Abstract
The in vitro activities of the lipopeptides palmitoyl (Pal)-Lys-Lys-NH2 and Pal-Lys-Lys against gram-positive cocci were investigated. Enterococci and streptococci demonstrated higher susceptibilities than staphylococci and Rhodococcus equi. A positive interaction was shown when the lipopeptides were combined with beta-lactams and vancomycin. These results suggest that lipopeptides are promising candidates for antimicrobial therapy for infections caused by gram-positive organisms.
The dramatic increase in antibiotic resistance by bacteria has prompted research to increase the arsenal of antimicrobial agents. Since the emergence of penicillinase-producing Staphylococcus aureus in the 1940s, gram-positive cocci have proved themselves adept at developing or acquiring mechanisms that confer resistance to all clinically available antibacterial classes. Several new drugs have emerged as possible therapeutic alternatives, such as oxazolidinones, lipopeptides, injectable streptogramins, ketolides, glycylcyclines, expanded-spectrum glycopeptides, and novel fluoroquinolones (5, 9, 17, 19). Among the compounds that are currently under investigation for their therapeutic potentials are antimicrobial peptides of the innate immune system and their synthetic derivatives. Widely distributed in nature, antimicrobial peptides are an essential defense component of invertebrates and vertebrates and control cell proliferation and invading pathogens (4, 10, 11).
Numerous studies of synthetic peptides have focused on designing analogue peptides with antimicrobial activities more potent than those of the natural peptides without damaging mammalian cells. Several attempts have been made to improve the antimicrobial activities of these peptides against bacterial cells while eliminating the cytotoxicity against mammalian cells, such as red blood cells, by changing the flexible-region chain length and changing the net charge and hydrophobicity and/or helicity (7, 16, 18). Short lipopeptides are monomeric in solution, while longer ones form oligomers; and this feature can potentiate the killing of microbes (3, 14). So far, different mechanisms of bactericidal activity have been identified. One of them, the most popular one, is mediated by the direct disruption of bacterial membrane electric potentials, which results in less of a likelihood for the development of cross-resistance. However, other reports have provided evidence that some lipopeptides are capable of killing bacteria via interference with synthesis of the cell wall (13, 14).
The aim of the present study was to evaluate the in vitro activities of two lipopeptides and their bactericidal effects for a large number of gram-positive cocci, included methicillin-resistant (MR) staphylococci and vancomycin-resistant (VR) enterococci, as well as to investigate their in vitro interactions with six clinically used antibiotics.
Organisms.
The quality control strains used in this study included methicillin-susceptible (MS) S. aureus ATCC 29213, MR S. aureus ATCC 43300, vancomycin-susceptible (VS) Enterococcus faecalis ATCC 29212, VR E. faecalis ATCC 51299, Rhodococcus equi ATCC 6939, and Streptococcus pyogenes ATCC 19615. Thirty nosocomial isolates of each species except VR E. faecalis and R. equi were tested. Fourteen strains of VR E. faecalis and 12 strains of R. equi were tested. The isolates were obtained from distinct patients from central Italy. The patients had unrelated sources of infection and were admitted to the Hospital Umberto I, Ancona, Italy, from January 2000 to December 2005.
Antimicrobial agents.
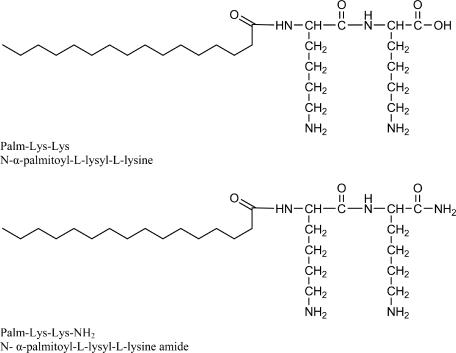
N-terminal palmitoyl (Pal)-lipidated peptides Pal-Lys-Lys-NH2 and Pal-Lys-Lys (Fig. 1) were synthesized manually by the solid-phase methodology by use of the 9-fluorenylmethoxy carbonyl-tert-butylene strategy (8). The crude lipopeptides were purified by solid-phase extraction by a previously described protocol (12).
FIG. 1.
Chemical structures of experimental compounds.
In addition, the following control agents were tested: amoxicillin, vancomycin, and doxycycline (Sigma-Aldrich, Milan, Italy); imipenem (Merck Sharp & Dohme, Milan, Italy); clarithromycin (Abbott, Rome, Italy); and linezolid (Pharmacia & Upjohn, Kalamazoo, MI).
Susceptibility testing.
MIC and minimal bactericidal concentration (MBC) determinations were performed by the procedures outlined by the Clinical and Laboratory Standards Institute (formerly NCCLS) (15). Experiments were performed in triplicate.
Bacterial killing assay.
ATCC control strains were used to study the in vitro killing effects of the lipopeptides. Aliquots of exponentially growing bacteria were resuspended in fresh Mueller-Hinton (MH) broth at approximately 107 cells/ml and were exposed to the lipopeptides at 2× MIC for 0, 5, 10, 15, 20, 25, 30, 40, 50, and 60 min at 37°C. After these times the samples were serially diluted in 10 mM sodium HEPES buffer (pH 7.2) to minimize the carryover effect and were plated onto MH agar plates to obtain viable colonies.
Synergy studies.
In interaction studies, six strains of MS S. aureus, six strains of VS E. faecalis, and six strains of S. pyogenes were used to test the antibiotic combinations by a checkerboard titration method by using 96-well polypropylene microtiter plates. The fractional inhibitory concentration (FIC) index for combinations of two antimicrobials was calculated according to the following equation: FIC index = FICA + FICB = (A/MICA) + (B/MICB), where A and B are the MIC of drug A and the MIC of drug B in the combination, respectively; MICA and MICB are the MIC of drug A and the MIC of drug B alone, respectively; and FICA and FICB are the FIC of drug A and the FIC of drug B, respectively. The FIC indices were interpreted as follows: <0.5, synergy; 0.5 to 4.0, indifferent; and >4.0, antagonism (6).
Hemolysis of hRBCs.
Fresh human red blood cells (hRBCs) with EDTA anticoagulant were rinsed three times with phosphate-buffered saline (PBS; 35 mM phosphate buffer, 0.15 M NaCl, pH 7.3) by centrifugation at 800 × g for 10 min and were resuspended in PBS. The lipopeptides dissolved in PBS were then added to 50 μl of a solution of the stock hRBCs in PBS to reach a final volume of 100 μl (final erythrocyte concentration, 4% [vol/vol]). The resulting suspension was incubated with agitation for 60 min at 37°C. The samples were then centrifuged at 800 × g for 10 min. The release of hemoglobin was monitored by measuring the absorbance of the supernatant at 540 nm. Controls for 0% hemolysis (blank) and 100% hemolysis consisted of hRBCs suspended in PBS and 1% Triton, respectively.
All isolates were inhibited by lipopeptides at concentrations of 1 to 16 mg/liter. For the control strains S. aureus ATCC 29213, S. aureus ATCC 43300, E. faecalis ATCC 29212, E. faecalis ATCC 51299, R. equi ATCC 6939, and S. pyogenes ATCC 19615, Pal-Lys-Lys-NH2 showed MICs of 8 mg/liter, 8 mg/liter, 4 mg/liter, 4 mg/liter, 16 mg/liter, and 2 mg/liter, respectively, and MBCs of 16 mg/liter, 16 mg/liter, 8 mg/liter, 16 mg/liter, 32 mg/liter, and 4 mg/liter, respectively. Pal-Lys-Lys showed similar in vitro activities against all strains, with the exception of R. equi, for which the MIC and the MBC were 8 and 16 mg/liter, respectively. Overall, high rates of resistance to the clinically used antibiotics, with the exception of linezolid, were demonstrated for the multiresistant strains. The results are summarized in Table 1.
TABLE 1.
MICs and MBCs of lipopeptides and other clinically used antibiotics for clinical isolates
| Strain (no. of isolates) and agent | MIC (mg/liter)
|
MBC (mg/liter)
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |
| MR S. aureus (30) | ||||||
| Pal-Lys-Lys-NH2 | 2-16 | 4 | 8 | 2-32 | 8 | 16 |
| Pal-Lys-Lys | 2-32 | 4 | 8 | 2-32 | 8 | 16 |
| Imipenem | 1-128 | 16 | 128 | 8-128 | 64 | 256 |
| Doxycycline | 0.50-16 | 4 | 16 | 4-64 | 8 | 32 |
| Clarithromycin | 1-32 | 4 | 32 | 4-128 | 32 | 128 |
| Linezolid | 0.25-2 | 0.5 | 1 | 0.50-4 | 0.5 | 2 |
| Vancomycin | 0.12-4 | 0.5 | 2 | 0.50-4 | 0.5 | 2 |
| Amoxicillin | 4-128 | 32 | 128 | 8-128 | 64 | 256 |
| MS S. aureus (30) | ||||||
| Pal-Lys-Lys-NH2 | 2-16 | 4 | 8 | 2-16 | 8 | 16 |
| Pal-Lys-Lys | 2-16 | 4 | 8 | 2-32 | 8 | 16 |
| Imipenem | 0.25-4 | 0.5 | 2 | 0.5-32 | 4 | 8 |
| Doxycycline | 0.50-8 | 2 | 8 | 2-32 | 8 | 32 |
| Clarithromycin | 0.50-16 | 4 | 8 | 2-64 | 16 | 64 |
| Linezolid | 0.12-2 | 0.5 | 2 | 0.50-2 | 1 | 2 |
| Vancomycin | 0.12-2 | 0.5 | 1 | 0.50-2 | 0.5 | 2 |
| Amoxicillin | 0.25-8 | 1 | 4 | 0.50-32 | 8 | 16 |
| VS Enterococcus faecalis (30) | ||||||
| Pal-Lys-Lys-NH2 | 2-16 | 2 | 4 | 2-32 | 4 | 8 |
| Pal-Lys-Lys | 2-16 | 2 | 4 | 2-32 | 4 | 8 |
| Imipenem | 1-64 | 4 | 16 | 4-256 | 16 | 64 |
| Doxycycline | 1-32 | 4 | 32 | 4-128 | 16 | 64 |
| Clarithromycin | 4-128 | 8 | 32 | 8-256 | 32 | 128 |
| Linezolid | 0.50-4 | 1 | 2 | 0.5-4 | 2 | 4 |
| Vancomycin | 0.25-4 | 1 | 2 | 1-4 | 2 | 4 |
| Amoxicillin | 1-32 | 4 | 16 | 4-64 | 16 | 64 |
| VR Enterococcus faecalis (14) | ||||||
| Pal-Lys-Lys-NH2 | 2-16 | 2 | 4 | 2-32 | 4 | 16 |
| Pal-Lys-Lys | 2-32 | 2 | 4 | 2-64 | 4 | 16 |
| Imipenem | 4-128 | 16 | 64 | 8-256 | 64 | 128 |
| Doxycycline | 2-64 | 16 | 64 | 8-64 | 16 | 64 |
| Clarithromycin | 8-128 | 16 | 64 | 16-256 | 32 | 256 |
| Linezolid | 0.50-4 | 1 | 2 | 1-4 | 4 | 4 |
| Vancomycin | 32-128 | 32 | 64 | 64-256 | 64 | 256 |
| Amoxicillin | 4-128 | 16 | 64 | 8-256 | 64 | 128 |
| Streptococcus pyogenes (30) | ||||||
| Pal-Lys-Lys-NH2 | 1-8 | 1 | 2 | 2-16 | 2 | 4 |
| Pal-Lys-Lys | 1-8 | 1 | 2 | 2-16 | 2 | 4 |
| Imipenem | 0.25-2 | 0.50 | 1 | 0.5-4 | 1 | 4 |
| Doxycycline | 0.50-8 | 2 | 8 | 1-32 | 8 | 16 |
| Clarithromycin | 0.50-8 | 4 | 8 | 1-64 | 8 | 32 |
| Linezolid | 0.12-2 | 0.5 | 1 | 0.50-2 | 1 | 2 |
| Vancomycin | 0.25-2 | 0.5 | 1 | 0.50-2 | 1 | 2 |
| Amoxicillin | 0.06-2 | 0.25 | 2 | 0.5-16 | 4 | 8 |
| Rhodococcus equi (12) | ||||||
| Pal-Lys-Lys-NH2 | 2-16 | 4 | 16 | 4-32 | 8 | 32 |
| Pal-Lys-Lys | 2-16 | 2 | 16 | 2-16 | 4 | 16 |
| Imipenem | 0.25-2 | 0.25 | 1 | 2-16 | 8 | 16 |
| Doxycycline | 0.50-4 | 1 | 2 | 8-256 | 32 | 128 |
| Clarithromycin | 0.50-2 | 0.50 | 2 | 16-128 | 32 | 64 |
| Linezolid | 0.25-2 | 0.50 | 1 | 4-32 | 8 | 16 |
| Vancomycin | 0.25-2 | 0.50 | 1 | 2-16 | 8 | 16 |
| Amoxicillin | 0.25-4 | 1 | 2 | 2-32 | 8 | 32 |
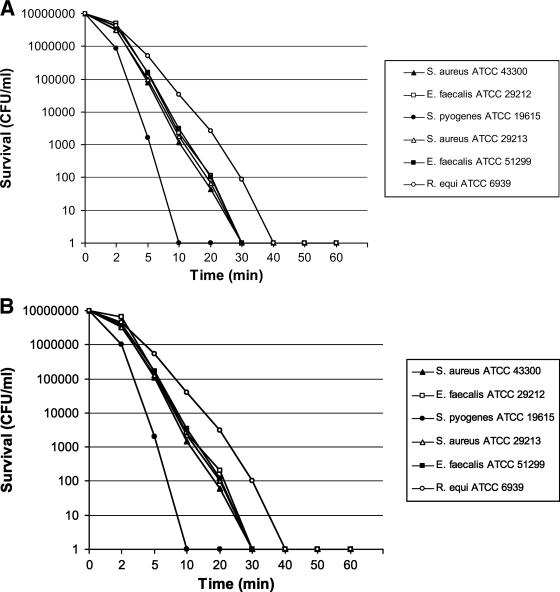
Killing by both lipopeptides was shown to be very rapid: the activities against the staphylococci and the enterococci were complete after 30 min of exposure at a concentration of 2× MIC, against R. equi they were complete after 40 min at the same concentration, and finally, against S. pyogenes they were complete after 10 min at the same concentration (Fig. 2).
FIG. 2.
Time-kill kinetics of lipopeptides against the quality control bacterial strains. (A) Pal-Lys-Lys-NH2; (B) Pal-Lys-Lys.
In the combination studies modest synergy against all strains of bacteria tested was observed when Pal-Lys-Lys-NH2 and Pal-Lys-Lys were combined with amoxicillin, imipenem, and vancomycin. In contrast, the other experiments with clarithromycin, doxycycline, and linezolid gave FIC indices between 0.917 and 1.833 (Table 2).
TABLE 2.
Results of studies of interaction between lipopeptides and other drugsc
| Peptide and combination agent | Mean (range) FIC index
|
||||||
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Enterococcus faecalis | Rhodococcus equi | Streptococcus pyogenes | ||||
| Pal-Lys-Lys-NH2 | |||||||
| Imipenem | 0.46 (0.31-0.75)a | 0.31 (0.19-0.50)a | 0.31 (0.19-0.50)a | 0.46 (0.31-0.75)a | |||
| Doxycycline | 1.17 (0.75-2.00)b | 1.83 (1.50-2.00)b | 1.83 (1.50-2.00)b | 1.46 (1.00-2.00)b | |||
| Clarithromycin | 1.50 (1.00-2.00)b | 1.17 (0.75-2.00)b | 1.46 (1.00-2.00)b | 1.46 (1.00-2.00)b | |||
| Linezolid | 1.50 (1.00-2.00)b | 1.46 (1.25-2.00)b | 1.17 (0.75-2.00)b | 1.50 (1.000-2.00)b | |||
| Vancomycin | 0.46 (0.31-0.75)a | 0.31 (0.19-0.50)a | 0.39 (0.31-0.50)a | 0.39 (0.31-0.50)a | |||
| Amoxicillin | 0.31 (0.19-0.50)a | 0.39 (0.31-0.50)a | 0.31 (0.19-0.50)a | 0.39 (0.31-0.50)a | |||
| Pal-Lys-Lys |
|
||||||
| Imipenem | 0.31 (0.18-0.50)a | 0.31 (0.19-0.50)a | 0.39 (0.31-0.50)a | 0.31 (0.19-0.50)a | |||
| Doxycycline | 1.50 (1.00-2.00)b | 1.83 (1.50-2.00)b | 1.17 (1.00-1.50)b | 1.50 (1.00-2.00)b | |||
| Clarithromycin | 1.17 (0.75-2.00)b | 1.83 (1.50-2.00)b | 1.46 (1.00-2.00)b | 1.46 (1.00-2.00)b | |||
| Linezolid | 0.91 (0.750-1.25)b | 1.17 (1.00-1.50)b | 0.92 (0.75-1.25)b | 1.29 (0.75-2.00)b | |||
| Vancomycin | 0.46 (0.31-0.75)a | 0.39 (0.31-0.50)a | 0.46 (0.31-0.75)a | 0.46 (0.31-0.75)a | |||
| Amoxicillin | 0.31 (0.19-0.50)a | 0.39 (0.31-0.50)a | 0.31 (0.19-0.50)a | 0.31 (0.19-0.50)a | |||
Synergy.
Indifference.
The ranges of concentrations tested were 0.125 to 64 mg/liter for lipopeptides and 0.25 to 256 mg/liter for the other antimicrobial agents. Six strains for each genus were tested. Antagonism was absent for all combinations tested.
Finally, our data revealed that both lipopeptides showed low levels of hemolytic activity, despite their high levels of activity against gram-positive cocci. In fact, hemolytic activity was observed at concentrations higher than the MICs (50 mg/liter for Pal-Lys-Lys and 20 mg/liter for Pal-Lys-Lys-NH2).
In the present study, in vitro experiments with Pal-Lys-Lys-NH2 and Pal-Lys-Lys were performed to determine their bactericidal activities and to determine whether synergism, antagonism, or indifference would be the predominant response when these peptides were tested in combination with other antibiotics clinically used against gram-positive cocci.
Overall, our data showed that enterococci and streptococci were highly susceptible to both lipopeptides, while staphylococci and R. equi showed lower levels of susceptibility. Interestingly, they were demonstrated to be equally active against both susceptible and multiresistant clinical isolates. Time-kill studies showed a rapid bactericidal effect, even if the inactivation of the staphylococci appears to be slower than that observed for the other gram-positive cocci.
Many studies with membrane-active peptides have demonstrated the important role of hydrophobicity and structure for their biological function. Recent studies have shown that the attachment of palmitic acid to the N terminus of positively charged short peptides, whose activities against microorganisms are inert, endowed them with a broad spectrum of potent antimicrobial activities and with low levels of hemolytic activity against a highly diluted solution of erythrocytes (3, 13, 14). Furthermore, previous studies showed that oligomer formation seems to be an important requirement for antimicrobial activity because many pathogens, including bacteria, are surrounded by the plasma membrane, which is an external barrier which mainly contains polysaccharide compounds. Therefore, to reach the cytoplasmic phospholipid membranes (a possible target of the lipopeptides), they need to traverse the microorganism cell wall. Similar to other antimicrobial peptides, the main target of the lipopeptides is the biological membrane. All the lipopeptides possessed high cell-permeant activities, which correlated with the hydrophobic ties of the peptides (1-3, 13, 14). The extent of their membrane-permeant activities correlated with their biological function, suggesting that the plasma membrane was one of their major targets. It is also worth noticing that amidation of the C terminus of the lipopeptide results in higher hydrophobicities for these substances. There was no difference in the MICs and the MBCs between amidated and nonamidated compounds (except for those for R. equi), whereas the total MIC and MBC ranges were slightly lower for Pal-Lys-Lys-NH2 than for Pal-Lys-Lys. This finding suggests that the additional hydrophobicity on the C terminus of the lipopeptides does not significantly influence the antibacterial activities of the peptides. Similar results were achieved for the nonamidated compound, which was also characterized by lower hemolytic properties.
The basic interesting information provided by this study suggests that these new lipopeptides can be used as adjuvants and potential candidates for the future design of drugs with activities against infections caused by gram-positive organisms.
Acknowledgments
This work was supported by the Italian Ministry of Education, University and Research (PRIN 2003).
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Avrahami, D., and Y. Shai. 2002. Conjugation of a magainin analogue with lipophilic acids controls hydrophobicity, solution assembly, and cell selectivity. Biochemistry 41:2254-2263. [DOI] [PubMed] [Google Scholar]
- 2.Avrahami, D., and Y. Shai. 2003. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to d,l-amino acid-containing antimicrobial peptides: a plausible mode of action. Biochemistry 42:14946-14956. [DOI] [PubMed] [Google Scholar]
- 3.Avrahami, D., and Y. Shai. 2004. A new group of antifungal and antibacterial lipopeptides derived from non-membrane active peptides conjugated to palmitic acid. J. Biol. Chem. 279:12277-12285. [DOI] [PubMed] [Google Scholar]
- 4.Cannon, M. 1987. A family of wound healers. Nature 328:478. [DOI] [PubMed] [Google Scholar]
- 5.Cormican, M. G., and R. N. Jones. 1996. Emerging resistance to antimicrobial agents in gram-positive bacteria. Enterococci, staphylococci and nonpneumococcal streptococci. Drugs 51:S6-S12. [DOI] [PubMed] [Google Scholar]
- 6.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-393. In V. Lorian (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins Co., Baltimore, MD.
- 7.Epand, R. M., and H. J. Vogel. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11-28. [DOI] [PubMed] [Google Scholar]
- 8.Fields, G. B., and R. L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161-214. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez, A., T. Bischoff, S. Tallent, G. Sheke, B. Ostrowski, M. B. Edmond, and R. P. Wenzel. 2003. Antibiotic resistance in the community. J. Hosp. Infect. 55:156-157. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, R. E. W. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 11.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamysz, W., B. Kochańska, A. Kędzia, J. Ochocińska, Z. Maćkiewicz, and G. Kupryszewski. 2002. Statherin SV2 and its analogue. Synthesis and evaluation of antimicrobial activity. Pol. J. Chem. 76:801-806. [Google Scholar]
- 13.Makovitzki, A., and Y. Shai. 2005. pH-dependent antifungal lipopeptides and their plausible mode of action. Biochemistry 44:9775-9784. [DOI] [PubMed] [Google Scholar]
- 14.Malina, A., and Y. Shai. 2005. Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide. Biochem. J. 390:695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 16.Putsep, K., C. I. Branden, H. G. Boman, and S. Normark. 1999. Antibacterial peptide from H. pylori. Nature 398:671-672. [DOI] [PubMed] [Google Scholar]
- 17.Raad, I., A., Alrahwan, and K. Rolston. 1998. Staphylococcus epidermidis: emerging resistance and need for alternative agents. Clin. Infect. Dis. 26:1182-1187. [DOI] [PubMed] [Google Scholar]
- 18.Steiner, H., D. Hultmark, A. Engstrom, H. Bennich, and H. G. Boman. 1981. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292:246-248.7019715 [Google Scholar]
- 19.Woodford, N. 2005. Biological counterstrike: antibiotic resistance mechanism of gram-positive cocci. Clin. Microbiol. Infect. 11:S2-S21. [DOI] [PubMed] [Google Scholar]