Abstract
We show that quinolone resistance in Helicobacter pylori has reached an alarming level in Germany. Our data suggest that the use of quinolones requires prior antimicrobial susceptibility testing, especially for isolates from patients who have already undergone previous unsuccessful eradication treatments, and also underline the further need for surveillance studies to monitor antibiotic resistance in H. pylori.
Helicobacter pylori infection causes gastritis and can result in complications such as peptic ulcer diseases, mucosa-associated lymphoid tissue lymphoma, or gastric cancer (22). In Germany, antibiotic chemotherapy that includes amoxicillin, clarithromycin, and a proton pump inhibitor is recommended for the eradication of these bacteria in infected individuals (14). After treatment failures, quinolone-based triple therapies have been shown to be highly effective and therefore have been proposed as rescue regimens (4, 20).
The aim of this study was to estimate the prevalence of quinolone resistance in H. pylori by testing the antimicrobial susceptibilities of clinical isolates obtained between 2001 and 2005. We analyzed H. pylori isolates from patients who had already been treated (n = 805; 86%) or who had not been treated (n = 126; 14%). Susceptibilities to ciprofloxacin, metronidazole, and clarithromycin were determined by the Etest method, according to the manufacturer's instructions and a modified protocol, as described previously (9). Isolates were classified as resistant to ciprofloxacin and clarithromycin when the MIC was >1 mg/liter and were classified as resistant to metronidazole when the MIC was >8 mg/liter (9, 12).
Due to the predominance of previously treated patients, the overall rates of resistance to metronidazole (Mtzr; 67.8%), clarithromycin (Clar; 62.9%), and to both antimicrobials (Mtzr Clar; 39.8%) were expectedly high. We also observed a large number (n = 150; 16.1%) of ciprofloxacin-resistant isolates; detailed analysis showed that the majority of these isolates (n = 95; 10.2%) were also resistant to metronidazole and clarithromycin, whereas only a minority (n = 17; 1.8%) were exclusively resistant to ciprofloxacin.
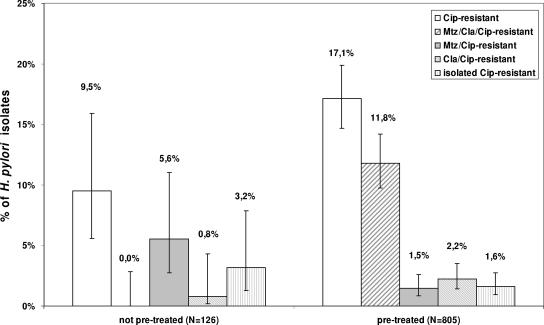
Previous unsuccessful eradication trials appear to be an important risk factor for the development of overall quinolone resistance and triple-drug resistance, since those patterns of resistance were more common among isolates from previously treated patients (Cipr, 17.1%; Mtzr Clar Cipr, 11.8%) than among those derived from previously untreated individuals (Cipr, 9.5%; Mtzr Clar Cipr, 0.0%). A chi-square test confirmed these differences to be significant at an error level of 5% (Fig. 1).
FIG. 1.
Influence of previous eradication trials on ciprofloxacin resistance in H. pylori. The resistance patterns of clinical isolates from patients who had not been previously treated (n = 126) were compared to those of isolates who had previously been treated (n = 805). The 95% confidence intervals were constructed by a Bayesian approach by using a noninformative prior function. Abbreviations: Cip, ciprofloxacin; Mtz; metronidazole; Cla, clarithromycin.
Next, we also analyzed the average MICs of ciprofloxacin and compared the annual differences in the proportions of quinolone-resistant isolates.
The geometric mean of the ciprofloxacin MICs showed a minimal decrease from 0.15 mg/liter in 2001 to 0.14 mg/liter in 2003, followed by an abrupt rise to 0.27 mg/liter in 2004 and a final increase to 0.38 mg/liter in 2005. Switching of the regression of the logarithms of the MICs with the year as an explanatory variable confirmed this finding of a structural break.
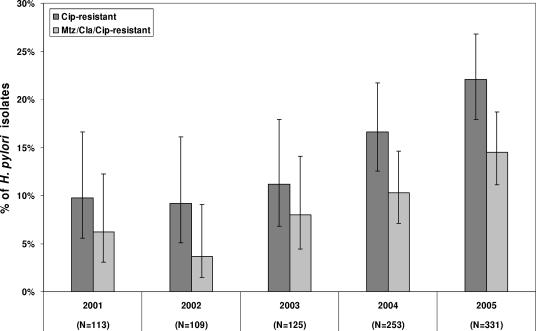
As a consequence, the proportions of Cipr H. pylori isolates rose from 11.2% in 2003 to 16.6% in 2004 and further increased to 22.1% in 2005. The rate of Mtzr Clar Cipr isolates more than doubled between 2001 (6.2%) and 2005 (13.7%) (Fig. 2). Logistic regression analysis showed a significant positive trend for the proportions of Cipr isolates and Mtzr Clar Cipr isolates. Similar high rates of quinolone resistance were reported from Korea (12) and Italy (2), whereas rates below 10% were reported from France (23), The Netherlands (6), Spain (24), and Portugal (3).
FIG. 2.
Proportions of Cipr isolates and Mtzr Clar Cipr isolates for H. pylori over the last 5-year period. The 95% confidence intervals were constructed by a Bayesian approach by using a noninformative prior function. Abbreviations: Cip, ciprofloxacin; Mtz; metronidazole; Cla, clarithromycin.
Quinolones exert their antimicrobial effects by affecting the A subunit of the DNA gyrase, the only known target enzyme in H. pylori (17, 25). Mutations in the quinolone resistance-determining region of the gyrA gene, which mainly involve amino acid substitutions at positions 87 and 91, confer resistance to these antibiotics (12, 19, 23). Thus, we amplified and sequenced 569-bp gyrA fragments (GenBank accession no. AE000583) of a selection of 60 resistant isolates by using primers 32fw (5′-ATATTGTAGAAGTGGGGATTGAT-3′) and 600rv (5′-ATGCACTAAAGCGTCTATGATT-3′) (Hermann GmbH, Freiburg, Germany) and by applying modified conditions, as described elsewhere (7).
All resistant strains harbored known gyrA mutations, as described earlier by Moore et al. (17) (Table 1). The natural transformation of ciprofloxacin-susceptible H. pylori strain 26695 by using the gyrA PCR products of the various resistant isolates was performed by a modified protocol described previously (8, 26) and confirmed the critical role of gyrA mutations in quinolone resistance in H. pylori (data not shown). There was no apparent correlation between the MICs for the resistant isolates and their underlying gyrA genotype. Other known resistance mechanisms, such as efflux pumps, have been discussed; but their presence in H. pylori seems to be rather unlikely (1, 23).
TABLE 1.
Ciprofloxacin-resistant H. pylori clinical isolates and in vitro mutants and corresponding ciprofloxacin MICs and gyrA genotypes
| Isolate type and no. of strains | MICa (mg/liter) | gyrA mutation/amino acid exchange |
|---|---|---|
| Clinical isolates | ||
| 26 | 4-32 | C261A/Asn87→Lys |
| 4 | 4-32 | C261G/Asn87→Lys |
| 14 | 16-32 | A272G/Asp91→Gly |
| 12 | 16-32 | G271A/Asp91→Asn |
| 4 | 8-32 | G271T/Asp91→Tyr |
| In vitro mutantsb | ||
| 3 | 32 | A272G/Asp91→Gly |
| 1 | 32 | C261A/Asn87→Lys |
MICs were determined by the Etest method.
Single-step in vitro mutants were generated by culturing H. pylori strain 26695 in the presence of CIP-5 disks.
In agreement with the results of others (23), we obtained in vitro ciprofloxacin-resistant mutants after a single passage by culturing the susceptible strain 26695 in the presence of CIP-5 Sensi-Discs (5 μg ciprofloxacin) (Becton Dickinson, Germany). Four randomly picked ciprofloxacin-resistant mutants showed MICs >32 mg/liter and harbored resistance-mediating gyrA mutations similar to those that we detected in the resistant isolates (Table 1). According to a protocol described elsewhere, we determined a rate for the development of ciprofloxacin resistance mutations of about 10−8 per cell division, thereby confirming previously published data (25).
We hypothesize that the development of ciprofloxacin resistance might be a rapid one-step event that is likely to occur more frequently due to the increasing use of fluoroquinolones (23). In Germany, the rate of prescription of quinolones has continuously increased for outpatients, and it is well known that fluoroquinolone resistance results from the previous use of these antibiotics (5, 10, 11).
However, only a minority of the previously treated patients (n = 32) harboring Cipr H. pylori strains whom we studied received quinolones in previous treatment trials, thereby attributing a pivotal role to the consumption of quinolones for the treatment of other infections (e.g., urogenital infections or respiratory diseases).
Since all patients have undergone only a single microbiological investigation, it was not possible to evaluate if resistance might have arisen in previously sensitive strains during one or more eradication trials.
It is still controversial whether antimicrobial susceptibility testing should be performed before the implementation of H. pylori eradication therapy. Pretreatment susceptibility testing was shown to be cost saving and associated with higher eradication rates (21); other data question the use of prior susceptibility testing before the use of first- and second-line therapies (16, 18). These conflicting results are probably due to the different study settings and the different patients evaluated. For Germany, we have shown that antimicrobial susceptibility testing should be implemented after the first treatment failure due to increasing double resistance to metronidazole and clarithromycin, thereby allowing antibiogram-adapted eradication therapies (13).
In conclusion, the data presented here show that resistance to quinolones and triple resistance to metronidazole, clarithromycin, and ciprofloxacin in H. pylori have reached alarming levels in Germany. Our results underline the prerequisite of in vitro susceptibility testing when the application of quinolones for the eradication of H. pylori is considered, particularly in patients previously treated unsuccessfully (2, 15).
Furthermore, more studies on the development of resistance due to quinolone-based eradication regimens or the use of quinolones for the treatment of unrelated diseases are needed.
Acknowledgments
We thank Christine Ganter, Beate Hobmaier, Christine Melzl, and Marianne Vetter-Knoll for excellent technical assistance and Christian Bogdan for critical reading of the manuscript.
This work was supported by the Robert-Koch-Institut by grant 1369-239 of the German Ministry of Health to M. Kist.
Footnotes
Published ahead of print on 16 October 2006.
REFERENCES
- 1.Bina, J. E., R. A. Alm, M. Uria-Nickelsen, S. R. Thomas, T. J. Trust, and R. E. W. Hancock. 2000. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob. Agents Chemother. 44:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branca, G., T. Spanu, G. Cammarota, A. M. Schito, A. Gasbarrini, G. B. Gasbarrini, and G. Fadda. 2004. High levels of dual resistance to clarithromycin and metronidazole and in vitro activity of levofloxacin against Helicobacter pylori isolates from patients after failure of therapy. Int. J. Antimicrob. Agents 24:433-438. [DOI] [PubMed] [Google Scholar]
- 3.Cabrita, J., M. Oleastro, R. Matos, A. Manhente, J. Cabral, R. Barros, A. I. Lopes, P. Ramalho, B. C. Neves, and A. S. Gurreiro. 2000. Features and trends in Helicobacter pylori antibiotic resistance in Lisbon area, Portugal (1990-1999). J. Antimicrob. Chemother. 46:1029-1031. [DOI] [PubMed] [Google Scholar]
- 4.Cammarota, G., R. Cianci, O. Cannizzaro, L. Cuoco, G. Pirozzi, A. Gasbarrini, A. Armuzzi, M. A. Zocco, L. Santarelli, F. Arancio, and G. Gasbarrini. 2000. Efficacy of two one-week rabeprazol/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 14:1339-1343. [DOI] [PubMed] [Google Scholar]
- 5.Carratala, J., A. Fernandez-Sevilla, F. Tubau, F. Manresa, and F. Gudiol. 2004. Emergence of fluoroquinolone-resistant Escherichia coli in fecal flora of cancer patients receiving norfloxacin prophylaxis. Antimicrob. Agents Chemother. 40:503-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debets-Ossenkopp, Y. J., A. J. Herscheid, R. G. J. Pot, E. J. Kuipers, J. G. Kusters, and C. M. Vandenbroucke-Grauls. 1999. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxycillin, tetracycline and trovafloxacin in The Netherlands. J. Antimicrob. Chemother. 43:511-515. [DOI] [PubMed] [Google Scholar]
- 7.Fujimura, S., S. Kato, K. Iinuma, and A. Watanabe. 2004. In vitro activity of fluoroquinolone and the gyrA gene mutation in Helicobacter pylori strains isolated from children. J. Med. Microbiol. 53:1019-1023. [DOI] [PubMed] [Google Scholar]
- 8.Gerrits, M. M., M. Berning, A. H. M. Van Vliet, E. J. Kuipers, and J. G. Kusters. 2003. Effects of 16S rRNA gene mutations on tetracycline resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 47:2984-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glupczynski, Y., F. Mégraud, M. Lopez-Brea, and L. P. Andersen. 2001. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 20:820-823. [DOI] [PubMed] [Google Scholar]
- 10.Kern, W. V. 2004. Antibiotika und Chemotherapeutika, p. 257-280. In U. Schwabe and D. Paffrath (ed.), Arzneiverordnungs-Report 2004. Springer, Heidelberg, Germany.
- 11.Kern, W. V., M. Steib-Bauert, K. de With, S. Reuter, H. Bertz, U. Frank, and H. von Baum. 2005. Fluoroquinolone consumption and resistance in haematology-oncology patients: ecological analysis in two university hospitals. J. Antimicrob. Chemother. 55:57-60. [DOI] [PubMed] [Google Scholar]
- 12.Kim, J. M., J. S. Kim, N. Kim, H. C. Jung, and I. S. Song. 2005. Distribution of fluoroquinolone MICs in Helicobacter pylori strains from Korean patients. J. Antimicrob. Chemother. 56:965-967. [DOI] [PubMed] [Google Scholar]
- 13.Kist, M., and E. Glocker. 2004. ResiNet—a nationwide German sentinel study for surveillance and analysis of antimicrobial resistance in Helicobacter pylori. Eurosurveill. Quart. (1-3):44-46. [Google Scholar]
- 14.Malfertheiner, P., F. Mégraud, C. O'Morain, A. P. S. Hungin, R. Jones, A. Axon, D. Y. Graham, G. Tytgat, and The European Helicobacter pylori Study Group (EHPSG). 2002. Current concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. Aliment. Pharmacol. Ther. 16:167-189. [DOI] [PubMed] [Google Scholar]
- 15.Marzio, L., D. Coraggio, S. Capodicasa, L. Grossi, and G. Capello. 2006. Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter 11:237-242. [DOI] [PubMed] [Google Scholar]
- 16.Miwa, H., A. Nagahara, A. Kurosawa, T. Ohkusa, R. Ohkura, M. Hojo, N. Enomoto, and N. Sato. 2003. Is antimicrobial susceptibility testing necessary before second-line treatment for Helicobacter pylori infection? Aliment. Pharmacol. Ther. 17:1545-1551. [DOI] [PubMed] [Google Scholar]
- 17.Moore, R. A., B. Beckthold, S. Wong, A. Kureishi, and L. E. Bryan. 1995. Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of Helicobacter pylori. Antimicrob. Agents Chemother. 39:107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neri, M., A. Milano, G. Di Bonaventura, R. Piccolomini, M. P. Caldarella, C. Balatsinou, D. Lapenna, and F. Cuccurullo. 2003. Role of antibiotic sensitivity testing before first-line Helicobacter pylori eradication treatments. Aliment. Pharmacol. Ther. 18:821-827. [DOI] [PubMed] [Google Scholar]
- 19.Nishizawa, T., H. Suzuki, K. Kurabayashi, T. Masaoka, H. Muraoka, M. Mori, E. Iwasaki, I. Kobayashi, and T. Hibi. 2006. Gatifloxacin resistance and mutations in gyrA after unsuccessful Helicobacter pylori eradication in Japan. Antimicrob. Agents Chemother. 50:1538-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nista, E. C., M. Candelli, M. A. Zocco, I. A. Cazzato, F. Cremonini, V. Ojetti, M. Santoro, R. Finizio, G. Pignataro, G. Cammarota, G. Gasbarrini, and A. Gasbarrini. 2005. Moxifloxacin-based strategies for first-line treatment of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 21:1241-1247. [DOI] [PubMed] [Google Scholar]
- 21.Romano, M., R. Marmo, A. Cuomo, T. De Simone, C. Mucherino, M. R. Iovene, F. Montella, M. A. Tufano, C. Del Vecchio Blanco, and G. Nardone. 2003. Pretreatment antimicrobial susceptibility testing is cost saving in the eradication of Helicobacter pylori. Clin. Gastroenterol. Hepatol. 1:273-278. [PubMed] [Google Scholar]
- 22.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175-1186. [DOI] [PubMed] [Google Scholar]
- 23.Tankovic, J., C. Lascols, Q. Sculo, J.-C. Petit, and C.-J. Soussy. 2003. Single and double mutations in gyrA but not in gyrB are associated with low- and high-level fluoroquinolone resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 47:3942-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toro, C., J. Garcia-Samaniego, J. Carbo, A. Iniguez, T. Alarcon, M. Lopez-Brea, and M. Baquero. 2001. Prevalence of primary Helicobacter pylori resistance to eight antimicrobial agents in a hospital in Madrid. Rev. Esp. Quimioter. 14:172-176. [PubMed] [Google Scholar]
- 25.Wang, G., T. J. Wilson, Q. Jiang, and D. E. Taylor. 2001. Spontaneous mutations that confer antibiotic resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 45:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Y., K. P. Roos, and D. E. Taylor. 1993. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J. Gen. Microbiol. 139:2485-2493. [DOI] [PubMed] [Google Scholar]