Abstract
We reported the analytical interference of anti-Escherichia coli protein (EP) antibodies in human sera and residual EP in a recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay as a possible source of false positives in severe acute respiratory syndrome serodiagnosis. The rate of false positives was significantly reduced by adding mouse anti-EP antiserum in the blocking step.
Severe acute respiratory syndrome (SARS) is an emerging infectious disease caused by a zoonotic coronavirus (CoV) named SARS-CoV (12). The viral nucleocapsid (N) protein is composed of 422 amino acids with an estimated molecular mass of 46 kDa (3, 10). Currently, several recombinant N protein (rNP)-based serodiagnostic systems that use either antigen-capturing (4, 8, 15) or indirect (6, 17, 19, 22) enzyme-linked immunosorbent assay (ELISA) systems have been developed. However, small portions of false positives have been reported (11, 20, 23), making this diagnostic tool less favorable for the early detection of possible resurfacing SARS infections. As the presence of anti-EP antibodies (Ab) in sera of healthy humans has been widely reported (5, 9, 14), herein we hypothesize that the interactions between the residual Escherichia coli proteins (REP) present in the coating antigen and the naturally occurring anti-E. coli protein (anti-EP) antibodies in healthy humans may serve as a potential interference (21).
In this study, 14 overlapping fragments encoded by the complete open reading frame of the N gene of strain HK-39849 (24), designated rNP1 to rNP14, were expressed using a pRSET protein expression system (Invitrogen) and purified by use of nickel-charged Sepharose FastFlow matrix (Amersham Biosciences) according to the manufacturer's instructions. As was found in other studies (2, 7, 13, 16), rNP5 (amino acids 72 to 422) shows the highest antigenicity, which is comparable to that of the full-length rNP (data not shown). We have chosen rNP5 as the antigen for the subsequent immunoassays due to its relatively high expression level (7.2 μg/ml). The purified rNP5 was analyzed by use of silver-staining-based sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with serum of a convalescent SARS patient showing a single prominent band observed at about 42 kDa (Fig. 1). The purity of rNP5 was 94.6% as determined by light densitometry (Bio-Rad).
FIG. 1.
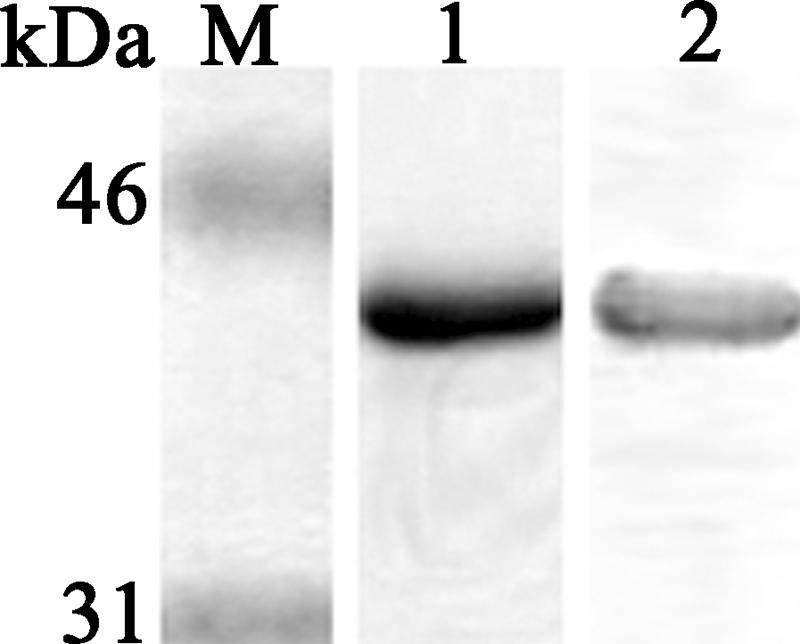
Characterization of the purified rNP5. Lanes: 1, 1 μg of purified rNP5 in silver-staining-based sodium dodecyl sulfate-polyacrylamide gel electrophoresis; 2, Western blot analysis of purified rNP5. SARS-positive serum (1:1,000) from a convalescent SARS patient was used for detection. Molecular mass marker M is shown on the left in kilodaltons.
The rNP5-based ELISA was first assessed by screening serum samples from 300 healthy individuals and 8 convalescent SARS patients. Briefly, wells were immobilized with 50 ng of rNP5 and washed before a standard blocking procedure with blocking buffer (3% milk powder in phosphate-buffered saline with 0.05% Tween 20). Human serum samples (1:100 [vol/vol]) were then applied, and incubation was performed at 37°C for 25 min, followed by incubation of horseradish peroxidase (HRP)-mouse anti-human immunoglobulin G (IgG) (1:1,000 [vol/vol]; Zymed) for 25 min. Absorbance was measured at 450 nm after the addition of TMB solution (Zymed) and 12% sulfuric acid. The relative level of SARS antibodies is determined by calculating the relative absorbance (AR) according to the equation (sample absorbance − blank absorbance)/(positive control absorbance − blank absorbance), while the cutoff value of the assay, 0.2, was defined by the summation of means of AR of the 300 control serum samples and 2 times the standard deviation. All of the eight serum samples from the SARS patients were positive both in the rNP ELISA and in that with a commercial ELISA kit (Beijing Huada GBI Biotechnology) with viral lysate used as the antigen. However, 16 of the 300 (5.4%) serum samples were regarded as false positives, as these samples showed positive in our rNP ELISA but negative in that with the commercial ELISA kit.
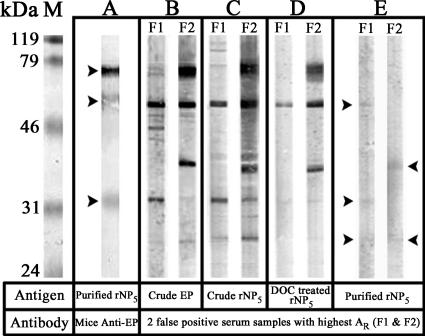
To demonstrate the potential existence of REP in the system, mouse anti-EP antiserum was raised by intramuscular immunization of 10 BALB/c mice with a crude preparation of EP. The presence of REP in the purified rNP5 was illustrated by Western blotting with the mouse anti-EP antiserum (1:300 [vol/vol]) followed by goat anti-mouse IgG (heavy plus light chains)-HRP conjugate (1:1,000 [vol/vol]; Zymed) (Fig. 2A). To demonstrate the presence of anti-EP Ab in the false-positive serum samples, Western blots were performed on the two false positives showing the highest AR values, designated F1 and F2, against crude EP and rNP5 collected at different stages of purification and detected by HRP-mouse anti-human IgG (1:250 [vol/vol]; Zymed) (Fig. 2B to E). Although the intensities of the corresponding bands of REP in purified rNP5 were relatively weak (Fig. 2E), these bands may accumulatively contribute to the overall optical density readings in the rNP5 ELISA, leading to the false-positive signals.
FIG. 2.
Western blot analysis showing the presence of residual E. coli proteins and anti-E. coli antibodies. The antigen and primary antibody used in each panel are indicated below the panels. A molecular mass marker is shown on the left in kilodaltons. (A) Recognition of REP in 2.5 μg of purified rNP5 by mouse anti-EP Ab, as indicated with arrows. (B) Recognition of crude EP (5 μg) with false-positive serum samples F1 and F2 (1:30 [vol/vol]). (C to E) Recognition of REP in rNP5 at different purification stages with false positives F1 and F2 (1:30 [vol/vol]). The presence of REP in the purified rNP5 is indicated with arrows. DOC, deoxycholate.
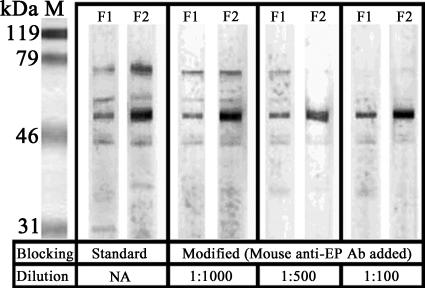
In trying to reduce the analytical interference of human anti-EP Ab and REP in rNP5, mouse anti-EP antiserum was used as an additional blocking reagent. The immunoblocking capacity of mouse anti-EP antiserum was demonstrated by adding different dilutions of mouse anti-EP antiserum in the blocking step of Western blotting procedures with serum samples F1 and F2 against crude EP (Fig. 3). When such a modified blocking step was applied in the rNP ELISA, a dilution of mouse anti-EP antiserum of 1:1,000 was found to have the maximum blocking efficiency (data not shown). The influence of the modification on the sensitivity of the system was found to be minimal, as indicated by the AR values for the eight SARS-positive sera, which remained unaffected with or without immunoblocking (Table 1, last column). Therefore, such a dilution of mouse anti-EP antiserum was added to the blocking buffer in the modified blocking procedure. The AR values of the 16 false positives in the standard rNP ELISA decreased significantly (P < 0.01, paired Student's t test) with the modified blocking step, while no significant decrease was observed if normal mice serum was used instead (Table 1). With the modified blocking step, the blocking efficiency to the 16 false-positive sera was 25.07%, and only 4 of the 16 of these samples (4/300; 1.3%) showed an AR value higher than the cutoff (0.2) (Table 1), indicating a significant improvement on the specificity of the rNP ELISA system. The remaining 1.3% false positives after immunoblocking may be due to the cross-reactivity of rNP with antibodies elicited by infection with other coronaviruses (1, 20).
FIG. 3.
Immunoblocking ability of mouse anti-EP antiserum. Western blot analysis of false positives F1 and F2 against crude EP with a standard blocking procedure or the application of different dilutions of mouse anti-EP Ab in the blocking procedure. The numbers and intensities of the bands decreased as the applied dilution of mouse anti-EP Ab increased, revealing the potential use of mouse anti-EP Ab to block the binding between EP and human anti-EP Ab specifically. NA, not applicable.
TABLE 1.
Differences in AR values with and without immunoblocking procedure and in blocking efficiencies of mouse anti-EP Ab in indicated groupsa
| Serum data group (no. of sera) | Blocking agent | Mean (SD) of difference | Significance at 95% levelb | % Blocking efficiency |
|---|---|---|---|---|
| False-positive | Anti-EPc | 0.0712 (0.0328) | Yes** | 25.07 |
| sera (16) | NMSd | 0.016 (0.0272) | No | |
| Sera from healthy individuals (300) | Anti-EP | 0.0079 (0.0209) | Yes** | 0.0283 |
| Sera from SARS patients (8) | NAe | NA (NA) | NA | 0.0150 |
The difference between the two blocking steps is defined by a paired Student's t test with a significance level of 95%. The AR values of the 16 false positives in the standard rNP ELISA decreased significantly after the modified immunoblocking procedure. The blocking efficiency {Σ[(AR-standard − AR-modified)/AR-standard]/n × 100%, in which AR-standard and AR-modified represent the AR values with standard and modified blocking, respectively, and n represents the total number of samples} of the 16 false positives is obviously higher than those of the other two serum data groups.
**, P < 0.01.
Anti-EP, mouse anti-EP antiserum.
NMS, normal mouse serum.
NA, not applicable.
In parallel with the widely documented presence of anti-EP Ab in healthy human sera, its interaction with REP in E. coli-expressed antigen-based ELISA systems and its contribution to false positives have also been reported (18). Despite the fact that the rNP-based ELISA systems have been widely used as a tool for detecting SARS-CoV infection, the high rate of false positives may lead to misleading conclusions in diagnosis and seroprevalence studies of SARS. In conclusion, we suggest the immunoblocking of mouse anti-EP antiserum as an alternative way to reduce the number of false positives caused by the analytical interference of REP in E. coli-expressed antigen and anti-EP Ab in human sera.
Acknowledgments
This work was supported by Research Grant Council grant HKU 7553/03 M.
C. W. Yip participated in the design of the study, carried out all the experiments, and drafted the manuscript. C. W. Yip, C. C. Hon, F. Zeng, and K. Y. C. Chow analyzed the results and edited the manuscript. F. C. C. Leung is the principal investigator and coordinated and supervised the study. K. H. Chan and J. S. M. Peiris coordinated the collection of clinical specimens.
No conflicts of interest are declared.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Chan, K. H., V. C. C. Cheng, P. C. Y. Woo, S. K. P. Lau, L. L. M. Poon, Y. Guan, W. H. Seto, K. Y. Yuen, and J. S. M. Peiris. 2005. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin. Diagn. Lab. Immunol. 12:1317-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, Z., D. Pei, L. Jiang, Y. Song, J. Wang, H. Wang, D. Zhou, J. Zhai, Z. Du, B. Li, M. Qiu, Y. Han, Z. Guo, and R. Yang. 2004. Antigenicity analysis of different regions of the severe acute respiratory syndrome coronavirus nucleocapsid protein. Clin. Chem. 50:988-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow, K. Y., C. C. Hon, R. K. Hui, R. T. Wong, C. W. Yip, F. Zeng, and F. C. Leung. 2003. Molecular advances in severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Genomics Proteomics Bioinformatics 1:247-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di, B., W. Hao, Y. Gao, M. Wang, Y.-D. Wang, L.-W. Qiu, K. Wen, D.-H. Zhou, X.-W. Wu, E.-J. Lu, Z.-Y. Liao, Y.-B. Mei, B.-J. Zheng, and X.-Y. Che. 2005. Monoclonal antibody-based antigen capture enzyme-linked immunosorbent assay reveals high sensitivity of the nucleocapsid protein in acute-phase sera of severe acute respiratory syndrome patients. Clin. Diagn. Lab. Immunol. 12:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths, E., P. Stevenson, R. Thorpe, and H. Chart. 1985. Naturally occurring antibodies in human sera that react with the iron-regulated outer membrane proteins of Escherichia coli. Infect. Immun. 47:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan, M., H. Y. Chen, S. Y. Foo, Y. J. Tan, P. Y. Goh, and S. H. Wee. 2004. Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients. Clin. Diagn. Lab Immunol. 11:287-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He, Q., K. H. Chong, H. H. Chng, B. Leung, A. E. Ling, T. Wei, S.-W. Chan, E. E. Ooi, and J. Kwang. 2004. Development of a Western blot assay for detection of antibodies against coronavirus causing severe acute respiratory syndrome. Clin. Diagn. Lab. Immunol. 11:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He, Q., Q. Du, S. Lau, I. Manopo, L. Lu, S. W. Chan, B. J. Fenner, and J. Kwang. 2005. Characterization of monoclonal antibody against SARS coronavirus nucleocapsid antigen and development of an antigen capture ELISA. J. Virol. Methods 127:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriksen, A. Z., and J. A. Maeland. 1987. Serum antibodies to outer membrane proteins of Escherichia coli in healthy persons and patients with bacteremia. J. Clin. Microbiol. 25:2181-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krokhin, O., Y. Li, A. Andonov, H. Feldmann, R. Flick, S. Jones, U. Stroeher, N. Bastien, K. V. Dasuri, K. Cheng, J. N. Simonsen, H. Perreault, J. Wilkins, W. Ens, F. Plummer, and K. G. Standing. 2003. Mass spectrometric characterization of proteins from the SARS virus: a preliminary report. Mol. Cell. Proteomics 2:346-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maache, M., F. Komurian-Pradel, A. Rajoharison, M. Perret, J. L. Berland, S. Pouzol, A. Bagnaud, B. Duverger, J. Xu, A. Osuna, and G. Paranhos-Baccala. 2006. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid-based Western blot assay were rectified by the use of two subunits (S1 and S2) of spike for detection of antibody to SARS-CoV. Clin. Vaccine Immunol. 13:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, and K. Y. Yuen. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu, M., J. Wang, H. Wang, Z. Chen, E. Dai, Z. Guo, X. Wang, X. Pang, B. Fan, J. Wen, J. Wang, and R. Yang. 2005. Use of the COOH portion of the nucleocapsid protein in an antigen-capturing enzyme-linked immunosorbent assay for specific and sensitive detection of severe acute respiratory syndrome coronavirus. Clin. Diagn. Lab. Immunol. 12:474-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanches, M. I., R. Keller, E. L. Hartland, D. M. Figueiredo, M. Batchelor, M. B. Martinez, G. Dougan, M. M. Careiro-Sampaio, G. Frankel, and L. R. Trabulsi. 2000. Human colostrum and serum contain antibodies reactive to the intimin-binding region of the enteropathogenic Escherichia coli translocated intimin receptor. J. Pediatr. Gastroenterol. Nutr. 30:73-77. [DOI] [PubMed] [Google Scholar]
- 15.Shi, Y., Y. Yi, P. Li, T. Kuang, L. Li, M. Dong, Q. Ma, and C. Cao. 2003. Diagnosis of severe acute respiratory syndrome (SARS) by detection of SARS coronavirus nucleocapsid antibodies in an antigen-capturing enzyme-linked immunosorbent assay. J. Clin. Microbiol. 41:5781-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan, Y.-J., P.-Y. Goh, B. C. Fielding, S. Shen, C.-F. Chou, J.-L. Fu, H. N. Leong, Y. S. Leo, E. E. Ooi, A. E. Ling, S. G. Lim, and W. Hong. 2004. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin. Diagn. Lab. Immunol. 11:362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timani, K. A., L. Ye, L. Ye, Y. Zhu, Z. Wu, and Z. Gong. 2004. Cloning, sequencing, expression, and purification of SARS-associated coronavirus nucleocapsid protein for serodiagnosis of SARS. J. Clin. Virol. 30:309-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warnes, A., A. R. Fooks, and J. R. Stephenson. 1994. Production of measles nucleoprotein in different expression systems and its use as a diagnostic reagent. J. Virol. Methods 49:257-268. [DOI] [PubMed] [Google Scholar]
- 19.Woo, P. C., S. K. Lau, H. W. Tsoi, K. H. Chan, B. H. Wong, X. Y. Che, V. K. Tam, S. C. Tam, V. C. Cheng, I. F. Hung, S. S. Wong, B. J. Zheng, Y. Guan, and K. Y. Yuen. 2004. Relative rates of non-pneumonic SARS coronavirus infection and SARS coronavirus pneumonia. Lancet 363:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo, P. C., S. K. Lau, B. H. Wong, K. H. Chan, W. T. Hui, G. S. Kwan, J. S. Peiris, R. B. Couch, and K. Y. Yuen. 2004. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polypeptide. J. Clin. Microbiol. 42:5885-5888. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Yip, C. W., C. C. Hon, F. Zeng, K. Y. Chow, F. C. Leung, et al. 2004. Prevalence of non-pneumonic infections with SARS-correlated virus. Lancet 363:1825-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu, F., M. Q. Le, S. Inoue, H. T. C. Thai, F. Hasebe, M. del Carmen Parquet, and K. Morita. 2005. Evaluation of inapparent nosocomial severe acute respiratory syndrome coronavirus infection in Vietnam by use of highly specific recombinant truncated nucleocapsid protein-based enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 12:848-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu, S., M. Qiu, Z. Chen, X. Ye, Y. Gao, A. Wei, X. Wang, L. Yang, J. Wang, J. Wen, Y. Song, D. Pei, E. Dai, Z. Guo, C. Cao, J. Wang, and R. Yang. 2005. Retrospective serological investigation of severe acute respiratory syndrome coronavirus antibodies in recruits from mainland China. Clin. Diagn. Lab. Immunol. 12:552-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng, F. Y., C. W. Chan, M. N. Chan, J. D. Chen, K. Y. Chow, C. C. Hon, K. H. Hui, J. Li, V. Y. Li, C. Y. Wang, P. Y. Wang, Y. Guan, B. Zheng, L. L. Poon, K. H. Chan, K. Y. Yuen, J. S. Peiris, and F. C. Leung. 2003. The complete genome sequence of severe acute respiratory syndrome coronavirus strain HKU-39849 (HK-39). Exp. Biol. Med. (Maywood) 228:866-873. [DOI] [PubMed] [Google Scholar]