Abstract
Human noroviruses (NVs) are a common cause of nonbacterial gastroenteritis. The disease is difficult to control due to its widespread nature and the lack of antivirals or vaccines against NVs. The recent identification of human histo-blood group antigens (HBGAs) as NV receptors opens a new way for the discovery and design of antivirals against NVs. A saliva-based enzyme immune assay (EIA) was used to screen a synthetic-compound library for inhibition of the binding of norovirus-like particles to HBGA receptors. Among 5,000 compounds tested in the first round of screening, 153 compounds exhibited >50% inhibition of the binding of VA387 (an NV that binds to A, B, and H epitopes) to the A antigen in saliva at ∼50 μg/ml, and 14 of the 153 compounds revealed strong inhibition, with a 50% effective concentration of <15 μM. Ten and 11 of the 14 compounds also revealed inhibition of the binding of VA387 to the B and H antigens, respectively. Seven and 6 of the 14 compounds also blocked the binding of the prototype Norwalk virus (A and H binder) to the A and H antigens, respectively. One compound significantly inhibited the binding of MOH (A and B binder) to the A and B antigens, but no compound revealed any inhibitory effect on the binding of a Lewis binding strain (VA207) to the Lewis antigens. The EIA is a high-throughput method for large-scale library screening for antivirals against NVs. Studies to further characterize the lead compounds and to screen additional compounds for other NVs are ongoing in our laboratory.
Noroviruses (NVs), previously called Norwalk-like viruses, are a leading cause of epidemics of acute nonbacterial gastroenteritis, affecting people of all ages worldwide (3, 21, 22). Viruses in this group are spread by fecal-oral pathways, through person-to-person transmission, or by contamination of environmental surfaces, water, or food. The viruses are highly contagious, usually resulting in large outbreaks in crowded communities or institutions such as schools, restaurants, hospitals, child care centers, nursing homes for the elderly, cruise ships, and military settings. NVs are difficult to study, because there is no cell culture or animal model available for them, and the disease is difficult to control because of a lack of vaccines or effective antivirals against NVs. It is therefore a public health priority to develop an effective strategy for the prevention and treatment of NV infection.
NVs are small (∼38 nm in diameter), nonenveloped, single-stranded, positive-sense RNA viruses (12, 15, 22, 23) belonging to the family Caliciviridae. The NV genome encodes three open reading frames (ORF), one of which, ORF-2, encodes one major structural protein of ∼60 kDa that spontaneously forms virus-like particles (VLPs) when expressed in baculovirus or in other expression systems (13, 14). These VLPs are morphologically and antigenically indistinguishable from the native forms of viruses found in human stools, providing valuable materials for the development of immunological assays, for the study of virus-host interaction, for use as a candidate vaccine, and for determination of the structure and capsid assembly of NVs (4, 8, 9, 14). In this study we utilized NV VLPs in the screening of potential antivirals against NV gastroenteritis.
NVs have recently been reported to recognize human histo-blood group antigens (HBGAs) as receptors (7, 10, 18, 20, 24). HBGAs are complex carbohydrates linked to glycoproteins or glycolipids that are present on the surfaces of red blood cells and mucosal epithelial cells or as free oligosaccharides in biological fluids such as blood, saliva, milk, and intestinal contents (19). The HBGA system is controlled by multiple gene families that contain silent alleles, and three major HBGA families, the Lewis, secretor, and ABO families, are involved in NV infection (18, 19). The recognition of HBGAs by NVs has been found to be highly specific; different NVs recognize different HBGAs, and so far eight distinct receptor-binding patterns have been identified (5-8, 10, 11, 20). According to potentially shared antigenic epitopes among different NVs (the A, B, H, and Lewis epitopes), the eight binding patterns can be sorted into two groups: the A/B and the Lewis (nonsecretor) binding groups (26). Strains in the A/B binding groups bind to the A and/or B or H epitopes but not the Lewis epitopes, while strains in the Lewis binding group recognize the Lewis and H epitopes but not the A and B epitopes.
The association of HBGAs with NV infection was first demonstrated by a retrospective study of volunteers challenged with Norwalk virus, in which type O individuals had the highest risk of infection and type B individuals had the lowest (10, 20). Direct evidence of H antigens as receptors for NV infection came from another human challenge study (18). Among 77 individuals challenged with NV, none of the 22 volunteers with nonsecretor status was infected following the challenge, and none of the saliva from the nonsecretors bound to Norwalk virus. Volunteers with a B blood type had the lowest infection rates compared with volunteers with other blood types, and the B saliva either did not bind to Norwalk virus or bound weakly. Although direct evidence for other NVs remains lacking, we strongly believe that the same principle of host range will apply, in which the attachment of NVs to the intestinal epithelium via a matched HBGA receptor is a prerequisite for NV infection. Inhibition of this interaction may result in the prevention or control of the viral infection or disease. In this study we describe a search for such antivirals by screening a compound library using a saliva-based blocking assay.
This saliva-based blocking assay offers several advantages for the screening of anti-NV agents. First, the assay is a solid-phase blocking assay that is simple and suitable for large-scale screening. Second, the HBGAs used in the assay were from healthy human volunteers, so they are likely to represent the natural forms of NV receptors. Third, according to our previous studies (8), the assay is highly sensitive and specific. Finally, we have assembled a panel of saliva samples from different individuals representing different blood types, allowing us to validate the results obtained in the primary screening, which used only a single saliva sample representing one blood type.
Since different NVs may share the same antigenic epitopes, such as the H epitope, shared by the A/B binding and Lewis binding strains, we initiated the study with one well-characterized NV strain, VA387, for our primary screening (8, 16). This strain has the broadest spectrum of binding to human HBGAs, recognizing A, B and H epitopes of the human HBGAs (8). Following the primary screening against the type A antigen, we extended the study of the hit compounds to determine their abilities to inhibit (i) the binding of the same strain to the B and H antigens and (ii) the binding of other NV strains to variable antigens. Our results have demonstrated that the saliva-based blocking assay is a simple and sensitive method for anti-NV drug screening and evaluation.
MATERIALS AND METHODS
Compound library.
The compound library tested in this study was “The Diversity Screening Set” (Timtec Inc., Newark, DE), a collection of diverse, highly pure, rationally selected drug-like small-molecule compounds ranging from 200 to 850 Da. The compounds were received as powders, which were dissolved in dimethyl sulfoxide to a stock concentration of 10 mM and stored at −20°C before use. Dimethyl sulfoxide concentrations in the reaction mixture were managed so as never to be higher than 0.5% (vol/vol). Most of the compounds in the library can dissolve at 50 μg/ml in the solvent used. For those compounds that do not dissolve (∼5%), we decreased the concentration to 10 to 25 μg/ml.
Preparation of NV VLPs.
Baculovirus-expressed recombinant capsid proteins of four NV strains representing four distinct receptor binding patterns were studied: one strain in genogroup I (Norwalk virus) and three strains in genogroup II (VA387, VA207, and MOH) (9). The procedures for production of NV VLPs in Spodoptera frugiperda (Sf9) insect cells have been published previously (14, 16). Briefly, a cDNA from the 3′ end of the genome containing the viral capsid gene (ORF-2) was cloned from viral RNA extracted from stool specimens. The recombinant baculoviruses carrying the viral capsid genes were constructed from the cloned cDNAs using the Bac to Bac expression system according to the manufacturer's instructions (Invitrogen Life Technologies, Carlsbad, CA). VLPs were partially purified by sucrose gradient centrifugation and were stored at −80°C. Protein concentrations were determined by measuring the optical density at 280 nm (OD280) using a GeneQuant spectrophotometer (Pharmacia, MI) and by comparison with a bovine serum albumin standard in a sodium dodecyl sulfate-polyacrylamide gel (25). The purity of VLPs used in this study was more than 90% as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis.
Saliva samples.
Saliva samples were collected from healthy adult volunteers under a human subject research protocol approved by the Institutional Review Board at the Cincinnati Children's Hospital Medical Center. Except for gender and race, no personal information was collected. A total of 5 to 10 ml of saliva was collected from each volunteer, and the samples were processed immediately after collection. Since saliva samples from the volunteers may contain mucosal immunoglobulin A (IgA) against NV, they were first boiled at 100°C for 10 min, to avoid potential NV-specific antibodies that might interfere in the receptor binding assays, and then centrifuged at 10,000 × g for 5 min. The clear supernatant was stored at −80°C until use. However, the NV seropositivity status was not determined, and the effectiveness of inactivation of IgA by boiling was unknown. The HBGA types of the saliva samples were determined by enzyme immune assays (EIAs) with monoclonal antibodies specific to human HBGAs (8). A saliva sample from a type A donor was selected for the primary screening, and a total of 25 saliva samples of known ABO, secretor, and Lewis types were selected for further studies to confirm the primary screening results.
A saliva-based EIA to screen compounds for blocking NV binding to human HBGA receptors.
A saliva-based EIA was developed to measure the inhibitory activities of compounds against the binding of VA387 VLPs to the A antigen. Standard 96-well microtiter plates (Dynex Immulon; Dynatech, Franklin, MA) were coated with the type A saliva sample at a dilution of 1:2,000 in phosphate-buffered saline (PBS) (pH 7.4). Unbound A antigen was removed by a wash with PBS, and the plates were blocked with 5% dried milk (BLOTTO) for 1 h at 37°C. A total volume of 100 μl of baculovirus-expressed VA387 VLP at a concentration of ∼100 ng/ml in PBS was incubated with or without compounds at 37°C for 30 min; then the reaction mixture was added to the microtiter plates coated with the type A saliva. After 1 h of incubation at 37°C, the bound VLPs were detected using a pooled guinea pig anti-NV antiserum (dilution, 1:3,333) obtained by cross-immunization of the animals with nine different NV strains (8), followed by the addition of horseradish peroxidase-conjugated goat anti-guinea pig IgG (ICN, Aurora, OH) at a dilution of 1:5,000. For each step, the plates were washed five times with PBS containing 0.5% Tween 20. The bound horseradish peroxidase conjugates were detected by the TMB kit (Kirkegaard & Perry Laboratories), and the OD450 was read using an EIA spectrum reader (Tecan). The hits from the primary screening were retested with serial dilutions of the compounds (from ∼0.1 to ∼100 μM), and the blocking activity of each compound was expressed as the 50% effective concentration (EC50) by comparison of the signals in wells with the compounds with the signals in negative-control wells without compounds minus the background noise (the blank control wells that contain all components except compounds and VLPs).
Following the primary screening, compounds with significant blocking activities against VA387 binding to the A antigen were further tested for their inhibitory activities on (i) VA387 binding to type B and type H antigens, (ii) Norwalk virus binding to the A and H antigens, (iii) MOH binding to the A and B antigens, and (iv) VA207 binding to the Lewis antigens. The same format and condition as for the assays described above, except for variable concentrations of VLPs (100 to 500 ng/ml), were used. This is because the receptor binding affinities of individual strains differ (8), and based on titration results, we selected a concentration to give an OD450 of ∼1 in the wells without compounds for each strain.
MTS cytotoxicity assay.
Human cervical carcinoma (HeLa) cells were grown in a humidified 5% CO2 incubator at 37°C in RPMI 1640 medium supplemented with 1% l-glutamine, 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (2). Human colon carcinoma (Caco-2) cells were grown in Earle's minimal essential medium supplemented with 10% fetal bovine serum, 1% l-glutamine, minimal essential medium nonessential amino acids, HEPES buffer, penicillin, and streptomycin (27). The cells were seeded at 5 × 104 per ml onto a 96-well plate and incubated overnight before testing. The MTS cytotoxicity assay was performed using CellTiter 96 aqueous nonradioactive cell proliferation kits (Promega, Madison, WI). The assay solution contained the tetrazolium compound 3-(4,5-dimethylthiazole-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), and an electron-coupling reagent, phenazine ethosulfate. The cytotoxicities of individual compounds were determined by the decrease in cellular reduction of MTS into the colored product. Briefly, after the incubations with compounds at various concentrations for 3 days, the culture medium was replaced with fresh medium with 100 μl of MTS-phenazine methosulfate/well, incubated at 37°C for 2 h, and measured with a plate reader at an absorbance of 490 nm. The 50% cytotoxic concentrations (CC50s) were determined as the concentrations of compounds that caused 50% inhibition of cell growth compared to the growth of control cells cultured without compound. The selective index was calculated as the values for the CC50 divided by the EC50s determined by the saliva-blocking assay described above (17).
RESULTS
Development of a saliva-based receptor blocking assay for screening of compounds against the binding of NVs to human HBGA receptors.
Recombinant VLPs from four NV strains representing four distinct receptor binding patterns (9), in two major receptor binding groups (Table 1), were used in the screening experiments. Under our standard protocol for saliva binding assays, VLPs from most of the strains usually resulted in ODs of >2.5 with their corresponding antigenic epitopes. These values are too high for a sensitive screening. To optimize the blocking assays, we titrated all reagents used in the assays, including the VLPs, the first and second antibodies, the blocking reagent (dried cow milk), and a panel of saliva samples from different donors. The final condition for blocking assays, which resulted in an OD450 of ∼1.0 in the absence of compounds and a 50% OD450 reduction in the presence of compounds, was selected as the cutoff for the primary screening. For comparison of daily results, eight control wells containing everything but compounds were included in each plate, and the assays were considered valid when the daily OD450 variations in these wells were less than 20%. Less than 1% of the total screening runs failed in our study. The ODs of the eight blank controls that did not contain compounds and VLPs ranged from 0 to 0.02, with little variation among different saliva samples used.
TABLE 1.
Four receptor binding patterns of four NV strains with HBGAs in salivaa
| Strain (genogroup) | Binding group(s) | Binding to antigen
|
|||
|---|---|---|---|---|---|
| Nonsecretor (Le) | Secretor
|
||||
| O (H) | A | B | |||
| VA387 (G II) | A/B | − | +++ | ++++ | ++++ |
| Norwalk (G I) | A/B | − | +++ | ++++ | − |
| MOH (G II) | A/B | − | − | ++++ | ++++ |
| VA207 (G II) | Le | +++ | ++ | + | +/− |
Symbols −, +/−, ++, +++, and ++++ indicate relative binding affinities, from lowest to highest, for individual strains in the saliva-binding EIAs. These values are not comparable between strains.
Primary screening of 5,000 compounds.
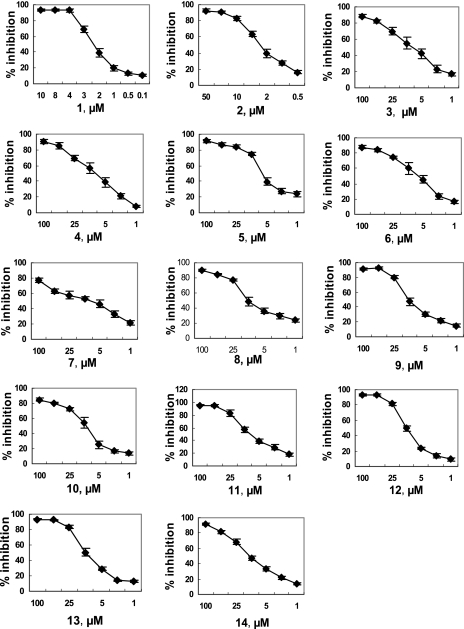
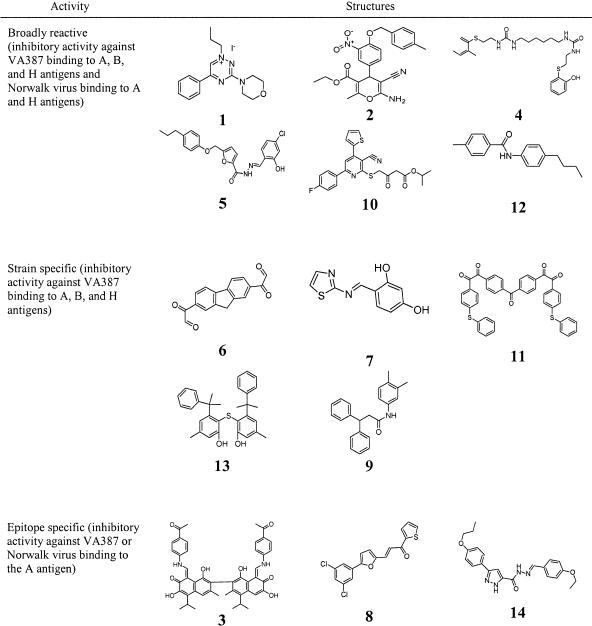
The optimal protocol described above was used to screen the compound library. The identities of the compounds were not revealed to the researcher until screening was completed. Among 5,000 compounds tested in the first round of screening, 212 (∼4%) compounds exhibited more than 50% inhibitory effects on the binding of VA387 to the A antigen at a concentration of 50 μg/ml. This concentration is estimated to be ∼100 μM for most compounds in the library. In repeating tests with serial dilutions of the compounds (from ∼0.1 to ∼100 μM), the inhibitory activities of 153 of the 212 compounds were confirmed with typical dose responses. Among the 153 compounds, 77 showed weak inhibition, with EC50s greater than 50 μM; 61 compounds exhibited moderate inhibition, with EC50s ranging from 15 to 50 μM; and 14 compounds revealed strong inhibition, with EC50s less than 15 μM against VA387 binding to type A antigen (Table 2). The most potent inhibitor found was compound 1, with an EC50 of 2.2 μM (Fig. 1; Table 2). The titration curves of the inhibitory activities of the 14 strong inhibitors on VA387 binding to the A antigen are shown in Fig. 1, and the chemical structures of the compounds are shown in Table 3.
TABLE 2.
Inhibitory effects of the 14 lead compounds against binding of NVs to the corresponding HBGAs
| Compounda | EC50b (μM) for the following virus and HBGA:
|
CC50c (μM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| rVA387
|
rNorwalk
|
rVA207
|
rMOH
|
||||||
| A | B | H | A | H | Le | A | B | ||
| 1 | 2.2 | 5.8 | 6.8 | 15.6 | 18.9 | >100 | >100 | >100 | 158.0 |
| 2 | 4.8 | 18.6 | 11.9 | 22.9 | 24.5 | >100 | 16.5 | 13.3 | 78.6 |
| 3 | 7.8 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 82.7 |
| 4 | 8.1 | 13.1 | 14.2 | 14.1 | 22.6 | >100 | >100 | >100 | 112.6 |
| 5 | 9.1 | 13.5 | 7.2 | 18.2 | 36.3 | >100 | >100 | >100 | 46.7 |
| 6 | 9.3 | 44.6 | 41.1 | >100 | >100 | >100 | >100 | >100 | 52.7 |
| 7 | 9.6 | 26.0 | 7.6 | >100 | >100 | >100 | >100 | >100 | 323.6 |
| 8 | 10.9 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 157.3 |
| 9 | 11.7 | >100 | 47.5 | >100 | >100 | >100 | >100 | >100 | 40.3 |
| 10 | 11.8 | 7.9 | 7.5 | 35.0 | 19.8 | >100 | >100 | >100 | 220.3 |
| 11 | 12.8 | 35.3 | 26.1 | >100 | >100 | >100 | >100 | >100 | 262.8 |
| 12 | 12.8 | 32.5 | 28.2 | 53.0 | 47.8 | >100 | >100 | >100 | 180.2 |
| 13 | 13.6 | 49.5 | 48.1 | >100 | >100 | >100 | >100 | >100 | 70.6 |
| 14 | 13.7 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 12.3 |
The solubilities of compounds 1 to 8 and of compounds 10 to 13 are greater than 400 μM; the solubilities of compounds 9 and 14 are greater than 100 μM.
Effective concentration required to achieve 50% inhibition of the binding of a norovirus to the corresponding HBGA. Experiments were repeated three times; the data in the table are the average EC50s.
The concentration of a compound that causes 50% inhibition of cell growth. Experiments were repeated three times; the data in the table are the average CC50s.
FIG. 1.
Titration of inhibitory activities of compounds against the binding of VA387 to the A antigen. All compounds that revealed significant blocking activities against VA387 binding to the A antigen (>50% reduction rate) were further titrated to determine the EC50s (effective concentrations causing 50% reduction of binding activity). The results for the 14 compounds with the greatest blocking activities among 153 compounds tested are shown. The concentrations of the compounds used in the assays were adjusted according to their blocking activities in the primary screening. The concentration ranges for compounds 1 and 2 were 0.1 to 10 μM and 0.5 to 50 μM, respectively. The concentration range for compounds 3 to 14 was 1 to 100 μM. Triplicate tests for each compound were performed, and the mean binding activity reduction and standard deviation are presented. The EC50 of each compound (Table 4) was calculated based on the data presented in this figure.
TABLE 3.
Structures and selective inhibitory activities of the 14 lead compounds against binding of NVs to the corresponding HBGA receptors
Two cell lines, HeLa and Caco-2 cells, were used to evaluate the cytotoxicities of the 14 lead compounds using the MTS assay (1). None of the 14 compounds had a significant inhibitory effect on the growth of Caco-2 cells below a compound concentration of 200 μM (data not shown). When HeLa cells were used, 11 of the 14 compounds had weak cytotoxicity, with CC50s greater than 50 μM, and only two compounds showed strong cytotoxicity, with CC50s around 10 μM.
Validation of receptor-blocking activities of compounds by using a panel of type A saliva samples.
A single dosage of the 14 lead compounds at their EC50s was used to inhibit VA387 binding to a panel of saliva samples from eight randomly selected type A individuals, and the inhibitory effects of all compounds have been confirmed (Table 4). Significant inhibition was observed for all samples in the presence of compounds, and the reduction rates of the compounds also were comparable to those found in the primary screening. Most compounds had narrow variation, with <15% differences in reduction activity from the primary screening results (Table 2), indicating that the EC50s determined in the primary screening are highly reproducible.
TABLE 4.
Inhibitory activities of the 14 lead compounds against the binding of VA387 to eight saliva samples from randomly selected type A individuals
| Compound | Concn (μM) of compounda | % Inhibition of binding to saliva sample:
|
Avg % inhibition (mean ± SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 1 | 2.2 | 48.1 | 56.2 | 46.5 | 47.1 | 52.2 | 55.4 | 41.2 | 47.1 | 49.3 ± 5.0 |
| 2 | 4.8 | 60.1 | 61.7 | 66.2 | 64.3 | 67.6 | 65.5 | 60.2 | 63.3 | 61.1 ± 2.8 |
| 3 | 7.8 | 62.2 | 50.2 | 56.4 | 54.3 | 51.2 | 52.2 | 47.5 | 60.3 | 53.8 ± 5.1 |
| 4 | 8.1 | 58.1 | 56.5 | 65.1 | 64.3 | 58.7 | 62.3 | 57.5 | 61.4 | 59.3 ± 3.2 |
| 5 | 9.1 | 66.1 | 70.1 | 72.5 | 62.3 | 62.4 | 65.7 | 61.1 | 55.5 | 62.9 ± 5.4 |
| 6 | 9.3 | 61.2 | 51.0 | 52.3 | 49.3 | 48.1 | 54.6 | 54.7 | 63.2 | 53.8 ± 5.4 |
| 7 | 9.6 | 45.4 | 44.3 | 53.2 | 55.1 | 39.7 | 42.0 | 46.1 | 31.7 | 45.3 ± 7.4 |
| 8 | 10.9 | 38.3 | 42.5 | 49.6 | 60.3 | 50.1 | 44.9 | 52.4 | 52.7 | 49.0 ± 6.8 |
| 9 | 11.7 | 41.8 | 35.7 | 57.1 | 55.5 | 53.3 | 55.4 | 60.2 | 53.6 | 51.4 ± 8.4 |
| 10 | 11.8 | 55.7 | 40.8 | 58.2 | 55.9 | 44.3 | 59.5 | 56.2 | 37.6 | 50.9 ± 8.6 |
| 11 | 12.8 | 34.7 | 43.3 | 55.1 | 40.0 | 44.4 | 48.6 | 46.2 | 52.2 | 46.1 ± 6.5 |
| 12 | 12.8 | 43.2 | 53.4 | 60.3 | 54.9 | 55.4 | 64.2 | 57.3 | 49.8 | 54.3 ± 6.4 |
| 13 | 13.6 | 42.2 | 48.1 | 48.4 | 56.5 | 50.1 | 55.1 | 40.4 | 49.7 | 48.9 ± 5.6 |
| 14 | 13.7 | 54.3 | 51.3 | 62.4 | 51.4 | 41.3 | 50.5 | 55.2 | 43.7 | 51.1 ± 6.6 |
EC50 obtained in the primary screening.
Specificity of inhibitory activities by the compounds.
In our preliminary studies to develop the saliva blocking assays, significant blocking activities were observed when the compounds and VLPs were added to the plate at the same time. However, higher blocking activities were observed when the compounds were preincubated with the VLPs before addition to the plate. Furthermore, when compounds were added to saliva-coated plates as a first step and removed before addition of the VLPs, no inhibitory activity was observed; this result was repeatedly obtained in testing of different VLPs with more than 70 active compounds (data not shown). These results suggested that the compounds play their inhibitory role via interaction with the VLPs, not with the HBGAs.
Inhibition of VA387 binding to type B and type H antigens by the 14 lead compounds.
We first tested the inhibitory activity of the 14 compounds on VA387 binding to the B and H epitopes using one saliva sample for each antigen. Ten of the 14 compounds revealed dose-dependent inhibition against the binding to both the B and H antigens and one compound only to the H antigen (Table 2). Four of the 10 compounds that blocked both B and H antigen binding revealed strong inhibition (<15.0 μM) against both antigens.
The overall blocking activities of the 14 compounds were higher to A than B and/or H antigens with one exception (compound 10), indicating these compounds interact preferentially at the A binding site of the viral capsid (Table 2). Significant numbers of compounds did not have any inhibitory activity against VA387 binding to the B (5 compounds) and H (4 compounds) antigens, indicating these compounds may act on only the A binding site of the capsid. These results supported our previous prediction of different binding sites for different HBGAs on the norovirus capsids.
Inhibition of binding activities of variable strains to different receptors.
The 14 lead compounds were further evaluated using different NV VLPs representing various HBGA receptor binding types. The prototype Norwalk virus represents a receptor binding pattern (binding to A and H but not to B) distinct from that of VA387 (8). Seven of the 14 compounds blocked Norwalk virus binding to the A antigen, and 6 of the 7 also inhibited Norwalk virus binding to the H antigens (Table 2). These results indicate a similar binding interface on the VLPs between the two strains.
We also tested a Lewis binding strain (VA207) that is distinct from the A/B binding strains. None of the 14 compounds tested had inhibitory activities toward VA207 binding (Table 2). This result further supports our classification of the two binding groups of NVs. Finally, we tested another G II strain (MOH) that shares the A and B epitope binding (but not O) with VA387. However, only compound 2 revealed significant inhibitory activity against MOH binding to type A and B salivas, with EC50s of 16.5 and 13.3, respectively (Table 2). This result is unexpected; future studies to elucidate the difference of the structure and receptor binding interface between the two strains are needed.
DISCUSSION
In this study, we have adapted a saliva-based receptor binding assay to design a receptor blocking assay and used this blocking assay to screen a compound library for inhibition of NV binding to HBGA receptors. Among ∼5,000 compounds screened, 153 (∼3%) revealed inhibitory activities against VA387 binding to the A antigen, and the 14 (∼0.3%) most potent inhibitors had EC50s less than 15 μM, an excellent starting point for further studies to develop a useful antiviral for NV gastroenteritis. We also extended the study to examine the broadness of the inhibitory activities of lead compounds and observed differences depending on the individual NV strains and HBGAs used, indicating potential “strain-specific,” “epitope-specific,” or broadly reactive compounds (Tables 2 and 3). Further, we have demonstrated that the inhibitory activities of the lead compounds were reproducible by testing against a group of saliva samples from different individuals. Finally, we have demonstrated that most of the inhibitors were nontoxic to selected cell lines at relatively high concentrations. In conclusion, the saliva-based blocking assay is a rapid, sensitive, and low-cost method for screening of antiviral drugs against NVs.
Human NVs are believed to replicate and cause disease in the intestinal tract. The attachment through the HBGA receptors on the intestinal epithelia could be a necessary first step of infection, and inhibition of this step could be an effective treatment of the disease. This treatment may not significantly benefit patients who have already contracted the disease, but it is particularly important for controlling the outbreak of NV gastroenteritis. If we can administer a high concentration of the inhibitors to all individuals at risk in an outbreak immediately after the identification of the index patient (prophylactic therapy), we may provide effective protection and significantly control the outbreak. In fact, NVs require only 10 to 100 infectious particles to initiate an infection, and the viral inocula may not be high titered under natural conditions, because NVs are transmitted either by person-to-person contact or by contaminated surfaces, food, or water. Thus, it may not be difficult for the inhibitors to compete with the intake virus that initiates the infection. The treatment may also reduce the symptoms of patients even when it is given after the onset of disease, if the compounds have sufficient affinity to compete with the virus for the receptors. Furthermore, if the compounds are highly stable and remain functional, they may further reduce the infection by blocking the progeny viruses for subsequent cycles of replication.
However, we do expect to encounter some hurdles in developing these inhibitors into a useful antiviral. Because the human intestinal tract contains a large surface area and the HBGA receptors are highly abundant on the mucosal surface, it would be difficult for a compound to block all receptor binding sites on the surfaces. In addition, the human intestinal tract is a complicated environment containing various components (salts, bile acids, enzymes, digested and undigested food, and nutrients) and extreme chemophysical conditions that could interfere with the compound's ability to function as an antiviral against NVs. Therefore, in addition to high affinity and high specificity, other properties, such as the ability of the compounds to survive in the intestinal tract and the biosafety of the compounds for the host, are also important for our selection and design experiments. Finally, we still are facing a challenge in further evaluation of the efficacy of the compounds for human use because of the lack of a cell culture and an animal model for NVs. Efforts to establish a cell culture and an animal model have been continued in several laboratories, and promising results have been obtained. Nevertheless, human volunteer studies have been performed for NV research; thus, our final choice would be direct evaluation of the efficacy of the compounds by a volunteer challenge study if we can demonstrate that the compounds are safe for laboratory animals.
High affinity is our first criterion for selection. In this study, 14 compounds reached EC50s of <15 μM and 2 had EC50s of <5 μM. These inhibitors clearly are candidates for further investigation. At this stage, compounds with lower affinity should not be excluded, because the potential chemical-structure modification/redesign of these compounds may lead to the discovery of compounds with higher affinity. In addition, a compound targeting a unique binding site on the norovirus capsids is valuable. We plan to screen other types of compound libraries, such as a natural product library and a carbohydrate library, with more strains against different HBGA receptors, which may allow us to accumulate a large number of active compounds with variable affinities and specificities. This collection of compounds would be valuable for our future studies in understanding the mechanism and potential targets of individual compounds by quantitative structure-activity relationship studies. Furthermore, in a separate study we are trying to determine the structure of the receptor binding interface of NV capsids by crystallographic analysis. A compound targeting a potentially unique site on the capsid would be valuable for this study, especially by cocrystallization of the compound with the viral capsids.
The specificity of compounds remains an issue of our study. In the initial experiments to develop the screening method, we noticed that if we included BLOTTO (dried cow milk) in the step of incubation of compounds with VLPs, a significant decrease in the inhibitory activities of the compounds was observed. This is understandable, because the cow milk contains large amounts of variable proteins, lipids, and glycans, which can easily interfere with the assay, particularly when a low (micromolar) concentration of compounds is used, as in our initial screening. To avoid any false-negative results, we removed the BLOTTO addition step from the EIA for all screening experiments in this study.
Thus, we are aware that compounds with nonspecific inhibitory activity might be selected in our primary screening, and stringent tests are required for further selection. However, in this study we clearly observed that compounds reacted with specific strains but not others or reacted with the same strains but on different antigens, indicating that these compounds are specific inhibitors. According to our recent classification of NVs based on the HBGA receptor binding patterns, strains within receptor binding patterns or receptor binding groups may share antigenic epitopes. Thus, compounds may be selected as type common or type specific for particular receptor binding patterns or receptor binding groups based on their relative reactivities to different strains and different antigens. Most compounds found in this study followed the principle of the classification, but we also observed compounds with variable specificities that are not consistent with others. At this stage we do not know if those compounds are extremely strain specific or the opposite, because the mechanism of these compounds and their targets on the viral capsids remain unknown; further study focusing on the structure-function relationship in terms of receptor binding specificity is necessary.
Most of the 14 lead inhibitors were nontoxic to selected cell lines at relatively high concentrations. In addition, in our characterization of the mechanism by which compounds block NV binding to HBGAs, we have demonstrated that most of the compounds act specifically on the viruses, not on the HBGA receptors. This is a good indication for these compounds, because they are unlikely to have direct contact with the human HBGAs, whereas if they bound to the HBGA receptors on the cell surfaces, they would have undesirable side effects on the host cells. This is particularly important for compounds with low affinity; in such cases, a highly efficient drug can be designed by increasing the dosage of the compounds.
In addition to biosafety, the stability of compounds in the intestinal tract should be equally important, because we are looking for compounds that are able to block all viruses, including the inoculum and progeny viruses, during the viral infection. The latter may be more difficult to control, because they may be present in large quantity and may persist during the entire course of infection. Thus, high stability of compounds is important for prevention of the generation and release of any new viruses into the lumen of the intestine before they enter new cells for a new cycle of infection. The library used in our screening contains selective drug-like compounds, which are of high quality in terms of compound stability. However, serial pharmacodynamic and pharmacokinetic studies of the compounds will be performed for antiviral agent selection and development.
In summary, we have identified a group of new compounds with different chemical skeletons that are potential candidates for further development as antivirals for NVs. In addition, we have identified a large number of compounds with variable specificity and affinity, which might be a second tier of candidates for further selection and design. Since the screening method is simple, easy to perform, and inexpensive, we plan to screen more compounds to build up a large panel of inhibitors for our future studies. Finally, the concept of design of an antiviral drug based on blocking of the receptor-ligand interaction has been widely accepted. Although the evaluation of new drugs for NVs is a challenge because of the lack of cell cultures or animal models, the rapid progress of this study is encouraging.
Acknowledgments
The research described in this article was supported by the U.S. Department of Defense (PR033018) and the National Institutes of Health (R01 AI37093 and R01 AI55649).
We thank Tibor Farkas, Ming Tan, and Pengwei Huang for helpful discussions of the paper. We also thank Weiming Zhong for technical assistance with this study.
Footnotes
Published ahead of print on 23 October 2006.
REFERENCES
- 1.Buttke, T. M., J. A. McCubrey, and T. C. Owen. 1993. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J. Immunol. Methods 157:233-240. [DOI] [PubMed] [Google Scholar]
- 2.Dong, M., X. Feng, B. Wang, L. Wu, and T. Ikejima. 2001. Two novel furostanol saponins from the rhizomes of Dioscorea panthaica Prain et Burkill and their cytotoxic activity. Tetrahedron 57:501-506. [Google Scholar]
- 3.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 4.Green, K. Y., J. F. Lew, X. Jiang, A. Z. Kapikian, and M. K. Estes. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 31:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington, P. R., L. Lindesmith, B. Yount, C. L. Moe, and R. S. Baric. 2002. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J. Virol. 76:12335-12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington, P. R., J. Vinje, C. L. Moe, and R. S. Baric. 2004. Norovirus capture with histo-blood group antigens reveals novel virus-ligand interactions. J. Virol. 78:3035-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennessy, E. P., A. D. Green, M. P. Connor, R. Darby, and P. MacDonald. 2003. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 188:176-177. [DOI] [PubMed] [Google Scholar]
- 8.Huang, P., T. Farkas, S. Marionneau, W. Zhong, N. Ruvoen-Clouet, A. L. Morrow, M. Altaye, L. K. Pickering, D. S. Newburg, J. LePendu, and X. Jiang. 2003. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J. Infect. Dis. 188:19-31. [DOI] [PubMed] [Google Scholar]
- 9.Huang, P., T. Farkas, W. Zhong, M. Tan, S. Thornton, A. L. Morrow, and X. Jiang. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 79:6714-6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutson, A. M., R. L. Atmar, D. Y. Graham, and M. K. Estes. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185:1335-1337. [DOI] [PubMed] [Google Scholar]
- 11.Hutson, A. M., R. L. Atmar, D. M. Marcus, and M. K. Estes. 2003. Norwalk virus-like particle hemagglutination by binding to H histo-blood group antigens. J. Virol. 77:405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, X., D. Y. Graham, K. N. Wang, and M. K. Estes. 1990. Norwalk virus genome cloning and characterization. Science 250:1580-1583. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, X., D. O. Matson, G. M. Ruiz-Palacios, J. Hu, J. Treanor, and L. K. Pickering. 1995. Expression, self-assembly, and antigenicity of a snow mountain agent-like calicivirus capsid protein. J. Clin. Microbiol. 33:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51-61. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, X., W. M. Zhong, T. Farkas, P. W. Huang, N. Wilton, E. Barrett, D. Fulton, R. Morrow, and D. O. Matson. 2002. Baculovirus expression and antigenic characterization of the capsid proteins of three Norwalk-like viruses. Arch. Virol. 147:119-130. [DOI] [PubMed] [Google Scholar]
- 17.Khabar, K. S., F. al-Zoghaibi, M. Dzimiri, M. Taha, A. al-Tuwaijri, and M. N. al-Ahdal. 1996. MTS interferon assay: a simplified cellular dehydrogenase assay for interferon activity using a water-soluble tetrazolium salt. J. Interferon Cytokine Res. 16:31-33. [DOI] [PubMed] [Google Scholar]
- 18.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 19.Marionneau, S., A. Cailleau-Thomas, J. Rocher, B. Le Moullac-Vaidye, N. Ruvoen, M. Clement, and J. Le Pendu. 2001. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83:565-573. [DOI] [PubMed] [Google Scholar]
- 20.Marionneau, S., N. Ruvoen, B. Le Moullac-Vaidye, M. Clement, A. Cailleau-Thomas, G. Ruiz-Palacios, P. Huang, X. Jiang, and J. Le Pendu. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noel, J. S., R. L. Fankhauser, T. Ando, S. S. Monroe, and R. I. Glass. 1999. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J. Infect. Dis. 179:1334-1344. [DOI] [PubMed] [Google Scholar]
- 23.Prasad, B. V., M. E. Hardy, T. Dokland, J. Bella, M. G. Rossmann, and M. K. Estes. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287-290. [DOI] [PubMed] [Google Scholar]
- 24.Rockx, B. H., H. Vennema, C. J. Hoebe, E. Duizer, and M. P. Koopmans. 2005. Association of histo-blood group antigens and susceptibility to norovirus infections. J. Infect. Dis. 191:749-754. [DOI] [PubMed] [Google Scholar]
- 25.Tan, M., P. Huang, J. Meller, W. Zhong, T. Farkas, and X. Jiang. 2003. Mutations within the P2 domain of norovirus capsid affect binding to human histo-blood group antigens: evidence for a binding pocket. J. Virol. 77:12562-12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan, M., and X. Jiang. 2005. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 13:285-293. [DOI] [PubMed] [Google Scholar]
- 27.White, L. J., J. M. Ball, M. E. Hardy, T. N. Tanaka, N. Kitamoto, and M. K. Estes. 1996. Attachment and entry of recombinant Norwalk virus capsids to cultured human and animal cell lines. J. Virol. 70:6589-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]