechanisms of FC resistance are possible, one may wonder if synergy is achievable for all FC-resistant isolates. We thus used a panel of FC-resistant Cryptococcus neoformans clinical isolates and various techniques to evaluate the influence of the FC resistance mechanism on the in vitro interaction between AMB and FC.
Ten FC-resistant isolates were studied. Susceptibility to fluorouracil (FU) and FC alone was tested twice. Checkerboard studies of AMB plus FC were performed twice based on the CLSI (formerly NCCLS) M27-A2 methodology (19). All experiments included quality control strains. Endpoint definition is a critical issue for assessing checkerboard interactions (25). Thus, the same endpoint (50% inhibition) was used for both drugs alone and in combination. Fractional inhibitory concentration indices (FICI) were calculated (9), and interactions were defined according to current recommendations (1). Although different approaches could be used to analyze in vitro antimicrobial combinations, we chose the FICI model instead of a surface response modeling (11) due to its simplicity and its wide use.
Killing curve testing and result interpretation were done as described elsewhere previously (28). FC was tested at 64 μg/ml and AMB was tested at 0.125 μg/ml alone and in combination. For three isolates with various FU susceptibilities (isolate 550, FU susceptible; isolate 98.0673, FU intermediate; and isolate 533, FU resistant), additional AMB concentrations and time points were tested. Killing experiments for these three isolates were performed twice. Preliminary results showed that isolate 550 was less susceptible to AMB than isolates 533 and 98.0673. Similar antifungal activity was found for AMB at 0.19 μg/ml against isolate 550 and for AMB at 0.12 μg/ml against isolates 533 and 98.0673.
Etest studies were performed once as described previously (16, 28). Additional tests performed as described previously by White et al. (29) gave similar results.
The 3.2-kb PCR-amplified fragment (primers FCY2-5′ [5′-CCGAAGTGCCTTTTATTGCAGAG] and FCY2-3′ [3′-GATGCCTATGAACAACGGTGGT]) containing the whole KNH99 FCY2 gene was cloned into the NotI site of a plasmid containing a hygromycin resistance cassette (13). The resulting plasmid, pNE344, was SspI digested and used to transform C. neoformans by biolistic DNA delivery (26). Transformants were selected on hygromycin agar medium.
Susceptibilities to FC and AMB alone and in combination by checkerboard experiments are presented in Table 1. Using the same breakpoints for FU as those for FC, four isolates were considered to be resistant to FU (MICs ≥ 32 μg/ml), and two were considered to be intermediate (MICs = 8 to 16 μg/ml). Sixty percent of the interactions were synergistic. Antagonism was not observed. There was no strict relationship between synergism and FU susceptibility.
TABLE 1.
MICs of single drugs and interaction of AMB with FC evaluated by the checkerboard method against 10 FC-resistant C. neoformans isolates, according to their susceptibility to FU
| Isolate | MIC (μg/ml) of drug alone
|
MICs (μg/ml) of drugs in combination
|
Lowest FICI in combination | Interactionb | |||
|---|---|---|---|---|---|---|---|
| FC | FUa | AMB | FC | AMB | |||
| 98.0176 | 64 | 0.008 | 0.5 | 0.5 | 0.12 | 0.258 | SYN |
| 18476 | 256 | 0.25 | 0.25 | 1 | 0.12 | 0.504 | IND |
| 285 | 32 | 4 | 1 | 0.25 | 0.5 | 0.508 | IND |
| 550 | 128 | 0.5 | 0.5 | 0.5 | 0.06 | 0.129 | SYN |
| 98.0673 | 64 | 16 | 1 | 16 | 0.25 | 0.500 | SYN |
| 99.1026 | 32 | 8 | 0.5 | 1 | 0.06 | 0.156 | SYN |
| 98.1121 | 256 | 256 | 0.5 | 0.25 | 0.5 | 1.001 | IND |
| 276 | 256 | 256 | 0.5 | 4 | 0.06 | 0.141 | SYN |
| 280 | 256 | 256 | 1 | 0.5 | 0.5 | 0.502 | IND |
| 533 | 256 | 256 | 0.5 | 0.5 | 0.12 | 0.252 | SYN |
FU was not tested in combination.
SYN, synergistic; IND, indifferent.
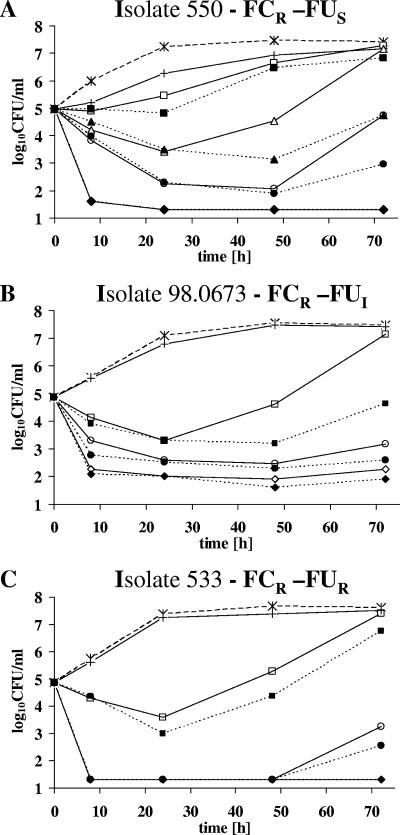
Figure 1A shows the killing curves for isolate 550. AMB at 0.19 μg/ml plus FC were significantly better than either drug alone and were synergistic. For isolate 98.0673, AMB at 0.125 μg/ml plus FC were significantly better than either drug alone (Fig. 1B), and the interaction was synergistic. For isolate 533, interactions were indifferent (Fig. 1C).
FIG. 1.
Time-kill study with AMB alone and in combination with FC on C. neoformans (A) isolate 550, (B) isolate 98.0673, and (C) isolate 533. Concentrations alone and in combination were 64 μg/ml for FC and 0.125 to 0.5 μg/ml for AMB. × (dashed line), control; + (solid line), FC; □ (solid line), AMB at 0.125 μg/ml; ▪ (dotted line), AMB at 0.125 μg/ml plus FC; ▵ (solid line), AMB 0.19 μg/ml; ▴ (dotted line), AMB at 0.19 μg/ml plus FC; ○ (solid line), AMB at 0.25 μg/ml; • (dotted line), AMB at 0.25 μg/ml plus FC; ⋄ (solid line), AMB at 0.5 μg/ml; ⧫ (dotted line), AMB at 0.5 μg/ml plus FC. The limit of detection was 1.3 log10 CFU per milliliter. FCR, FC resistant; FUS, FU susceptible; FUI, FU intermediate; FUR, FU resistant.
Table 2 shows time-kill results for AMB at 0.125 μg/ml and FC alone or in combination for nine isolates. The combination exhibited synergy for four isolates and indifference for five isolates. The combination of AMB and FC against isolate 99.1026 (FU intermediate) was more active than the most active single drug, although the decrease of 1.8 log10 CFU/ml did not comply with the synergy definition. Antagonism was never observed.
TABLE 2.
Log10 CFU per milliliter after 72 h of incubation of FC at 64 μg/ml and AMB at 0.125μg/ml alone and in combination against FC-resistant isolates of C. neoformans evaluated by time-kill methodologya
| Isolate | Suscepti- bility
|
Log10 CFU/ml
|
Interaction | ||||
|---|---|---|---|---|---|---|---|
| FC | FU | Control | FC | AMB | FC + AMB | ||
| 98.0176 | R | S | 7.8 | 7.4 | 6.5 | 3.7 | SYN |
| 18476 | R | S | 7.9 | 7.5 | 6.7 | 3.9 | SYN |
| 550 | R | S | 7.4 | 7.2 | 7.2b | 4.7b | SYN |
| 98.0673 | R | I | 7.5 | 7.4 | 7.2 | 4.6 | SYN |
| 99.1026 | R | I | 7.7 | 7.1 | 5.2 | 3.4 | IND |
| 98.1121 | R | R | 7.5 | 7.5 | 1.5 | 1.3 | IND |
| 276 | R | R | 7.3 | 7.3 | 7.3 | 7.2 | IND |
| 280 | R | R | 7.5 | 7.4 | 5.6 | 5.2 | IND |
| 533 | R | R | 7.6 | 7.5 | 7.4 | 6.5 | IND |
R, resistant; S, susceptible; I, intermediate; SYN, synergistic; IND, indifferent.
Data presented are for a concentration of AMB of 0.19 μg/ml.
The interaction of AMB plus FC was evaluated by Etest for 10 FC-resistant and 5 FC-susceptible isolates (data not shown). FC MICs for all FC-resistant strains were >32 μg/ml. AMB MICs ranged from 0.094 to 0.5 μg/ml. All interactions were indifferent.
The genome of C. neoformans serotype D (17) was blasted with the FCY2 gene sequence of Saccharomyces cerevisiae, encoding the purine-cytosine permease (10). The closest homologue gene was named FCY2. The deduced sequence of 523 amino acids shared 68.5% similarity with the sequence of the known S. cerevisiae cytosine permease. Two isolates (FU-susceptible isolate 550 and FU-resistant isolate 280) were transformed in four independent experiments, and 10 transformants were obtained for each isolate.
Transformants of isolate 550 showed a significant decrease in the FC MIC (0.25 to 4 μg/ml). Transformant MICs of isolate 280 did not change. Cytosine permease enzymatic activity was not specifically measured. For the FU-susceptible isolate (isolate 550), transformation led to FC susceptibility, indicating permease activity after transformation.
In vitro primary FC resistance has been reported in up to 7% of C. neoformans isolates (5, 7, 20). It is thus of clinical interest to know whether AMB plus FC are possibly antagonistic against FC-resistant isolates.
The in vitro combination of AMB and FC against FC-resistant C. neoformans isolates has been evaluated for a few strains (12, 18, 23-25), and synergy and antagonism (12) have been reported. In mice infected with an FC-resistant isolate, it has been shown that AMB plus FC were more effective than monotherapies (22).Whether the FC resistance mechanism influences the potential synergy remains unknown.
FC uptake by yeasts occurs via cytosine permeases. FC is then deaminated by cytosine deaminases into FU. Although it is not known if FU enters the cell via a specific transporter in basidiomycetes, this is the case for Saccharomycetales (14). In the case of a cytosine permease or cytosine deaminase defect, cells are FC resistant but remain FU susceptible. Isolates that are FC resistant due to enzymatic defects downstream of the cytosine deaminase are also FU resistant. FC-resistant isolates used in this study included strains of various FU susceptibilities (3, 4), allowing us to assess if the interaction of AMB with FC is influenced by different genetic backgrounds.
As no standardized method for the testing of antifungal combinations in vitro exists, three different techniques were used. Indifference was observed with the Etest, possibly due to difficulties in testing C. neoformans with RPMI medium (8). Synergy by checkerboard was observed for FU-susceptible strains but also for some FU-resistant strains. In contrast, time-kill curves showed synergy only for strains that were not resistant to FU. The present results suggest that the synergy of AMB plus FC depends on the FC resistance mechanism.
These results could strengthen the current recommendation for induction treatment of cryptococcal meningitis by demonstrating that adding FC to AMB cannot harm and can even be beneficial even if the C. neoformans isolate is FC resistant.
Nucleotide sequence accession number.
The FCY2 sequence was deposited in GenBank under accession number DQ190470.
Acknowledgments
We are grateful to J. E. Bennett, NIH, Bethesda, MD, for sharing his C. neoformans 5FC-resistant clinical isolates and to Thierry Noel, Faculté de Pharmacie—Université de Bordeaux 2 for critical reading of the manuscript and helpful discussions.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.American Society for Microbiology. 2006. Instructions to authors. Antimicrob. Agents Chemother. 50:1-21. [Google Scholar]
- 2.Bennett, J. E., W. E. Dismukes, R. J. Duma, G. Medoff, M. A. Sande, H. Gallis, J. Leonard, B. T. Fields, M. Bradshaw, H. Haywood, Z. A. McGee, T. R. Cate, C. G. Cobbs, J. F. Warner, and D. W. Alling. 1979. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N. Engl. J. Med. 301:126-131. [DOI] [PubMed] [Google Scholar]
- 3.Block, E. R., A. E. Jennings, and J. E. Bennett. 1973. 5-Fluorocytosine resistance in Cryptococcus neoformans. Antimicrob. Agents Chemother. 3:649-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block, E. R., A. E. Jennings, and J. E. Bennett. 1973. Variables influencing susceptibility testing of Cryptococcus neoformans to 5-fluorocytosine. Antimicrob. Agents Chemother. 4:392-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt, M. E., M. A. Pfaller, R. A. Hajjeh, R. J. Hamill, P. G. Pappas, A. L. Reingold, D. Rimland, and D. W. Warnock. 2001. Trends in antifungal drug susceptibility of Cryptococcus neoformans isolates in the United States: 1992 to 1994 and 1996 to 1998. Antimicrob. Agents Chemother. 45:3065-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwer, A. E., A. Rajanuwong, W. Chierakul, G. E. Griffin, R. A. Larsen, N. J. White, and T. S. Harrison. 2004. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 363:1764-1767. [DOI] [PubMed] [Google Scholar]
- 7.Cuenca-Estrella, M., T. M. Diaz-Guerra, E. Mellado, and J. L. Rodriguez-Tudela. 2001. Flucytosine primary resistance in Candida species and Cryptococcus neoformans. Eur. J. Clin. Microbiol. Infect. Dis. 20:276-279. [DOI] [PubMed] [Google Scholar]
- 8.Dannaoui, E., M. Abdul, M. Arpin, A. Michel-Nguyen, M. A. Piens, A. Favel, O. Lortholary, and F. Dromer. 2006. Results obtained with various antifungal susceptibility testing methods do not predict early clinical outcome in patients with cryptococcosis. Antimicrob. Agents Chemother. 50:2464-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliopoulos, G. M., and R. C. Moellering. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins Co., Baltimore, MD.
- 10.Ferreira, T., D. Brethes, B. Pinson, C. Napias, and J. Chevallier. 1997. Functional analysis of mutated purine-cytosine permease from Saccharomyces cerevisiae. A possible role of the hydrophilic segment 371-377 in the active carrier conformation. J. Biol. Chem. 272:9697-9702. [DOI] [PubMed] [Google Scholar]
- 11.Greco, W. R., G. Bravo, and J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 12.Hamilton, J. D., and D. M. Elliott. 1975. Combined activity of amphotericin B and 5-fluorocytosine against Cryptococcus neoformans in vitro and in vivo in mice. J. Infect. Dis. 131:129-137. [DOI] [PubMed] [Google Scholar]
- 13.Hua, J., J. D. Meyer, and J. K. Lodge. 2000. Development of positive selectable markers for the fungal pathogen Cryptococcus neoformans. Clin. Diagn. Lab. Immunol. 7:125-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz, J. E., F. Exinger, P. Erbs, and R. Jund. 1999. New insights into the pyrimidine salvage pathway of Saccharomyces cerevisiae: requirement of six genes for cytidine metabolism. Curr. Genet. 36:130-136. [DOI] [PubMed] [Google Scholar]
- 15.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, PA.
- 16.Lewis, R. E., D. J. Diekema, S. A. Messer, M. A. Pfaller, and M. E. Klepser. 2002. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 49:345-351. [DOI] [PubMed] [Google Scholar]
- 17.Loftus, B. J., E. Fung, P. Roncaglia, D. Rowley, P. Amedeo, D. Bruno, J. Vamathevan, M. Miranda, I. J. Anderson, J. A. Fraser, J. E. Allen, I. E. Bosdet, M. R. Brent, R. Chiu, T. L. Doering, M. J. Donlin, C. A. D'Souza, D. S. Fox, V. Grinberg, J. Fu, M. Fukushima, B. J. Haas, J. C. Huang, G. Janbon, S. J. Jones, H. L. Koo, M. I. Krzywinski, J. K. Kwon-Chung, K. B. Lengeler, R. Maiti, M. A. Marra, R. E. Marra, C. A. Mathewson, T. G. Mitchell, M. Pertea, F. R. Riggs, S. L. Salzberg, J. E. Schein, A. Shvartsbeyn, H. Shin, M. Shumway, C. A. Specht, B. B. Suh, A. Tenney, T. R. Utterback, B. L. Wickes, J. R. Wortman, N. H. Wye, J. W. Kronstad, J. K. Lodge, J. Heitman, R. W. Davis, C. M. Fraser, and R. W. Hyman. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medoff, G., M. Comfort, and G. S. Kobayashi. 1971. Synergistic action of amphotericin B and 5-fluorocytosine against yeast-like organisms. Proc. Soc. Exp. Biol. Med. 138:571-574. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 20.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, G. V. Doern, and D. J. Diekema. 2005. Global trends in the antifungal susceptibility of Cryptococcus neoformans (1990 to 2004). J. Clin. Microbiol. 43:2163-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, W. E. Dismukes, et al. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz, P., F. Dromer, O. Lortholary, and E. Dannaoui. 2006. Efficacy of amphotericin B in combination with flucytosine against flucytosine-susceptible or flucytosine-resistant isolates of Cryptococcus neoformans during disseminated murine cryptococcosis. Antimicrob. Agents Chemother. 50:113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz, P., F. Dromer, O. Lortholary, and E. Dannaoui. 2003. In vitro interaction of flucytosine with conventional and new antifungals against Cryptococcus neoformans clinical isolates. Antimicrob. Agents Chemother. 47:3361-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shadomy, S., G. Wagner, E. Espinel-Ingroff, and B. A. Davis. 1975. In vitro studies with combinations of 5-fluorocytosine and amphotericin B. Antimicrob. Agents Chemother. 8:117-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Te Dorsthorst, D. T., P. E. Verweij, J. Meletiadis, M. Bergervoet, N. C. Punt, J. F. Meis, and J. W. Mouton. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 46:2982-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Horst, C. M., M. S. Saag, G. A. Cloud, R. J. Hamill, J. R. Graybill, J. D. Sobel, P. C. Johnson, C. U. Tuazon, T. Kerkering, B. L. Moskovitz, W. G. Powderly, and W. E. Dismukes. 1997. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N. Engl. J. Med. 337:15-21. [DOI] [PubMed] [Google Scholar]
- 28.Vitale, R. G., J. Afeltra, and E. Dannaoui. 2005. Antifungal combinations. Methods Mol. Med. 118:143-152. [DOI] [PubMed] [Google Scholar]
- 29.White, R. L., D. S. Burgess, M. Manduru, and J. A. Bosso. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 40:1914-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]