Abstract
Recombinant human prolactin (rhPRL) was administered to huPBL-SCID mice to determine its effects on production of human immunoglobulin (Ig). The huPBL-SCID mice were injected intraperitoneally (i.p.) with 10 μg rhPRL every other day for a total of 10 injections. The results reconfirmed that rhPRL significantly increased the numbers of human CD3+ T cells and human CD19+ B cells in spleens, lymph nodes, and thymuses of huPBL-SCID mice. The huPBL-SCID mice were then concurrently given various doses of diphtheria-tetanus (DT) vaccine and 10-μg i.p. injections of rhPRL and were examined for the presence of human DT-specific proliferation of lymph node cells in vitro and antibody production in vivo. rhPRL greatly improved the engraftment of functional human lymphocytes (CD3+ T cells and CD19+ B cells) in DT-immunized huPBL-SCID mice. The rhPRL-treated, DT-immunized huPBL-SCID mice produced significantly larger amounts of DT-specific antibodies in response to the vaccine. The predominant Ig isotype induced after immunization was IgG. Thus, rhPRL stimulation promotes human secondary IgG responses in huPBL-SCID mice.
Growth hormone and prolactin (PRL) have been shown to exert similar immunohematopoiesis-promoting effects to those of conventional hematopoietic cytokines (4, 19). Specific depression of PRL release by bromocriptine or the presence of anti-PRL antibodies was associated with decreased T-cell function (10). It was noted that PRL increased the proliferation of NK, T, and B cells in response to mitogenic stimuli, such as interleukin-2 (IL-2), phytohemagglutinin (PHA), and Staphylococcus aureus Cowan strain 1, respectively (8). Treatment with PRL in serum-free medium independently or synergistically with IL-2 enhanced the natural cytotoxicity of human NK and lymphokine-activated killer cells to tumor targets (7). PRL was reported to improve stem cell differentiation in a semisolid colony assay system (5). We also observed that PRL administration increased the antigen-specific proliferation of lymph node T cells in both normal and dwarf mice (20).
However, the effects of prolactin on B cells have not been studied as extensively as the effects on T cells. Most investigations come from systemic lupus erythematosus (SLE)-related studies. Elevated prolactin levels and serum anti-DNA antibodies have been found in 15 to 25% of patients with SLE (2, 11, 13-15, 29, 30). It has also been demonstrated that both nonstimulated and mitogen-stimulated lymphocytes from patients with lupus secrete more prolactin than do control lymphocytes (9, 12). Bromocriptine, a drug that blocks prolactin secretion by the anterior pituitary gland, was suggested to have a beneficial effect in patients with SLE in small clinical trials (3, 15). In order to study survival and activation of different populations of autoreactive B cells and the effects of prolactin on B cells, particularly anti-DNA production in SLE, an R4A-γ2b mouse model was established and well characterized (24, 28). Using this model, it was found that a twofold increase in serum prolactin induced a lupus-like illness similar to that seen in patients with SLE. In R4A-γ2b BALB/c mice, treatment with prolactin induced an increased number of transgene-expressing B cells, with a resulting rise in serum anti-DNA titers and immunoglobulin G (IgG) deposits in the glomeruli. The anti-DNA B-cell population present in prolactin-treated mice displayed a follicular B-cell phenotype, and the expansion of transgene-expressing B cells was evident in the follicles. The impact of prolactin on autoreactive B cells was abrogated in the absence of CD4+ T cells, demonstrating that the survival, expansion, and activation of anti-DNA B cells are T cell dependent (24, 28).
Until now, most experiments have been done in vitro or with animals, and we need further studies with humans or human-related experimental systems. The engraftment of normal human lymphocytes into mice with severe combined immune deficiency (SCID) offers an invaluable means for examining their development and immune function in an in vivo setting (6, 17). These mice lack mature T- and B-cell function and are incapable of rejecting a solid tissue graft. huPBL-SCID mice were injected intraperitoneally (i.p.) with mature human lymphocytes, and the human cells persisted in these mice for months, could be detected in the peritoneums and peripheral lymphoid organs of the mice, and were capable of mounting antigen-specific secondary responses to various recall antigens (18). Thus, we believe that this animal model is the best for evaluating the adjuvant effect of prolactin in vivo.
Here we assess the effects of recombinant human PRL (rhPRL) treatment on the human immunologic response following rechallenge with the diphtheria-tetanus (DT) vaccine in huPBL-SCID mice, an extension of our recent study which demonstrated that rhPRL improved the reconstitution of human lymphocytes (25) and the antitumor effects of NK cells in huPBL-SCID mice (34). We report here that rhPRL treatment also promotes the secondary Ig response to DT vaccine in this human/mouse chimera.
MATERIALS AND METHODS
Mice.
CB.17 SCID mice were obtained from the National Animal Production Center (Beijing, China) and used at 8 to 12 weeks of age. SCID mice were housed in microisolator cages, and all food, water, and bedding were autoclaved before use. SCID mice were kept under specific-pathogen-free conditions at all times. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (23a).
Creation of huPBL-SCID mice.
All healthy donors (13 donors, all male, 23 ± 1.3 years old) of human peripheral blood lymphocytes (PBLs) were immunized with DT vaccine in childhood according to the Immunization Protocol of the Ministry of Health of China, and samples obtained from the Shandong Blood Bank were screened for human immunodeficiency virus type 1 and hepatitis B virus, with all donors providing informed consent before donation. Leukapheresis was performed by the Shandong Blood Bank Apheresis Unit in accordance with the tenets of the Declaration of Helsinki. The mononuclear cells in leukocytes were further separated from other cell types by using a Ficoll gradient, giving >90% purity of lymphocytes. PBLs (50 × 106) were injected intraperitoneally in 0.5 ml phosphate-buffered saline into CB.17 SCID mice. All mice received 20 μl of aASGM1 (Wako Chemicals, Dallas, TX) intravenously the day before injection of human PBLs, which has been verified to improve engraftment by removing host NK cells (21). Human PBLs used in each experiment were from different donors, and there were four or five mice per experimental group.
Immunization protocol.
rhPRL was generously provided by William J. Murphy from NCI-Frederick, NIH, Frederick, MD, and its quality and usage have been verified in previous studies (32, 33). Mice were injected i.p. with 10 μg rhPRL every other day for 20 days (a total of 10 injections, starting on day 1), as previously reported (25). Mice without rhPRL treatment received i.p. injections of Hanks balanced salt solution (HBSS). Mice were immunized i.p. with different doses of a DT vaccine (Connaught Laboratories Inc., Swiftwater, PA) on day 1 and day 14. The DT vaccine consisted of both diphtheria toxin and tetanus toxin at concentrations of approximately 67 ng of diphtheria toxin and 50 ng of tetanus toxin per 1 μl of vaccine. Blood samples were collected on day 28 to determine the amount of human DT-specific antibodies or total immunoglobulin by using an enzyme-linked immunosorbent assay (ELISA).
ELISA for DT-specific antibodies.
A 96-well round-bottomed plate with 100 μl/well of DT solution at a final concentration of 100 ng/well was placed overnight at 4°C. After the plate was washed three times and dried, 200 μl blocking buffer was added and incubated for 2 h. The blocking buffer consisted of phosphate-buffered saline with 5% filtered chicken serum. The plate was washed again, and then the test samples or a normal human serum control was added, with dilution to 1:10, 1:100, and 1:1,000, and incubated for 2 h at 37°C. The plates were then washed again, and a mixture of goat anti-human IgG and IgM (alone or together) conjugated to alkaline phosphatase (100 μl) (Sigma) was added and incubated for another hour at room temperature. After a wash step, a phosphatase substrate solution (Sigma) was added at 100 μl/well, and the color was allowed to develop until the positive control reached an optical density (OD) of close to 1.0. The plates were then read at 450 nm. Data are presented as ODs for a dilution of serum of 1:10 or 1:100. Since there is no positive control monoclonal antibody to DT, the data have to be presented as OD values.
Flow cytometry analysis.
Spleens, thymuses, and lymph nodes (pooled mesenteric, axillary, and inguinal lymph nodes) were harvested on day 28 after huPBL transfer, and single-cell suspensions were prepared and analyzed by double-color flow cytometry analysis as previously described (22). Reagents used included fluorescein isothiocyanate-labeled anti-HLA-ABC and phycoerythrin-labeled anti-CD3 or -CD19. All antibodies were obtained from Becton Dickinson (Mountain View, CA). The cells were fixed in 2% paraformaldehyde and analyzed on an EPICS flow cytometer. Each fluorescence study had directly labeled double-negative isotype controls of normal rat immunoglobulin.
DT-specific proliferation and mitogen assays of lymph node cells.
On day 1 after huPBL transfer, the huPBL-SCID mice received rhPRL injections i.p. and then were immunized i.p. with DT. On day 28, the lymph node cells were harvested, and the cell suspension was stimulated with DT or a mitogen (PHA or lipopolysaccharide assay) in vitro. DT (10 ng/ml), PHA (10 μg/ml; Sigma), or lipopolysaccharide (10 μg/ml; Sigma) and lymph node cells (1 × 106/200 μl/well) were added to flat-bottomed 96-well plates (Costar). Three days (for mitogen assay) or 5 days (for DT-specific proliferation assay) later, proliferation was assayed by pulsing cells with 1 mCi (3.7 × 104 becquerels) of [3H]thymidine (6.7 Ci/mmol; New England Nuclear, Boston, MA) for 8 h and harvesting them with a MASH II apparatus (Microbiological Associates, Bethesda, MD). Student's t test was performed to determine statistical differences, with P values of <0.05 being considered significant.
Statistical analysis.
All experiments were performed at least three times and had at least three mice per group, and results of representative experiments are shown. Student's t test was performed to determine if values differed significantly (P < 0.05 or P < 0.01). Error limits are identified as standard deviations (SD) in both the tables and the figures.
RESULTS
rhPRL improves the development and function of human lymphocytes in immunized huPBL-SCID mice.
In agreement with our previously published results (25), rhPRL improved the engraftment of lymphocytes into thymuses, lymph nodes, and spleens, as the cellularities of these organs increased. In order to observe the effects of rhPRL on the secondary Ig response in huPBL-SCID mice, we further immunized the huPBL-SCID mice with DT vaccine. Some of the SCID recipients received 1 × 108 huPBL, followed by either rhPRL (10 μg i.p. every other day for 20 days) or HBSS alone. The huPBL-SCID mice were immunized i.p. with different doses of DT vaccine on day 1 after huPBL transfer and then reimmunized on day 14. Four weeks after cell transfer, cells from various lymphoid organs of the SCID recipients were harvested and analyzed for the presence of human cells, as detected by flow cytometric analysis. The results demonstrated that rhPRL improved the engraftment of lymphocytes into thymuses, lymph nodes, and spleens, showing that the cellularities of these organs increased, although the cellularities tended to vary depending on the donor. The amounts of human T cells (HLA-ABC+ CD3+) increased greatly in the thymus (8.92-fold), spleen (4.64-fold), and lymph nodes (6.28-fold) after rhPRL injections. The amounts of human B cells (HLA-ABC+ CD19+) also increased greatly in lymph nodes (5.08-fold) and the spleen (1.97-fold). The results further indicated that rhPRL may promote lymphocyte engraftment in mice being rechallenged with recall antigens.
We then examined the potential of lymphocyte proliferation in response to DT stimulation. It was noted that the PBLs from healthy human donors exhibited greater DT-specific proliferation in vitro after rhPRL treatment before the cells were transferred into SCID mice (160.00% increase) (Table 1), suggesting that rhPRL improves the recall response in vitro. We then treated the DT-immunized huPBL-SCID mice with rhPRL for 20 days, and on day 28, we harvested the lymph nodes and again analyzed the engrafted human lymph node cell response to DT stimulation in vitro. The cells from lymph nodes of rhPRL-treated, DT-immunized huPBL-SCID mice were stimulated with DT vaccine in vitro for 3 days. As shown in Table 2, the proliferation of lymphocytes from the rhPRL-treated huPBL-SCID mice was much greater in response to DT stimulation than that of cells without rhPRL treatment in vivo (163.36% increase), as [3H]thymidine incorporation significantly increased, indicating that rhPRL promoted the functional development, in addition to the engraftment, of human lymphocytes in vivo in immunized huPBL-SCID mice.
TABLE 1.
DT-specific proliferation of human PBLs in vitroa
| Group | DT stimulation | [3H]thymidine uptake (cpm)b |
|---|---|---|
| PBLs | − | 1,908 ± 87 |
| + | 6,981 ± 454 | |
| PBLs plus rhPRL | − | 1,829 ± 128 |
| + | 11,170 ± 528* |
Human PBLs were obtained from healthy donors, and the cell suspension was used for a DT-specific proliferation assay in vitro. DT (10 ng/ml) and lymphocytes (5 × 106/200 μl/well) were added to flat-bottomed 96-well plates (Costar). Five days later, proliferation was assayed by pulsing cells with 1 mCi (3.7 × 104 becquerels) of [3H]thymidine (6.7 Ci/mmol; New England Nuclear, Boston, MA) for 8 h and harvesting them with a MASH II apparatus (Microbiological Associates, Bethesda, MD). Student's t test was performed to determine statistical differences.
Data are means ± SD. *, P < 0.01, comparing the PBL-plus-hPRL group with the PBL group for responses to DT stimulation.
TABLE 2.
DT-specific proliferation of lymph node cells from DT-immunized huPBL-SCID micea
| Group | DT stimulation | [3H]thymidine uptake (cpm)b |
|---|---|---|
| PBLs | − | 2,565 ± 168 |
| + | 9,086 ± 272 | |
| PBLs plus rhPRL | − | 1,939 ± 203 |
| + | 14,843 ± 804* |
huPBL-SCID mice were created and then immunized with DT as described in the legend to Fig. 1. Ten micrograms of rhPRL or HBSS was injected i.p. every other day for a total of 20 days. On day 28 after huPBL transfer, lymph nodes (axillary, inguinal, and mesenteric) were harvested, and the cell suspension was used in a DT-specific proliferation assay in vitro. DT (10 ng/ml) and lymph node cells (1 × 106/200 μl/well) were added to flat-bottomed 96-well plates (Costar). Five days later, proliferation was assayed by pulsing cells with 1 mCi (3.7 × 104 Bq) of [3H]thymidine (6.7 Ci/mmol; New England Nuclear, Boston, MA) for 8 h and harvesting them with a MASH II apparatus (Microbiological Associates, Bethesda, MD). Student's t test was performed to determine statistical differences.
Data are means ± SD. *, P < 0.01, comparing PBL-plus-hPRL group with PBL group in response to DT stimulation.
PRL enhances specific secondary Ig production in response to DT vaccine in human/SCID chimeras.
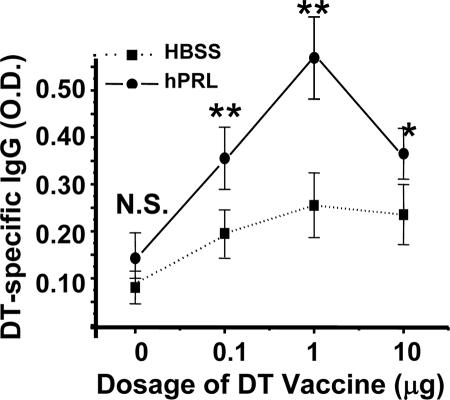
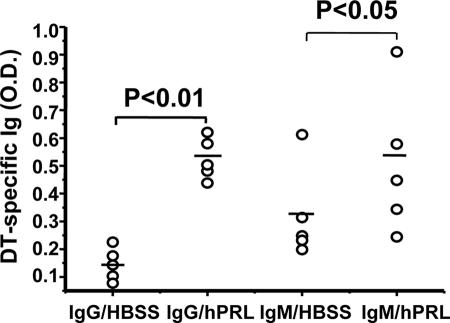
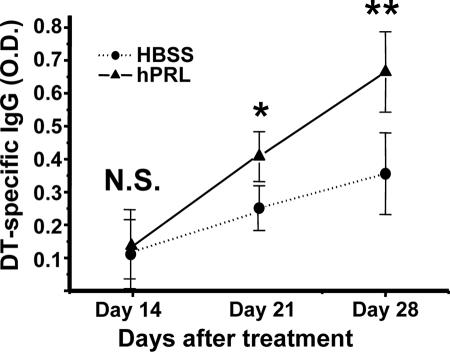
The huPBL-SCID mouse has been demonstrated to be capable of mounting a human secondary Ig response to a variety of immunogens after immunization (31). Because rhPRL may improve the engraftment of human lymphocytes into lymphoid organs and promote general lymphocyte function, as reported previously (26, 34), and the DT-specific proliferating response (Table 2) after in vivo treatment, we wanted to further observe if rhPRL acts as an adjuvant in DT immunization. It was noted that even without DT vaccine rechallenge, the baseline of DT-specific IgG was elevated after rhPRL treatment in huPBL-SCID mice (Fig. 1) (not a significant difference). The huPBL-SCID mice were then rechallenged with different doses of DT vaccine, and we found that rhPRL significantly enhanced IgG production if mice were stimulated with DT (Fig. 1), with an optimal dosage of 1 μg/mouse. The optimal dose of rhPRL significantly promoted IgG (P < 0.01) and IgM (P < 0.05) production, although there was large variation in Ig production levels for different mice (Fig. 2). The IgG production kinetics demonstrated that at longer times after the second immunization, there was more IgG production, and rhPRL significantly (P < 0.05 for day 21; P < 0.01 for day 28) promoted DT-specific IgG production after rechallenge by DT immunization compared with that at day 14 (Fig. 3), further suggesting that rhPRL may act as an adjuvant in human vaccine immunization.
FIG. 1.
rhPRL improves production of DT-specific IgG after DT vaccine rechallenge of huPBL-SCID mice. huPBL-SCID mice were created and immunized as described in Materials and Methods. Ten micrograms of rhPRL or HBSS was injected i.p. every other day for a total of 20 days. On day 28 after huPBL transfer, sera were analyzed using a DT-specific ELISA as described in Materials and Methods. The experiment had four or five mice per group. The results are expressed in optical density units, as assessed on a spectrophotometer at 450 nm from a 1:100 dilution of each sample. rhPRL significantly (P < 0.05 for 0 μg and 10 μg of DT vaccine; P < 0.01 for 0.1 μg and 1 μg of DT vaccine) promoted DT-specific IgG production after rechallenge with DT immunization. **, P < 0.01; *, P < 0.05 (comparing PRL with HBSS group in response to DT injection).
FIG. 2.
Production of DT-specific IgG and IgM after DT vaccine rechallenge of huPBL-SCID mice treated with rhPRL. huPBL-SCID mice were created and immunized as described in Materials and Methods. Ten micrograms of rhPRL or HBSS was injected i.p. every other day for a total of 20 days. On day 28 after huPBL transfer, sera were analyzed using a DT-specific ELISA as described in Materials and Methods. There were four or five mice per group. The results are expressed in optical density units, as assessed on a spectrophotometer at 450 nm from a 1:100 dilution of each sample. rhPRL significantly (P < 0.05 for IgM production; P < 0.01 for IgG production) promoted DT-specific antibody (IgG and IgM) production after rechallenge with DT immunization.
FIG. 3.
Time course of DT-specific IgG production in huPBL-SCID mice after treatment with rhPRL. huPBL-SCID mice were created and immunized as described in Materials and Methods. Ten micrograms of rhPRL or HBSS was injected i.p. every other day for a total of 20 days. On days 14, 21, and 28 after huPBL transfer, sera were analyzed using a DT-specific ELISA as described in Materials and Methods. The experiment shown had four or five mice per group. The results are expressed in optical density units, as assessed on a spectrophotometer at 450 nm from a 1:100 dilution of each sample. rhPRL significantly (P < 0.05 for day 21; P < 0.01 for day 28) promoted DT-specific IgG production after rechallenge with DT immunization compared with that on day 14.
Because total human IgG and IgM levels, and also DT-specific IgG and IgM levels, without challenge with DT vaccine, were reported in our previous publication (25), we compared these results in more detail. The results demonstrated that total human IgG and IgM increased about 20-fold after PRL injection, regardless of vaccination, but that DT-specific IgG increased only about twofold, and DT-specific IgM even decreased, without vaccination (25). With vaccination, the DT-specific IgG and IgM levels increased 3.7-fold and 1.75-fold, respectively (Fig. 1 and 2). Thus, PRL improved specific antibody production.
DISCUSSION
We report here that rhPRL can promote human lymphocyte engraftment into peripheral lymphoid organs and improve the secondary Ig response to DT vaccine, a T-cell-dependent vaccine, in a human/mouse model. This is the first report to demonstrate that rhPRL can promote the human B-cell response in an in vivo setting. These findings confirm and extend previous reports on the in vitro stimulatory effects of PRL on human B cells (16). Our results on the huPBL-SCID mouse response to recall antigens suggest that PRL may be of potential use as a B-cell adjuvant in vivo, particularly in promoting IgG responses. However, the baseline for DT-specific IgG was elevated by PRL even without DT vaccine (25), suggesting that the “adjuvant effects” of increasing the DT response cannot be separated from the impact of increased numbers of human cells. Since the increases of DT-specific IgG and IgM with DT vaccination were bigger than those without vaccination (Fig. 1 to 3), we think that PRL still has “adjuvant effects” in addition to improving effects on human cell survival or engraftment. Meanwhile, the “adjuvant effects” should include an increase in the critical mass of immune cells, possibly coming from recruitment, prolonged survival, or growth, in addition to promotion of the immune response (e.g., antigen-induced activation processes), so the adjuvant mechanisms of hPRL are complex and need further investigation in the future.
It is interesting that rhPRL activation allowed for the entry of human cells into the murine thymus. These findings are in agreement with a report that mature T cells could traffic to the murine thymus provided that they were activated (1), indicating that rhPRL may activate human T cells in vivo, which may explain why the secondary Ig response to DT vaccine is promoted by rhPRL treatment. Previously, it was reported that treatment of huPBL-SCID mice with human growth hormone resulted in the appearance of human cells in the SCID mouse thymus (23), and our present results demonstrate that rhPRL has the same effect on human T cells. Our preliminary data also demonstrate that when human prolactin and human IL-2 are given together, even greater thymic localization of human T cells is noted (data not shown), further suggesting that PRL play an important role in T-cell trafficking to the thymus.
A recent report suggested that human Ig production is initially polyclonal for up to 3 weeks after cell transfer, as determined by two-dimensional gel electrophoresis (27), suggesting that mice have the capability of producing a broader array of antibodies than was previously thought. It has been reported that huPBL-SCID mice are capable of responding to recall antigens, such as tetanus toxoid and diphtheria-tetanus toxoid (31), demonstrating that if huPBLs are obtained from previously immunized individuals, a predominantly IgG secondary response may appear when the mice are challenged again. In the present study, this model is again used practically to support the function of rhPRL on the DT-specific IgG secondary response.
Acknowledgments
rhPRL was generously supplied by William J. Murphy from NCI-Frederick, NIH.
This work was supported by the Natural Science Foundation of China (30571696) and the National 973 Science Program by the Ministry of Science and Technology of China (2004CB518807).
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Agus, D. B., C. D. Surh, and J. Sprent. 1991. Reentry of T cells to the adult thymus is restricted to activated T cells. J. Exp. Med. 173:1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, S. H., G. C. Sharp, G. Wang, C. Conley, Y. Takeda, S. E. Conroy, and S. E. Walker. 1996. Prolactin levels and antinuclear antibody profiles in women tested for connective tissue disease. Lupus 5:30-37. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Nemegyei, J., A. Cobarrubias-Cobos, F. Escalante-Triay, J. Sosa-Munoz, J. M. Miranda, and L. J. Jara. 1998. Bromocriptine in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled study. Lupus 7:414-419. [DOI] [PubMed] [Google Scholar]
- 4.Bazan, J. F. 1990. Structural design and molecular evolution of cytokine receptor superfamily. Proc. Natl. Acad. Sci. USA 87:6934-6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellone, G., M. Geuna, A. Carbone, S. Silvestri, R. Foa, G. Emanuelli, and L. Matera. 1995. Regulatory action of prolactin on the in vitro growth of CD34 positive human hemopoietic progenitor cells. J. Cell Physiol. 163:221-231. [DOI] [PubMed] [Google Scholar]
- 6.Bosma, G. C., R. P. Custer, and M. J. Bosma. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527-530. [DOI] [PubMed] [Google Scholar]
- 7.Cesano, A., E. Oberholtzer, M. Contarini, M. Geuna, G. Bellone, and L. Meteral. 1991. Independent and synergistic effect of interleukin-2 and prolactin in development of T- and NK-derived LAK effectors. Adv. Neuroimmunol. 1:158-172. [DOI] [PubMed] [Google Scholar]
- 8.Gala, R. R., and E. M. Shevach. 1993. Influence of prolactin and growth hormone on the activation of dwarf mouse lymphocytes in vivo. Proc. Soc. Exp. Biol. Med. 204:224-230. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez, M. A., J. F. Molina, L. J. Jara, M. L. Cuellar, C. Garcia, S. Gutierrez-Urena, A. Gharavi, and L. R. Espinoza. 1995. Prolactin and systemic lupus erythematosus: prolactin secretion by SLE lymphocytes and proliferative (autocrine) activity. Lupus 4:348-352. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann, D. P., J. W. Holaday, and E. W. Bernton. 1989. Inhibition of lymphocyte proliferation by antibodies to prolactin. FASEB J. 3:2194-2202. [DOI] [PubMed] [Google Scholar]
- 11.Jara, L. J., C. Gomez-Sanchez, L. H. Silveira, P. Martinez-Osuna, F. B. Vasey, and L. R. Espinoza. 1992. Hyperprolactinemia in systemic lupus erythematosus: association with disease activity. Am. J. Med. Sci. 303:222-226. [DOI] [PubMed] [Google Scholar]
- 12.Larrea, F., A. Martinez-Castillo, V. Cabrera, J. Alcocer-Varela, G. Queipo, C. Carino, and D. Alarcon-Segovia. 1997. A bioactive 60-kilodalton prolactin species is preferentially secreted in cultures of mitogen-stimulated and nonstimulated peripheral blood mononuclear cells from subjects with systemic lupus erythematosus. J. Clin. Endocrinol. Metab. 82:3664-3669. [DOI] [PubMed] [Google Scholar]
- 13.Lavalle, C., A. Graef, V. Baca, M. Ramirez-Lacayo, F. Blanco-Favela, and O. Ortiz. 1993. Prolactin and gonadal hormones: a key relationship that may have clinical, monitoring and therapeutic implications in systemic lupus erythematosus. Lupus 2:71-75. [DOI] [PubMed] [Google Scholar]
- 14.McMurray, R. W. 1996. Prolactin and systemic lupus erythematosus. Ann. Med. Interne 147:253-258. [PubMed] [Google Scholar]
- 15.McMurray, R. W., D. Weidensaul, S. H. Allen, and S. E. Walker. 1995. Efficacy of bromocriptine in an open label therapeutic trial for SLE. J. Rheumatol. 22:2084-2091. [PubMed] [Google Scholar]
- 16.Morales, P., M. V. Carretero, H. Geronimo, S. G. Copin, M. L. Gaspar, M. A. Marcos, and J. Martin-Perez. 1999. Influence of prolactin on the differentiation of mouse B-lymphoid precursors. Cell Growth Differ. 10:583-589. [PubMed] [Google Scholar]
- 17.Mosier, D. E., R. J. Gulizia, S. M. Baird, and D. B. Wilson. 1988. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335:256-259. [DOI] [PubMed] [Google Scholar]
- 18.Mosier, D. E. 1991. Adoptive transfer of human lymphoid cells to severely immunodeficient mice: models for normal human immune function, autoimmunity, lymphomagenesis, and AIDS. Adv. Immunol. 50:303-325. [DOI] [PubMed] [Google Scholar]
- 19.Murphy, W. J., R. Hallgeir, and D. L. Longo. 1995. Effects of growth hormone and prolactin: immune development and function. Life Sci. 57:1-14. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, W. J., S. K. Durum, M. R. Anver, and D. L. Longo. 1992. Immunologic and haematologic effects of neuroendocrine hormone: studies on DW/J dwarf mice. J. Immunol. 148:3799-3805. [PubMed] [Google Scholar]
- 21.Murphy, W. J., M. Bennett, M. R. Anver, M. Baseler, and D. L. Longo. 1992. Human-mouse lymphoid chimeras: host-vs.-graft and graft-vs.-host reactions. Eur. J. Immunol. 22:1421-1427. [DOI] [PubMed] [Google Scholar]
- 22.Murphy, W. J., K. C. Conlon, T. J. Sayers, R. H. Wiltrout, T. C. Back, J. R. Ortaldo, and D. L. Longo. 1993. Engraftment and activity of anti-CD3-activated human peripheral blood lymphocytes transferred into mice with severe combined immune deficiency. J. Immunol. 150:3634-3642. [PubMed] [Google Scholar]
- 23.Murphy, W. J., S. K. Durum, and D. L. Longo. 1992. Human growth hormone promotes engraftment of murine or human T cell into severe combined immune deficient mice. Proc. Natl. Acad. Sci. USA 89:4481-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 24.Peeva, E., D. Michael, J. Cleary, J. Rice, X. Chen, and B. Diamond. 2003. Prolactin modulates the naive B cell repertoire. J. Clin. Investig. 111:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun, R., J. Zhang, C. Zhang, J. H. Zhang, S. J. Liang, A. Y. Sun, J. F. Wang, and Z. G. Tian. 2004. Human prolactin improves engraftment and reconstitution of human peripheral blood lymphocytes in SCID mice. Cell. Mol. Immunol. 1:129-136. [PubMed] [Google Scholar]
- 26.Sun, R., H. Wei, J. Zhang, A. Li, W. Zhang, and Z. Tian. 2003 2002. Recombinant human prolactin improves antitumor effects of murine natural killer cells in vitro and in vivo. Neuroimmunomodulation 10:169-176. [DOI] [PubMed] [Google Scholar]
- 27.Tissot, J. D., P. Schneider, M. Schapira, and M. A. Duchosal. 1996. Human immunoglobulins produced in huPBL-SCID mice are polyclonal early after xenotransplantation. Cell. Immunol. 167:241-248. [DOI] [PubMed] [Google Scholar]
- 28.Venkatesh, J., E. Peeva, X. Xu, and B. Diamond. 2006. Cutting edge. Hormonal milieu, not antigenic specificity, determines the mature phenotype of autoreactive B cells. J. Immunol. 176:3311-3314. [DOI] [PubMed] [Google Scholar]
- 29.Vidaller, A., L. Llorente, F. Larrea, J. P. Mendez, J. Alcocer-Varela, and D. Alarcon-Segovia. 1986. T cell dysregulation in patients with hyperprolactinemia: effect of bromocriptine treatment. Clin. Immunol. Immunopathol. 38:337-343. [DOI] [PubMed] [Google Scholar]
- 30.Walker, S. E., R. W. McMurray, J. M. Houri, S. H. Allen, D. Keisler, G. C. Sharp, and J. A. Schlechte. 1998. Effects of prolactin in stimulating disease activity in systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 840:762-772. [DOI] [PubMed] [Google Scholar]
- 31.Walker, W., and G. Gallagher. 1994. The in vivo production of specific human antibodies by vaccination of human-PBL-SCID mice. Immunology 83:163-170. [PMC free article] [PubMed] [Google Scholar]
- 32.Welniak, L. A., Z. G. Tian, R. Sun, J. R. Keller, S. Richards, F. W. Ruscetti, and W. J. Murphy. 2000. Effects of growth hormone and prolactin on hematopoiesis. Leuk. Lymphoma 38:435-445. [DOI] [PubMed] [Google Scholar]
- 33.Woody, M. A., L. A. Welniak, R. Sun, Z. G. Tian, M. Henry, S. Richards, A. Raziuddin, D. L. Longo, and W. J. Murphy. 1999. Prolactin exerts hematopoietic growth promoting effects in vivo and partially counteracts myelosuppression by azidothymidine. Exp. Hematol. 27:811-816. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, J., R. Sun, H. Wei, and Z. Tian. 2005. Antitumor effects of recombinant human prolactin in human adenocarcinoma-bearing SCID mice with human NK cell xenograft. Int. Immunopharmacol. 5:417-425. [DOI] [PubMed] [Google Scholar]