Abstract
Three isolates of zygomycetes belonging to two different genera (Rhizopus oryzae and Absidia corymbifera) were used to produce a systemic infection in neutropenic mice. On days −2 and −1 and at 2 h prior to infection, the mice received either posaconazole (POS) at doses ranging from 20 to 80 mg/kg of body weight/day or amphotericin B (AMB) at 1 mg/kg/day. Antifungal drug efficacy was assessed by determination of the prolongation of survival, determination of the percentage of infected organs (brain, lung, spleen, and kidney), and histological examination for the number of infection foci and their sizes in brain and kidney tissues. AMB significantly prolonged the survival of mice infected with all isolates. POS significantly prolonged the survival of mice infected with zygomycetes. Cultured organs from mice infected with R. oryzae were all positive, while treated mice challenged with A. corymbifera generally showed lower percentages of infected organs compared with the percentages for the controls. Zygomycete isolates established an active infection (the presence of hyphae) in the brains and the kidneys of all controls. In mice challenged with R. oryzae, both antifungal drugs were effective at reducing the number and the size of infection foci in the kidneys. Only AMB reduced the numbers, but not the sizes, of infection foci in the brain. Finally, both drugs significantly reduced the numbers and the sizes of infection foci in both tissues of mice infected with A. corymbifera. Our data suggest that prophylaxis with POS has some potential to prevent zygomycosis.
Zygomycosis is a rare but highly aggressive filamentous fungal infection (4). The clinical manifestations of zygomycosis include primary rhinocerebral, pulmonary, gastrointestinal, cutaneous or subcutaneous, and allergic disease and disseminated disease (4, 6, 7, 13). The zygomycetes most commonly identified as etiologic agents of human diseases are Rhizopus species, Rhizomucor species, Mucor species, and Absidia species (13). The invasive forms of zygomycosis cause angioinvasion, followed by progressive, necrotic, and generally fatal infections in immunocompromised hosts, such as diabetic patients with ketoacidosis, neutropenic patients, patients taking corticosteroids, and subjects with burns or iron overload (4, 6, 7, 12, 13). The standard therapy for these life-threatening infections consists of removal of the predisposing factors, widespread surgical debridement, and high doses of intravenous amphotericin B (AMB) (13). Nevertheless, even with aggressive therapy, the rate of mortality is often above 50% (4, 6). It is clear that new strategies for the treatment of zygomycosis are urgently needed.
Posaconazole (POS) is a new broad-spectrum triazole with activity against many filamentous fungal pathogens, including zygomycetes (3, 8, 11, 13, 14). Experimental infection models and clinical data showed that POS might be useful for the treatment of zygomycosis (3, 11, 14). No data on the effects of POS prophylaxis against these infections are available. Therefore, in this study we evaluated the effect of POS prophylaxis against Rhizopus oryzae and Absidia corymbifera in an experimental model of neutropenic mice infection.
(This work was presented in part at the 16th Congress of the International Society for Human and Animal Mycology, Paris, France, 25 to 29 June 2006).
MATERIALS AND METHODS
Organisms.
Three clinical isolates were used in this study: Rhizopus oryzae 4570, R. oryzae 4745, and Absidia corymbifera 4535. Both isolates of R. oryzae were recovered from the sputum of two oncology patients with systemic zygomycosis, while A. corymbifera was obtained from the sputum of a patient with pulmonary zygomycosis. The isolates were stored as conidial suspensions at −80°C in 10% glycerol until they were used.
Antifungal drugs.
For both the in vitro and the in vivo studies, POS (Schering-Plough) was prepared in polyethylene glycol 200 (Sigma). For the in vitro studies, AMB (Sigma) was dissolved in dimethyl sulfoxide (Sigma), while for the in vivo studies, AMB (Fungizone; Bristol-Myers Squibb) was dissolved in sterile saline.
In vitro studies.
MICs were determined by the CLSI (formerly NCCLS) broth microdilution methodology (document M38-A) (9). Each strain was grown on Sabouraud dextrose agar (SDA) plates at 35°C for 5 days. The fungal colonies were then covered with 1 ml of sterile 0.85% saline and gently scraped with a sterile pipette. The resulting conidial suspensions were transferred to sterile tubes, and heavy particles were allowed to settle. To obtain a final inoculum of approximately 104 CFU/ml, the conidial suspension was further adjusted by use of a hemocytometer. The microdilution trays, which contained both drugs at concentrations ranging from 0.015 to 8.0 μg/ml, were incubated at 35°C for 21 to 26 h. The MIC endpoint for both drugs was the lowest drug concentration that prevented any discernible growth. Each strain was tested in quintuplicate.
In vivo studies. (i) Animals and immunosuppression.
Male CD1 mice (weight, 25 g; Charles River Laboratories, Calco, Italy) were used in all studies. The mice were rendered neutropenic by intraperitoneal (i.p.) administration of cyclophosphamide at 200 mg/kg of body weight on days −4, +1, and +4 postinfection.
The animal experiments were conducted with the approval of the University of Ancona ethics committee.
(ii) Prophylaxis and infection models.
Prophylaxis was started 2 days before the infection, with the last dose given 2 h prior to challenge, for a total of three daily doses (i.e., on days −2, −1, and 0). AMB was given i.p. at 1 mg/kg/day (200 μl), while POS was administered by oral gavage at doses of 20, 30, 50, and 80 mg/kg/day (200 μl). Control mice received 200 μl of polyethylene glycol 200 by oral gavage. Two hours after the last drug dose, the mice were infected intravenously (200 μl) with the spores of each isolate. Pilot studies were performed with R. oryzae 4570 and A. corymbifera to determine the 90% lethal dose by testing three inoculum sizes.
Final experiments were conducted by challenging the mice with 5 × 106 spores of R. oryzae 4570 per mouse, 2 × 106 (high inoculum) and 1 × 105 (low inoculum) spores of R. oryzae 4745 per mouse, and 5 × 105 spores of A. corymbifera per mouse. To confirm the inocula, dilutions were streaked onto SDA plates, and the colonies were counted following 24 h of incubation at room temperature. Each experiment was performed once.
(iii) Survival studies.
In the survival studies, the mice were observed through day 10 postinfection. Deaths were recorded daily. Moribund mice were killed, and their deaths were recorded as occurring on the next day. There were from 13 to 18 mice in each group.
(iv) Qualitative cultures.
The mice were treated and infected as reported above. The animals were killed on day 3 postinfection. The brain, lungs, spleen, and kidneys from each animal were aseptically removed and homogenized in 2 ml of sterile saline solution. Either diluted or undiluted homogenates (including the entire organ) were plated onto SDA plates. The limit of detection was 1 CFU/organ. The data are presented as the percentage of infected organs (positive cultures) with respect to the total number of organs observed for each group (3). There were eight mice in each group.
(v) Histopathology.
The mice were treated and infected as reported described above. On day 3 postinfection, the animals were killed. The brains and kidneys from the control and the treated animals were aseptically removed, fixed in 10% neutral buffered formalin solution, embedded in paraffin, and stained with Grocott-Gomori's methenamine-silver nitrate. For each stained section, the numbers and the sizes of the infection foci (clusters of hyphae) (15) were counted in 20 consecutive microscopic fields by at least two observers, who were unaware of the treatment group, using a Leitz Orthoplan light microscope equipped with a micrometric eyepiece (objective, NPL Fluotar 25/0.55; eyepiece, Periplan GW ×10/26). The total measured area of tissue was equal to 15.7 mm2 for each stained section. The data are presented as the mean number and the mean size of each infection focus from the treated and the untreated animals. There were five mice in each group.
Statistical analysis.
Differences in survival were analyzed by the log rank test and were plotted by the use of Kaplan-Meier curves. Qualitative cultures were compared by Fisher's exact test. Histopathology results were compared by analysis of variance with Bonferroni's correction for repeated measures. All P values <0.05 were considered significant.
RESULTS
The overall susceptibilities of the two zygomycete isolates are reported in Table 1. The median POS MICs were 1.0, 1.0, and 0.25 μg/ml for R. oryzae 4570, R. oryzae 4745, and A. corymbifera, respectively. The median AMB MICs were 2.0, 0.5, and 1.0 μg/ml for R. oryzae 4570, R. oryzae 4745, and A. corymbifera, respectively.
TABLE 1.
In vitro susceptibilities of zygomycete isolates to amphotericin B and posaconazolea
| Isolate | Drug | MIC (μg/ml)
|
|
|---|---|---|---|
| Median | Range | ||
| R. oryzae 4570 | POS | 1.0 | 1.0-2.0 |
| AMB | 2.0 | 2.0-4.0 | |
| R. oryzae 4745 | POS | 1.0 | 1.0 |
| AMB | 0.5 | 0.5-1.0 | |
| A. corymbifera 4535 | POS | 0.25 | 0.25-2.0 |
| AMB | 1.0 | 1.0-2.0 | |
Each test was run in quintuplicate and was repeated on two different days.
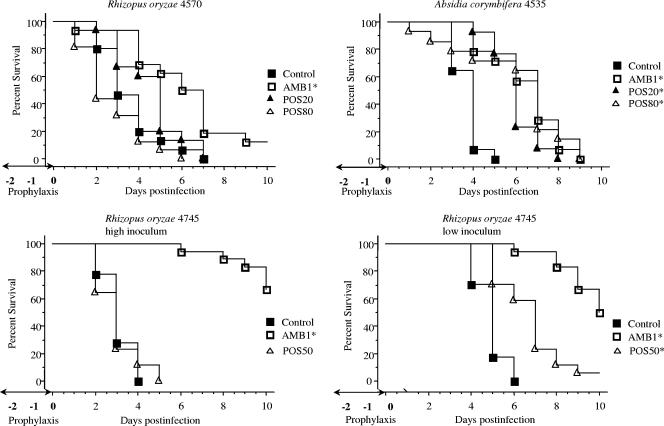
Figure 1 shows the results of the survival studies. In experiments with R. oryzae 4570 and A. corymbifera, the mice were given POS at 20 and 80 mg/kg/day. In mice infected with R. oryzae 4570, AMB significantly prolonged the survival of the mice compared with that of the control mice (P = 0.001). POS was not effective at any dose. For mice infected with A. corymbifera, both doses of POS significantly prolonged survival compared with that of the controls (P = 0.0001 for POS at 20 mg/kg/day; P = 0.001 for POS 80 at mg/kg/day). Similarly, AMB was effective (P = 0.0001).
FIG. 1.
Survival of neutropenic mice infected intravenously with 5 × 10 6 and 5 × 10 5 spores of R. oryzae 4570 and A. corymbifera 4535 per mouse, respectively, and with 2 × 10 6 (high inoculum) and 1 × 10 5 (low inoculum) spores of R. oryzae 4745 per mouse. The mice were treated orally with POS at 20, 50, or 80 mg/kg/day daily and with AMB i.p. at 1 mg/kg/day on days −2 and −1 and at 2 h prior to infection (three total daily doses). The animals were observed through day 10 postinfection, and deaths were recorded daily. Moribund mice were killed, and their deaths were recorded as occurring on the next day. There were 13 to 18 mice in each group. Asterisks indicate groups with prolonged survival compared with that of the controls (P < 0.05).
In the experiments with R. oryzae 4745, POS was administered at 50 mg/kg/day and the mice were infected with the high and the low inocula. While AMB was effective in mice infected with both inocula (P = 0.0001), POS significantly prolonged survival only for mice infected with the low inoculum (P = 0.001).
Then, we investigated the effect of prophylaxis on qualitative cultures of four different organs. These experiments were conducted with R. oryzae 4570 and A. corymbifera. In these studies, POS was given at 30 mg/kg/day. The results are presented in Table 2. In mice infected with R. oryzae 4570, neither AMB nor POS showed any potential for organ clearance. Actually, all tested organs of 100% of the treated animals were shown to be infected. In the animals with infections due to A. corymbifera, the percentage of positive organs for the treated mice was generally lower than that observed for the untreated control mice, but the only significant difference was observed for the brains of mice treated with AMB (12.5% versus 100% [P = 0.0014]).
TABLE 2.
Culture results for control and treated mice infected with R. oryzae 4570 and A. corymbiferaa
| Isolate and treatment groupb | % Positive culture
|
|||
|---|---|---|---|---|
| Brain | Lung | Spleen | Kidney | |
| R. oryzae 4570 | ||||
| Control | 100.0 | 100.0 | 100.0 | 100.0 |
| POS | 100.0 | 100.0 | 100.0 | 100.0 |
| AMB | 100.0 | 100.0 | 100.0 | 100.0 |
| A. corymbifera 4535 | ||||
| Control | 100.0 | 62.5 | 100.0 | 100.0 |
| POS | 62.5 | 12.5 | 100.0 | 75.0 |
| AMB | 12.5c | 25.0 | 100.0 | 62.5 |
Eight animals were present in each group.
POS was given at 30 mg/kg/day by oral gavage; AMB was given at 1 mg/kg/day i.p.
Significantly different from the values obtained for the control groups (P < 0.05).
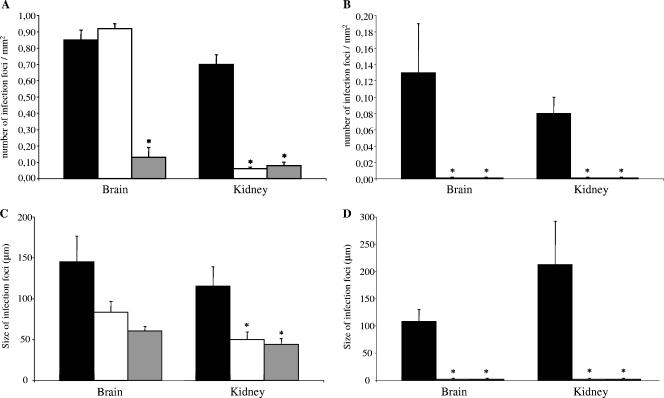
Then, the effects of prophylaxis were further investigated with brain and kidney tissues by histological studies. In these experiments, we examined the numbers and the sizes of the infection foci (clusters of hyphae). As in the previous experiment, the mice were given POS at 30 mg/kg/day. The results are reported in Fig. 2. In mice infected with R. oryzae 4570, POS was effective at reducing the number of infection foci compared with the numbers in the controls for the kidneys (P < 0.05) but not for the brain (Fig. 2A and Fig. 3). AMB was active in both organs (P < 0.05). In mice infected with A. corymbifera, either drug was effective at reducing the number of infection foci compared with the numbers in the controls for both organs (P < 0.05) (Fig. 2B).
FIG. 2.
Histopathological analysis of antifungal prophylaxis for systemic zygomycoses. The numbers of infection foci (clusters of hyphae) in mice infected with R. oryzae 4570 (A) and A. corymbifera 4535 (B) and the sizes of the infection foci in mice infected with R. oryzae 4570 (C) and A. corymbifera 4535 (D) are shown. Brains and kidneys were retrieved 3 days postinfection from control mice (n = 5; black histograms), posaconazole-treated mice (n = 5; white histograms), and amphotericin B-treated mice (n = 5; gray histograms). Tissue sections were prepared and stained with Grocott-Gomori's methenamine-silver nitrate. At least 20 microscopic fields were examined for each mouse, and the number of infection foci and their sizes were determined. Data are reported as the means ± standard errors of the means. Analysis of variance with Bonferroni's correction was used for statistical analysis. Asterisks indicate a significant difference between the treated and the untreated groups (P < 0.05).
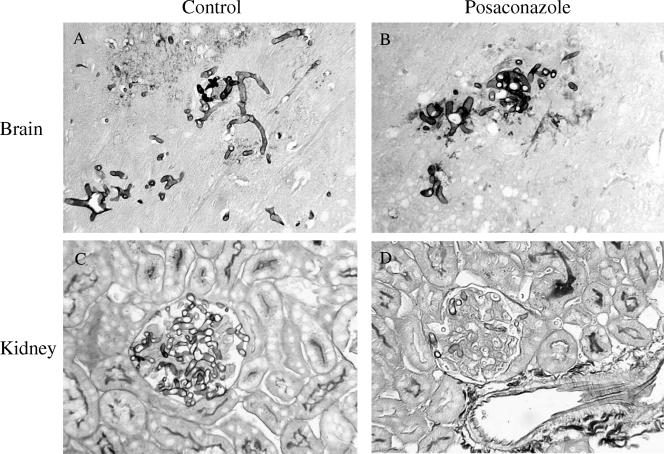
FIG. 3.
Representative results for histopathological sections of brain (A and B) and kidney (C and D) tissues stained with Grocott-Gomori's methenamine silver nitrate from mice infected with R. oryzae 4570. Control mice (A and C) show typical infection foci in both tissues. Posaconazole, given for three consecutive days at 30 mg/kg/day, was not effective in the brain (B), while it reduced the hyphal growth in the kidney (D). Magnification, ×130.
Prophylaxis with both drugs was effective at reducing the sizes of the foci in the kidneys of mice infected with R. oryzae 4570 (P < 0.05), while neither drug was effective in the brain (Fig. 2C). In mice infected with A. corymbifera, either drug was effective at reducing the sizes of the foci compared with those in the controls for both organs (P < 0.05) (Fig. 2D).
DISCUSSION
In this study, we investigated the effects of POS for the prophylaxis of experimental systemic zygomycosis. The indices used to assess the outcome of infection were (i) the rates of survival of the animals; (ii) the percentages of infected organs; and (iii) the results of histopathological examination, in which either the numbers or the sizes of infection foci were measured. In terms of survival, POS was effective against A. corymbifera and one isolate of R. oryzae. However, the protective benefit of POS against R. oryzae was inoculum dependent. These results are similar to those recently found by Ibrahim et al. (5). Those investigators found that caspofungin improved the survival of mice with diabetic ketoacidosis infected with a small inoculum but not with a large inoculum of R. oryzae.
AMB was effective against all isolates. These data are quite similar to those previously reported by Dannaoui et al. (3). They studied the effects of POS therapy with a nonimmunocompromised model of systemic infection sustained by three isolates of zygomycetes and found that the new triazole prolonged the survival of animals infected with A. corymbifera and Rhizopus microsporus var. rhizopodiformis but not those infected with R. oryzae (3).
Prophylaxis with POS was not effective in terms of organ sterilization in mice challenged with R. oryzae: 100% of all four organs tested yielded positive cultures. AMB prophylaxis was also ineffective. These results are in accordance with the findings of previous studies, which showed that AMB prolonged the survival of infected animals but did not clear the fungus from the organs (10). On the contrary, sterilization of the organs of mice given POS and infected with A. corymbifera was not a rare event. It must be noted, however, that the only significant difference between the treated and the control groups was found for the brains of mice treated with the polyene (12.5% versus 100% of positive cultures, respectively [P < 0.05]).
It has previously been reported that intravenous inoculation of mice with zygomycetes resulted in a generalized distribution of spores, particularly in the liver and the spleen, whereas the development of infection foci containing hyphae occurred in the brain and the kidney (1, 2). Therefore, we further investigated the effects of prophylaxis on the histopathological features of these two organs. Interestingly, we found that both POS and AMB were effective at reducing the numbers and the expansion of infection foci in the kidneys of mice challenged with R. oryzae, while only AMB was effective at reducing the numbers of fungal foci in the brain, although it was not effective at reducing the sizes. These findings seem to correlate well with the survival results. In fact, all moribund mice presented clinical evidence of neurological disorders, which probably represented the ultimate cause of death. Furthermore, histopathological analysis of the brains and the kidneys from mice infected with A. corymbifera and prophylactically treated with either POS or AMB showed a significant reduction in the numbers and the expansion of infection foci compared with those for the control group.
To our knowledge, this is the first study to have analyzed the effects of POS prophylaxis in experimental infections due to zygomycetes. We found that POS is effective against infections due to A. corymbifera in terms of the prolongation of survival and bringing about a reduction of active infections in the organs tested. Although the treatment was also observed to have beneficial effects in animals with infections due to R. oryzae, our data confirm that this opportunistic pathogen remains difficult to manage.
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Corbel, M. J., and S. M. Eades. 1975. Factors determining the susceptibility of mice to experimental phycomycosis. J. Med. Microbiol. 8:551-564. [DOI] [PubMed] [Google Scholar]
- 2.Corbel, M. J., and S. M. Eades. 1978. Observations on the localization of Absidia corymbifera in vivo. Sabouraudia 16:125-132. [PubMed] [Google Scholar]
- 3.Dannaoui, E., J. F. Meis, D. Loebenberg, and P. E. Verweij. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards, J., Jr. 1989. Zygomycosis, p. 1192-1199. In P. Hoeprich and M. Jordan (ed.), Infectious disease, 4th ed. J. B. Lippincott Co., Philadelphia, PA.
- 5.Ibrahim, A. S., J. C. Bowman, V. Avanessian, K. Brown, B. Spellberg, J. E. Edwards, Jr., and C. M. Douglas. 2005. Caspofungin inhibits Rhizopus oryzae 1,3-β-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 49:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim, A. S., J. E. J. Edwards, and S. G. Filler. 2003. Zygomycosis, p. 241-251. In W. E. Dismukes, P. G. Pappas, and J. D. Sobel (ed.), Clinical mycology. Oxford University Press, New York, NY.
- 7.Kauffman, C. A. 2004. Zygomycosis: reemergence of an old pathogen. Clin. Infect. Dis. 39:588-590. [DOI] [PubMed] [Google Scholar]
- 8.Keating, G. M. 2005. Posaconazole. Drugs 65:1553-1567. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 10.Odds, F. C., F. Van Gerven, A. Espinel-Ingroff, M. S. Bartlett, M. A. Ghannoum, M. V. Lancaster, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, and T. J. Walsh. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raad, I. I., J. R. Graybill, A. B. Bustamante, O. A. Cornely, V. Gaona-Flores, C. Afif, D. R. Graham, R. N. Greenberg, S. Hadley, A. Langston, R. Negroni, J. R. Perfect, P. Pitisuttithum, A. Restrepo, G. Schiller, L. Pedicone, and A. J. Ullmann. 2006. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin. Infect. Dis. 42:1726-1734. [DOI] [PubMed] [Google Scholar]
- 12.Roden, M. M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaufele, M. Sein, T. Sein, C. C. Chiou, J. H. Chu, D. P. Kontoyiannis, and T. J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 13.Spellberg, B., J. Edwards, Jr., and A. Ibrahim. 2005. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 18:556-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Burik, J. A., R. S. Hare, H. S. Solomon, M. L. Corrado, and D. P. Kontoiyannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:e61-e65. [DOI] [PubMed] [Google Scholar]
- 15.Zhang, M. X., M. C. Bohlman, C. Itatani, D. R. Burton, P. W. H. I. Parren, S. C. St. Jeor, and T. R. Kozel. 2006. Human recombinant antimannan immunoglobulin G1 antibody confers resistance to hematogenously disseminated candidiasis in mice. Infect. Immun. 74:362-369. [DOI] [PMC free article] [PubMed] [Google Scholar]