Abstract
Helicobacter pylori antibodies were measured over 24 months in American Indian and Alaska Native persons who cleared their infections. Two months after treatment, 82% of H. pylori-negative persons remained seropositive. While there were declines in H. pylori antibodies for 12 months, after 24 months 71% of persons remained seropositive.
Helicobacter pylori eradication can be difficult, as reported rates of resistance to the commonly used antibiotics are 6% to 50% for clarithromycin and 12% to 61% for metronidazole (4, 5, 9). Tests to confirm treatment success, such as measuring H. pylori antibodies, are becoming clinically important. However, few serological studies have followed patients for longer than 12 months after eradication, and none have examined H. pylori antibodies over time in an Alaska Native (AN) or American Indian (AI) population. We measured H. pylori antibodies in AN/AI persons for 24 months after treatment. This study was approved by both the Centers for Disease Control and Prevention and Alaska Area Institutional Review Boards and the South Central Foundation Board of Directors.
H. pylori-infected persons were treated with an antibiotic regimen at the discretion of their medical providers. Blood was drawn and a urea breath test (UBT; Meretek Diagnostics, Inc., Nashville, TN) administered 2 months after treatment. Those who tested negative by UBT were enrolled in the 2-year follow-up study (7). Persons in the follow-up study were tested by UBT and had blood drawn 4, 6, 12, and 24 months after treatment. If a participant tested positive by UBT during follow-up, they were discontinued from the study.
Sera were tested for H. pylori-specific immunoglobulin G (IgG) by an in-house enzyme-linked immunosorbent assay (ELISA). This ELISA used 10 μg/ml of the high-molecular-weight cell-associated proteins described by Evans et al. as antigen (provided by Ezem, Inc., Westbury, NY) (2). Sera were diluted 1:200 and added to the plates. Antibodies were detected using alkaline phosphatase-labeled anti-human IgG (Sigma Chemical Co., St. Louis, MO) and p-nitrophenyl phosphate diluted in diethanolamine buffer (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). Optical density (OD) was measured at a 410-nm wavelength. To ensure assay reproducibility, a negative control serum and low- and highly positive control sera were tested on every plate (intra-assay variation, 9%, 5%, and 3%, respectively; interassay variation, 18%, 17%, and 11%, respectively).
Sera were positive for H. pylori-specific IgG if the OD was >0.5, negative if it was <0.3, and indeterminate if it was 0.3 to 0.5. We determined the cutoff values after repeated examination of 254 sera collected from Alaskan adults and children as part of a previous survey of H. pylori infection in Alaska (CDC, unpublished data). Using these cutoff values, the positive and negative predictive values of the ELISA optimized with sera from this unpublished survey were 89% and 93%.
Among 128 persons treated for an H. pylori infection who had sera available for testing, 90 (70%) eradicated their infection, as evidenced by a negative UBT. Among persons with H. pylori eradication, 79/90 (88%) had a decline in H. pylori-specific IgG between enrollment and 2 months after, compared to 22/38 (58%) persons who failed treatment. There was a decline in the mean H. pylori-specific IgG ODs in persons with H. pylori eradication but not in those without eradication, and persons with H. pylori eradication had smaller amounts of H. pylori antibody at 2 months than did those in whom H. pylori treatment failed (P < 0.0001). Despite the decline in H. pylori-specific IgG, 74 (82%) persons remained seropositive 2 months after eradication. Thus, the predictive value of 2-month positive serology identifying persons who failed treatment was only 30% (95% confidence interval [95% CI], 21 to 39%). The predictive value of 2-month negative serology identifying persons with successful treatment was 79% (95% CI, 57 to 100%).
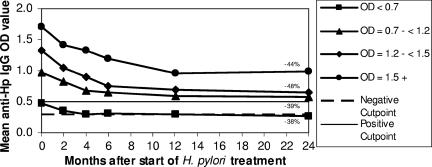
A total of 104 AN/AI participants were enrolled in the follow-up study. There were declines in H. pylori-specific IgG between enrollment and 2 months, 2 and 4 months, 4 and 6 months, and 6 and 12 months (P < 0.0002 for all four time intervals). Further declines were not seen after 12 months (P = 0.29 [12 months versus 24 months]). For all participants, the mean H. pylori-specific IgG declined 43%, from 1.13 OD units (95% CI, 1.04 to 1.23) at enrollment to 0.64 OD units (95% CI, 0.55 to 0.73) 24 months after the start of treatment. The percentages of decline were similar regardless of age and H. pylori-specific IgG OD at enrollment (Fig. 1).
FIG. 1.
Mean H. pylori-specific (anti-Hp) IgG ODs (measured at 0, 2, 4, 6, 12, and 24 months posttreatment) for 104 patients in follow-up after successful H. pylori eradication. Participants are categorized by the quartiles of their pretreatment H. pylori-specific IgG ODs. The percent decline in H. pylori-specific IgG OD from time zero to 24 months is indicated for each quartile. Data were obtained in Anchorage, AK, 1998-2002. For persons reinfected with H. pylori, the H. pylori-specific IgG OD was removed from the data beginning from the visit where reinfection was detected.
Two months after H. pylori treatment, 13% (11/86) of persons whose H. pylori infection was eradicated converted from seropositive for H. pylori antibodies to seronegative; this increased to 29% (18/63) 2 years after treatment. No persons with large amounts of antibody (>1.5 OD units) at enrollment became seronegative over 24 months, compared to 92% of those with smaller amounts of antibody (<0.7 OD units) (Table 1).
TABLE 1.
Percentages of seronegative persons among those who were urea breath test negative 2 years after H. pylori eradicationa
| OD units at study enrollment | % of seronegative persons (no. of seronegative persons/ total no. of persons) at 2 yrb |
|---|---|
| 0.0-<0.7 | 92 (12/13) |
| 0.7-<1.2 | 21 (3/14) |
| 1.2-<1.5 | 20 (3/15) |
| ≥1.5 | 0 (0/21) |
| Total | 29 (18/63) |
Participants are categorized by the quartiles of their pretreatment H. pylori-specific IgG ODs.
Data were collected in Anchorage, AK, 1998-2002. Data for 12 persons with indeterminant results at 2 years were removed.
This is the first study investigating H. pylori antibodies over time in an AI/AN population. We found that antibody dynamics posttreatment do not differ in this population compared with that in populations studied previously (3, 6, 8, 10). While the majority of studies followed patients for ≤12 months, our study followed participants for 24 months. In persons whose H. pylori infection was eradicated, we found a continuous decline in antibodies for 12 months, at which point the antibody decline ceased. We determined that less than one-third of persons became seronegative during the 24-month study period, similar to a study published by Cutler et al. that followed persons for >12 months (1).
The results of this study show that single IgG measurements should not be used to determine H. pylori treatment outcomes. In addition, antibodies remain circulating long after successful treatment, and therefore H. pylori-specific IgG antibodies should not be used to diagnose active infection in persons previously treated for an H. pylori infection. We have also been able to show that after treatment for an H. pylori infection, AI/AN persons have an antibody response that is similar to that for persons from other parts of the world.
Acknowledgments
Funding came from the Centers for Disease Control and Prevention, the Alaska Native Tribal Health Consortium, the Alaska Science and Technology Foundation, and the National Institutes of Health (DK53727, Native American Research Centers for Health, 1 U26 94 00005-01).
We thank Catherine Dentinger, Marilyn Getty, Jim Gove, Cindy Hamlin, and Susan Seidel for enrolling and monitoring study participants, Alice Muller for data management, and Bonnie Irwin for specimen management.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Cutler, A. F., V. M. Prasad, and P. Santogade. 1998. Four-year trends in Helicobacter pylori IgG serology following successful eradication. Am. J. Med. 105:18-20. [DOI] [PubMed] [Google Scholar]
- 2.Evans, D. J., D. G. Evans, D. Y. Graham, and P. D. Klein. 1989. A sensitive and specific serologic test for detection of Campylobacter pylori infection. Gastroenterology 96:1004-1008. [DOI] [PubMed] [Google Scholar]
- 3.Fallone, C. A., V. G. Loo, and A. N. Barkun. 1998. Utility of serology in determining Helicobacter pylori eradication after therapy. Can. J. Gastroenterol. 12:117-124. [DOI] [PubMed] [Google Scholar]
- 4.Kato, M., Y. Yamaoka, J. J. Kim, R. Reddy, M. Asaka, K. Kashima, M. S. Osato, F. A. K. El-Zaatari, D. Y. Graham, and D. H. Kwon. 2000. Regional differences in metronidazole resistance and increasing clarithromycin resistance among Helicobacter pylori isolates from Japan. Antimicrob. Agents Chemother. 44:2214-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim, J. J., R. Reddy, M. Lee, J. G. Kim, F. A. K. El-Zaatari, M. S. Osato, D. Y. Graham, and D. H. Kwon. 2001. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J. Antimicrob. Chemother. 47:459-461. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi, W., S. Tanabe, H. Imaizumi, K. Hibi, M. Kida, M. Ohida, I. Okayasu, and K. Saigenji. 2003. Effect of anti-Helicobacter pylori IgG antibody titer following eradication of Helicobacter pylori infection. Hepatogastroenterology 50:293-296. [PubMed] [Google Scholar]
- 7.McMahon, B. J., M. G. Bruce, T. W. Hennessy, D. L. Bruden, F. Sacco, H. Peters, D. A. Hurlburt, J. M. Morris, A. L. Reasonover, G. Dailide, D. E. Berg, and A. J. Parkinson. 2006. Reinfection after successful eradication of Helicobacter pylori: a 2-year prospective study in Alaska Natives. Aliment. Pharmacol. Ther. 23:1215-1223. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Perez, G. I., A. F. Cutler, and M. J. Blaser. 1997. Value of serology as a noninvasive method for evaluating the efficacy of treatment of Helicobacter pylori infection. Clin. Infect. Dis. 25:1038-1043. [DOI] [PubMed] [Google Scholar]
- 9.Vasquez, A., Y. Valdez, R. H. Gilman, J. J. McDonald, T. U. Westblom, D. Berg, H. Mayta, and V. Gutierrez. 1996. Metronidazole and clarithromycin resistance in Helicobacter pylori determined by measuring MICs of antimicrobial agents in color indicator egg yolk agar in miniwell format. J. Clin. Microbiol. 34:1232-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vikram, K., N. Anathakrishnan, and S. Badrinath. 2002. Chronological change in H. pylori antibody titers after treatment and its utility in follow-up. Am. J. Gastroenterol. 97:1563-1564. [DOI] [PubMed] [Google Scholar]