Abstract
Storage of high-quality cryopreserved peripheral blood mononuclear cells (PBMC) is often a requirement for multicenter clinical trials and requires a reproducibly high standard of practice. A quality assurance program (QAP) was established to assess an Australia-wide network of laboratories in the provision of high-quality PBMC (determined by yield, viability, and function), using blood taken from single donors (human immunodeficiency virus [HIV] positive and HIV negative) and shipped to each site for preparation and cryopreservation of PBMC. The aim of the QAP was to provide laboratory accreditation for participation in clinical trials and cohort studies which require preparation and cryopreservation of PBMC and to assist all laboratories to prepare PBMC with a viability of >80% and yield of >50% following thawing. Many laboratories failed to reach this standard on the initial QAP round. Interventions to improve performance included telephone interviews with the staff at each laboratory, two annual wet workshops, and direct access to a senior scientist to discuss performance following each QAP round. Performance improved substantially in the majority of sites that initially failed the QAP (P = 0.002 and P = 0.001 for viability and yield, respectively). In a minority of laboratories, there was no improvement (n = 2), while a high standard was retained at the laboratories that commenced with adequate performance (n = 3). These findings demonstrate that simple interventions and monitoring of PBMC preparation and cryopreservation from multiple laboratories can significantly improve performance and contribute to maintenance of a network of laboratories accredited for quality PBMC fractionation and cryopreservation.
Multicenter clinical trials of human immunodeficiency virus (HIV) and hepatitis C virus therapies and vaccines, as well as observational research, including natural history studies, require high-quality cryopreserved peripheral blood mononuclear cells (PBMC) for subsequent examination of immunological function. While real-time functional analysis of PBMC in local laboratories is stipulated by some multicenter clinical trials, batched analysis of cryopreserved PBMC at specialist laboratories is often preferable. It is well appreciated that PBMC populations are fragile and that poor handling during cryopreservation and thawing can compromise functional data relative to those obtained with freshly isolated cells (4, 8, 12-15). The process of PBMC isolation, storage, and recovery consumes considerable resources. Hence, it is reasonable to expect that investment of these resources should be linked to adequate, objectively assessed performance. In 1999, a multicenter quality assurance program (QAP) was established in laboratories across Australia to evaluate PBMC quality (yield, viability, and function) and to support participating laboratories to improve their performance. Since 2001, the QAP has used single-source blood donors (one HIV-positive and one HIV-negative donor) as well as local donors.
In the Australian setting, there are established networks of clinical trial sites located in the major population centers which have conducted many successful multicenter trials, several of which involved PBMC storage for immunological substudies. These networks have generally operated internal QAPs to monitor different methods used in these studies. However, given the move toward uniform quality assurance, we modeled our QAP on that of the NIH AIDS Clinical Trials Group (ACTG) to ensure that all laboratories participating in an Australian laboratory network could isolate and freeze PBMC to an agreed standard.
The designated performance standard required in this QAP was procurement of at least 5 × 106 PBMC from each 9-ml acid citrate dextran (ACD) blood tube provided, a postthaw PBMC viability of >80%, and a yield of viable PBMC of >50%. The initial performance at most laboratories was poor, leading to the introduction of specific interventions to address this inadequacy. Consistent with the ACTG experience (15), a few laboratories failed to make sufficient improvements in performance. However, most laboratories made substantial improvements in response to these interventions. The impact of these interventions and their effect on laboratory performance in this single-donor QAP are reported.
MATERIALS AND METHODS
Specimens and shipping.
A single clinic (St. Vincent's Hospital, Sydney, Australia) collected the donor samples, and a QAP coordinator (Wayne Dyer) at the Australian Red Cross Blood Service (ARCBS) in Sydney coordinated sample distribution and assessment of thawed PBMC and implemented strategies to improve laboratory performance. QAP rounds were performed twice yearly.
One HIV-infected and one healthy donor gave approximately 300 to 400 ml of whole blood for each round of the QAP. Blood was collected into ACD tubes (27 to 36 ml blood per laboratory). Inclusion criteria for the HIV-infected volunteers were a CD4+ T-cell count of >300 cells/μl, hemoglobin within the normal range for age and gender, low plasma HIV RNA (<5,000 copies/ml), being either on or off antiretroviral therapy, and no history of receipt of an immunomodulatory agent (e.g., recombinant interleukin-2, alpha interferon [IFN-α], hydroxyurea, or corticosteroids) within 6 months of donation, and those for the healthy donor were CD4+ T-cell counts and hemoglobin within the normal range. Blood tubes were shipped at ambient temperature to the participating laboratories by overnight courier service from Sydney to major cities in Australia (Melbourne, Brisbane, Adelaide, and Perth). Ambient temperatures were monitored with a Tinytag Transit unit (Gemini Data Loggers, Chichester, United Kingdom) placed inside the specimen container. Blood processing commenced at the same time at each laboratory site, approximately 24 h after the blood draw. From the fifth QAP round onward, a fresh blood specimen collected from a local HIV-negative donor was also included in the assessment as a backup in case of poor results from the shipped blood specimens. Following PBMC isolation and cryopreservation, frozen cryovials were returned on dry ice to ARCBS in Sydney for analysis. These procedures were approved by the St. Vincent's Hospital Human Research Ethics Committee, and informed consent was given by each donor.
Isolation and freezing of PBMC.
A laboratory protocol for PBMC cryopreservation was provided to assist all laboratories to achieve the required performance standard, with the added advantage that adherence to a best practice protocol may result in overall improved skills in PBMC handling at each site. Briefly, whole-blood tubes were centrifuged at 600 × g for 10 min. The buffy coats (from a single donor) were pooled into a 50-ml centrifuge tube and diluted with either RPMI cell culture medium (supplemented with 20 IU/ml penicillin, 20 μg/ml streptomycin, 25 mM HEPES, and 2 mM l-glutamine) or phosphate-buffered saline to 30 ml, and 15 ml of Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden) was layered beneath the buffy coat and medium mixture. Tubes were centrifuged at 400 × g for 20 min at 20°C with the brake off. PBMC bands from each tube were removed, placed into 50-ml centrifugation tubes, and washed twice with RPMI or phosphate-buffered saline. PBMC were counted manually in a hemacytometer or using an automated cell counter, and 1-ml aliquots (minimum of 5 × 106 PBMC/vial) were frozen in cryopreservation medium (10% dimethyl sulfoxide [DMSO] in 20 to 90% fetal calf serum [FCS], with the balance made up with RPMI), using either a controlled-rate freezer or a precooled Mr. Frosty unit (Nalgene, Rochester, NY) placed in a −80°C freezer for at least 4 h. Frozen cells were stored in liquid nitrogen until return shipment on dry ice to the central testing laboratory at ARCBS in Sydney.
Assessment of PBMC viability and function.
Cryopreserved PBMC were rapidly thawed, with gradual dilution of the cryopreservation medium with warm RPMI plus 10% FCS to 20 ml. After a second wash, cells were counted manually in the presence of trypan blue to determine viability, and the viable cell yield was determined by dividing the sum of the lymphocyte and monocyte counts, performed on an automated cell counter (CellDyn 3200; Abbott, Abbott Park, IL), by the number of PBMC claimed to have been placed in each vial. Cryopreserved PBMC from each QAP round were thawed and assessed on the same day by the same assessor.
A lymphocyte proliferation assay (LPA) (measuring the CD4 T-cell response) was initially chosen to assess cell function and was later supplemented with an enzyme-linked immunospot (ELISPOT) assay (measuring the CD8 T-cell response), using methods described previously (5). The ELISPOT assay was included because this method is now widely used to assess immune function in clinical trials, it measures the response of a different cell population, and more importantly, the intra- and interassay variations in background readings were much less in the ELISPOT than in the LPA, enabling better comparison of results between QAP rounds. In this study, thawed PBMC (1 × 105/well) were added to IFN-γ-coated ELISPOT plates (Mabtech, Nacka Strand, Sweden) and cultured with one of the following: a pool of 23 defined epitopes from cytomegalovirus, Epstein-Barr virus, and influenza virus (CEF peptides; Pepscan Systems, Lelystad, The Netherlands), at 2 μg/ml of each peptide (3); phorbol 12-myristate 13-acetate (PMA; 20 ng/ml) combined with ionomycin (100 ng/ml) as a positive control for maximum stimulation; or medium alone (RPMI plus 10% FCS) as the negative control. After 18 h of incubation, the plates were developed according to the manufacturer's instructions, and resulting spots were visualized on an automated ELISPOT reader (AID, Strassberg, Germany).
PBMC for the LPA were resuspended in LPA medium (RPMI plus 15% human serum), and 1 × 105 cells/well were added in triplicate to round-bottomed 96-well plates containing tetanus toxoid (2 flocculation units/ml) or inactivated influenza virus (A/Sydney/5/97; 200 hemagglutinating units/ml), both of which were kindly provided by CSL (Parkville, VIC, Australia), or containing medium as a control. Plates were cultured for 6 days, [3H]thymidine (1 μCi/well; NEN, Perkin-Elmer, Wellesley, MA) was added during the final 6 h of culture prior to harvesting of cells onto glass fiber-bottomed plates (Perkin-Elmer), and β-emission of incorporated thymidine was counted in a liquid scintillation counter (Top Count; Perkin-Elmer). Results were expressed as stimulation indexes (cpm of stimulus wells/cpm control wells).
To achieve accreditation in the QAP, each laboratory was required to provide at least one PBMC specimen that passed the following performance indicators in at least two of the three most recent QAP rounds: ability to fractionate at least 5 × 106 PBMC from each 9-ml ACD blood tube, PBMC viability of >80%, and viable yield of >50%. Functional assay results were considered in the final assessment only if these indicated poor cell quality not identified by the viability and yield results.
Statistical analysis.
Correlations between viability, yield, and function were determined by linear regression. Fisher's exact test was used to test the association between the presence of cell clumps and below-standard viability and yield results. Paired t tests were used to evaluate changes in performance between specific QAP rounds, and unpaired t tests were used to compare results within QAP rounds. To determine the overall improvement in performance throughout the course of the QAP, the slope of change was first calculated for each laboratory by linear regression analysis, and then a single sample t test was used to determine if the aggregate improvement was significantly different from zero, which is a preferable method for analyzing serial determinants from each site compared to using paired tests (9). These tests were performed separately for both viability and yield data for the combined single-donor specimens from each laboratory.
RESULTS
Viability and yield of PBMC.
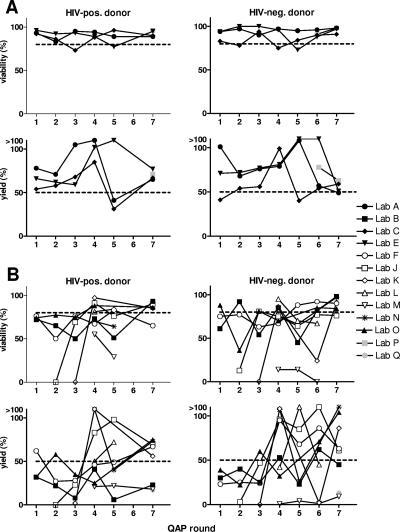
During the course of establishing this QAP, the number of participating laboratories increased from 9 to 13, with three laboratories dropping out of the QAP (results not reported) and five laboratories participating in all seven QAP rounds. Sequential viability and yield results for PBMC isolated from the HIV-positive and HIV-negative common donors are shown in Fig. 1. Fig. 1A shows results from laboratories that performed consistently throughout the course of the QAP, while the laboratories whose results are shown in panel B demonstrated different stages in achieving the desired quality standards. Considerable intra-QAP variability in yields was evident between laboratories, as well as inter-QAP variability in results from the same laboratory, whereas the spread in viability data narrowed to a relatively high standard over time (Fig. 1A).
FIG. 1.
Sequential assessment of viability and yield results from (A) laboratories that performed adequately throughout the QAP and (B) laboratories that either improved during the course of the QAP or failed to improve. Viabilities (upper panels) and yields (lower panels) of PBMC from HIV-positive (left panels) and HIV-negative (right panels) common donors supplied from each participating laboratory (same symbols in each panel) are shown. Dashed lines represent the required performance standards.
The complete records of results for each specimen tested, including the locally sourced donor PBMC, are displayed in Table 1. The ability to recover PBMC following isolation and freezing with a viability of >80% was achieved consistently by about half of the laboratories (n = 3) that participated in all seven QAP rounds. Two laboratories (J and K) performed poorly on the first attempt at the QAP exercise but made rapid improvements in both viability and yield in the subsequent round (P = 0.001 and P = 0.040, respectively), which were followed by a consistent overall trend in laboratory J (P = 0.014 and P = 0.015 for viability and yield, respectively). After the generally poor results in the second round, there was a gradual increase in viability toward the seventh QAP round as more laboratories attained the required performance standard (P = 0.013). Over the same time, the yield remained variable between sites and did not improve to the same extent (P = 0.107). Upon initiation of local donor specimen processing in the fifth QAP round, using data from laboratories that provided both local and single-donor PBMC, viability was higher in the local donor PBMC than in the single-donor PBMC (P = 0.030), but yield was not significantly increased (P = 0.148). However, this difference in quality was less obvious in subsequent rounds (P > 0.1). Overall, comparing results between the HIV-positive and HIV-negative donors, there was no significant difference in the average viability (P > 0.1) or yield (P > 0.1) over the course of the QAP.
TABLE 1.
Raw viability (V) and yield (Y) data (%) from the seven single-donor QAP roundsa
| Lab | Donor | QAP round
|
Improvement (slope)b,c
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
4
|
5
|
6
|
7
|
|||||||||||
| V | Y | V | Y | V | Y | V | Y | V | Y | V | Y | V | Y | V | Y | ||
| A§ | HIV+ | 93 | 78 | 83 | 142 | 95 | 105 | 94 | 111 | 89 | 41 | 89 | 65 | −0.13 | −5.88 | ||
| HIV− | 94 | 101 | 97 | 68 | 90 | 76 | 97 | 79 | 95 | 108 | 96 | 57 | 98 | 49 | 0.54 | −5.39 | |
| Local | 98 | 80 | 95 | 57 | 93 | 100 | |||||||||||
| B‡ | HIV+ | 72 | 32 | 65 | 22 | 50 | 8 | 73 | 41 | 51 | 6 | 93 | 23 | 2.94 | −1.24 | ||
| HIV− | 61 | 30 | 92 | 40 | 54 | 25 | 85 | 53 | 45 | 23 | 82 | 62 | 98 | 45 | 2.93 | 3.11 | |
| Local | 73 | 31 | |||||||||||||||
| C§ | HIV+ | 92 | 54 | 86 | 58 | 73 | 68 | 88 | 85 | 96 | 31 | 90 | 67 | 0.86 | 0.30 | ||
| HIV− | 83 | 41 | 78 | 54 | 94 | 56 | 75 | 99 | 84 | 40 | 90 | 54 | 91 | 59 | 1.38 | 1.36 | |
| Local | 84 | 66 | 88 | 44 | 85 | 73 | |||||||||||
| E§ | HIV+ | 96 | 66 | 92 | 62 | 93 | 59 | 89 | 102 | 77 | 119 | 95 | 77 | −0.96 | 4.49 | ||
| HIV− | 94 | 71 | 100 | 72 | 100 | 77 | 96 | 81 | 73 | 122 | 88 | 141 | 97 | 51 | −1.50 | 0.68 | |
| Local | 92 | 124 | 97 | 144 | 98 | 61 | |||||||||||
| F‡ | HIV+ | 76 | 62 | 50 | 27 | 76 | 28 | 67 | 46 | 83 | 49 | 65 | 67 | 0.56 | 3.21 | ||
| HIV− | 75 | 23 | 77 | 25 | 63 | 24 | 67 | 95 | 88 | 68 | 92 | 86 | 90 | 63 | 3.57 | 10.21 | |
| Local | 97 | 113 | 98 | 93 | 97 | 90 | |||||||||||
| J‡ | HIV+ | 0 | 0 | 69 | 23 | 91 | 83 | 76 | 98 | 90 | 69 | 14.31 | 15.37 | ||||
| HIV− | 13 | 81 | 47 | 75 | 96 | 64 | 85 | 77 | 116 | 76 | 61 | 8.34 | 3.20 | ||||
| Local | 84 | 69 | 94 | 74 | 96 | 61 | |||||||||||
| K‡ | HIV+ | 0 | 0 | 97 | 113 | 85 | 56 | 15.42 | 7.39 | ||||||||
| HIV− | 0 | 0 | 86 | 185 | 24 | 2 | 91 | 86 | 12.00 | 7.40 | |||||||
| Local | 64 | 58 | 35 | 13 | 97 | 108 | |||||||||||
| L | HIV+ | 81 | 50 | 84 | 72 | §§ | §§ | ||||||||||
| HIV− | 95 | 42 | 70 | 116 | 67 | 45 | −14.0 | 1.50 | |||||||||
| Local | |||||||||||||||||
| M | HIV+ | 55 | 21 | 29 | 22 | 18 | §§ | −1.14 | |||||||||
| HIV− | 14 | 0.6 | 14 | 4 | 0 | 1 | 9 | −7.00 | 2.22 | ||||||||
| Local | 96 | 127 | 65 | 26 | 95 | 49 | |||||||||||
| N‡ | HIV+ | 70 | 187 | 64 | 40 | 73 | §§ | −5.36 | |||||||||
| HIV− | 76 | 185 | 66 | 26 | 97 | 145 | 8.21 | 5.29 | |||||||||
| Local | 84 | 98 | 494 | ||||||||||||||
| O‡ | HIV+ | 74 | 32 | 77 | 58 | 75 | 35 | 88 | 25 | 86 | 75 | 2.22 | 5.33 | ||||
| HIV− | 88 | 39 | 36 | 22 | 81 | 60 | 70 | 32 | 85 | 71 | 84 | 104 | 2.94 | 10.25 | |||
| Local | 93 | 68 | 92 | 72 | |||||||||||||
| P | HIV+ | 96 | 71 | §§ | §§ | ||||||||||||
| HIV− | 91 | 78 | 98 | 63 | §§ | §§ | |||||||||||
| Local | 83 | 42 | 94 | 81 | |||||||||||||
| Q | HIV+ | 91 | 17 | §§ | §§ | ||||||||||||
| HIV− | 95 | 13 | §§ | §§ | |||||||||||||
| Local | 83 | 42 | 88 | 14 | |||||||||||||
| QAP round summary (mean ± SD) | HIV+ | 74 ± 11 | 54 ± 19 | 64 ± 32 | 47 ± 33 | 66 ± 30 | 40 ± 33 | 81 ± 13 | 68 ± 32 | 72 ± 21 | 51 ± 33 | 88 ± 9 | 57 ± 23 | ||||
| HIV− | 83 ± 13 | 51 ± 29 | 70 ± 33 | 47 ± 21 | 70 ± 32 | 46 ± 27 | 76 ± 23 | 71 ± 34 | 67 ± 25 | 61 ± 38 | 72 ± 31 | 60 ± 34 | 92 ± 7 | 58 ± 29 | |||
| Local | 85 ± 10 | 60 ± 29 | 84 ± 21 | 62 ± 30 | 94 ± 4 | 72 ± 28 | |||||||||||
For statistical analysis, the maximum yield was defined as 100%. Improvements in PBMC viability and yield from each laboratory (single-donor PBMC only) were calculated by linear regression analysis (slope), and overall improvement was determined from the slope data by a t test. Laboratories A, C, and E (§) retained a sufficient level of performance throughout each QAP round (P > 0.1). Most remaining laboratories showed substantial improvements (‡) in viability and yield results.
§§, insufficient data for slope analysis.
P values for a slope t test for all labs were 0.100 and 0.020 for viability and yield, respectively, and those for a t test for improved labs were 0.002 and 0.001 for viability and yield, respectively.
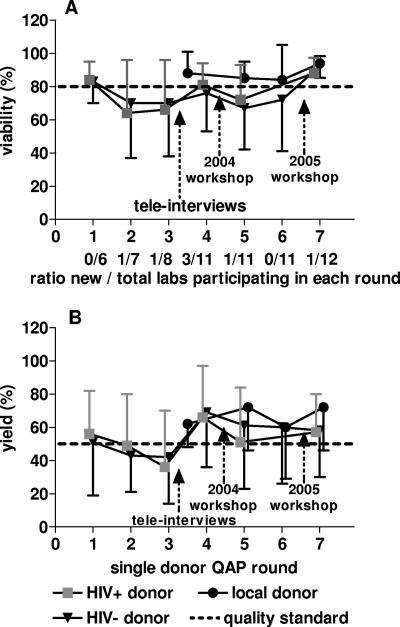
There was an overall trend toward improved viability and yield in the group of participating laboratories as a whole (Fig. 2), which can reasonably be attributed to specific interventions aimed at improving performance (presented below). Although the raw yield data (Table 1) displayed considerable overestimations of cell numbers for some samples submitted, for all analyses the maximum yield achievable was determined to be 100% (so as to not skew the average performance to a higher level and to reflect a theoretical range in yield between 0 and 100%). The number of new laboratories joining the QAP for the first time is also shown (Fig. 2). Poorer results from laboratories participating for the first time led to a reduction in the mean performance of the respective QAP round as a whole.
FIG. 2.
Summary (means and standard deviations) of viabilities (A) and viable yields (B) of PBMC prepared by the participating laboratories in each QAP round (yield data were calculated with the maximum yield being 100%). The ratio of new to total labs participating in each round is indicated. Initiatives to improve performance are indicated by arrows. The dashed lines represent the performance standards set for both viability and yield. Local donor results represent PBMC collected from an HIV-negative donor at the laboratory site.
Correlates of poor yield and viability.
One of the commonest findings across all QAP runs was that the presence of clumps in thawed PBMC samples was associated with low viability and yield. A significant association between the presence of cell clumps and a viability result of <80% was demonstrated in the third (P = 0.0114), fifth (P = 0.0086), and sixth (P = 0.0004) QAP rounds (Fisher's exact test), with a strong association (P < 0.0001) in the combined data from the second to seventh QAPs. A similar association between clumps and a yield result of <50% was evident in the second (P = 0.0101), third (P = 0.0114), fourth (P = 0.0037), and seventh (P = 0.0012) QAPs, with a strong association (P < 0.0001) in the combined data from the second to the seventh QAPs. Therefore, the possible causes of cell clumping in the thawed specimens were specifically targeted to improve performance in the QAP.
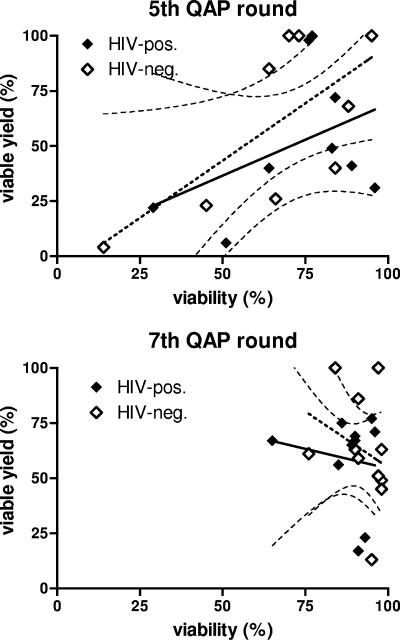
There was a direct correlation between yield and viability, as shown in results from the fifth QAP (Fig. 3). However, the overall improvement in viability in the seventh QAP round was not matched with the same improvement in yield (P > 0.1). Similarly, cell function results from the seventh QAP were of a uniformly high standard, with more variability in results from the fifth QAP. Given that cell clumps were removed before assessment of viability, yield, and function, the uniformly high viability and function results in the seventh QAP indicated that the likely cause of low yield (clumping [P < 0.0001]) did not impact cell quality (viability [P = 1.000]). In the earlier QAP rounds, we observed an association between reduced function and a high percentage of dead suspended cells and gross cell clumping. This suggested that the methods employed by the laboratory staff in the earlier QAP rounds impacted PBMC quality.
FIG. 3.
Association between viability and viable cell yield, revealing differences in quality of thawed PBMC between QAP rounds. Linear regression and 95% confidence interval curves are shown (for data taken from the single-donor PBMC only). In the fifth QAP round, there was a significant correlation between viability and yield for the HIV-negative single-donor specimens (dashed line) (r2 = 0.455; P = 0.046), but it was not significant for the HIV-positive donor specimens (solid line) (r2 = 0.175; P = 0.263); however, overall significance was reached when data from both donors were combined (r2 = 0.289; P = 0.022). Low yield was not associated with viability (uniformly high) in specimens from the seventh QAP round, consistent with improvements in PBMC quality from this round.
Assessment of PBMC function.
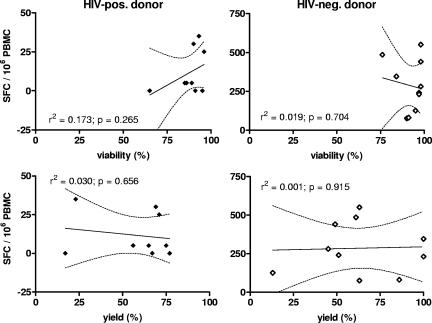
Functional data were assessed by LPA in the earlier QAP rounds and by ELISPOT assay in later QAP rounds. Functional ELISPOT data from the seventh QAP round demonstrated no significant association between viability or yield and cell function (Fig. 4). Responses to the CEF peptides from HIV-negative donor PBMC were uniformly detectable (285 ± 159 spots/106 PBMC) but below the detection limit of 50 spots/106 PBMC from the HIV-positive donor (CD4+ T-cell count, 713 ± 57). All samples from both donors responded maximally after exposure to PMA and ionomycin (data not shown). Data from the LPA (seventh QAP) were a little more variable, but the response to tetanus toxoid by PBMC from the HIV-negative donor was detectable, and the magnitude of this response was not associated with either viability or yield (data not shown). The response to influenza virus was similar to that to tetanus toxoid. The lack of correlation between viability, yield, or the presence of cell clumps and the functional data confirms the observation that PBMC samples from the seventh QAP were of uniformly high quality. However, a similar examination of the equivalent data from the fifth QAP round suggested a tendency toward lower function (LPA) and lower viability in the HIV-positive specimens (in parallel with correlations between cell clumps and viability, as mentioned above), but these observations did not reach statistical significance, possibly due to the generally low stimulation index values obtained from this QAP round. One observation from the fourth QAP round was an increase in background counts in the LPA associated with lower viability (P = 0.008). This was of concern because it suggested that lower viability was a reflection of lymphocyte quality, resulting in nonspecific activation, and not just contamination with granulocytes or other terminally differentiated cell populations.
FIG. 4.
As a consequence of uniformly high cell viability in the seventh QAP PBMC specimens, there was no association between viability or yield and immune function (determined by the IFN-γ response to the CEF peptide pool, as measured by ELISPOT assay). Linear regression and 95% confidence interval curves are shown (for data taken from the single-donor PBMC only). SFC, spot-forming cells.
Interventions to improve QAP performance.
Several interventions were made to improve QAP performance. These included detailed individual telephone interviews with participating laboratory staff and an annual centralized workshop in Sydney, Australia. The initial telephone interviews (conducted in 2003, between QAP rounds 3 and 4) revealed that laboratory staff members at certain sites were not strictly following the recommended protocol. Some of the practices identified were detrimental to PBMC quality and conflicted with the QAP protocol (summarized in Table 2).
TABLE 2.
Summary of practices that did not conform to the recommended protocol distributed to all participating labsa
| Procedure identified as being in conflict with the QAP protocol | Presence of procedure in laboratory
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | H | J | K | |
| Short Ficoll spin (<20 min) | X | ||||||||
| Inclusion of platelet deposit on tube adjacent to PBMC layer | X | X | X | X | X | ||||
| Non-cell culture-grade DMSO used | X | ||||||||
| Insufficient medium used for cell washing steps | X | ||||||||
| Inclusion of all white cells as the PBMC count | X | X | X | X | X | X | |||
| DMSO too concentrated (>10%) | X | ||||||||
| Freezing medium at ambient temperature when added to PBMC | X | ||||||||
| Ampoules not prechilled | X | X | X | ||||||
| PBMC kept in freezing medium too long before being frozen | X | ||||||||
| PBMC not stored at cryogenic temperatures (i.e., stored at >−150°C) | X | ||||||||
These practices were identified during telephone interview sessions. Each of these practices could have potentially reduced PBMC quality.
The second major intervention was an annual workshop. A wet workshop was held with at least one representative from each participating laboratory attending, including those from laboratories which had performed well. The workshop included an open forum to discuss the merits and disadvantages of variations in methods in relation to the QAP protocol. Emphasis was placed on methods to reduce cell clumping, either through minimizing granulocyte contamination or identifying any deviation from the protocol methods which may have inappropriately activated cells in the specimen. This included some of the issues raised during the telephone interviews (summarized in Table 2). A practical session also enabled participants to demonstrate their personal application of the protocol, thus exposing any potential misunderstanding that may have occurred.
To exclude the possibility that performance in laboratories outside Sydney was compromised by air transport of fresh blood specimens, a locally sourced HIV-negative blood donor specimen was collected by each laboratory and processed soon after collection. In order to resolve whether the ambient temperature range during transportation impacted quality, a temperature-monitoring device was sent with each interstate shipment of whole blood following the fourth QAP, and as a control, one was also sent with specimens to a Sydney laboratory. The temperature recorded in the local couriered sample within Sydney was remarkably consistent (22 to 24°C), whereas PBMC flown to cities other than Sydney experienced a temperature nadir as low as 12°C (which was identified to be due to warehouse conditions between the flight and morning delivery). However, the reduction in temperature during air transport did not correlate with performance in the QAP, since both adequate and inadequate performances were observed from laboratories both within and outside Sydney.
On the basis of paired data comparisons between single-donor results in specific QAP rounds, improved results appeared to be associated with both interventions (Fig. 2). The telephone interviews between the third and fourth QAPs resulted in improved viability (P = 0.067) and yield (P = 0.012), based on data from laboratories with results below standard in the third QAP. Likewise, matched data from laboratories with results below standard in the fifth QAP showed improved results in viability (P = 0.003) in the seventh QAP (after the 2005 workshop), but improvements in yield were not significant (P = 0.089). One confounding factor that potentially limited overall improvement between these QAP rounds involved high staff turnover at some laboratories.
In determining if the QAP resulted in overall improvements in viability and yield (single-donor data only) for laboratories that commenced the QAP with an acceptable performance standard (n = 3), this level of expertise did not change over the course of the QAP (viability, P = 0.947; yield, P = 0.675). Only two laboratories failed to make substantial improvements in either viability or yield, while another two did not improve their yield results (Table 1). However, the remaining laboratories with results below standard on the initial attempt at the QAP exercise made substantial improvements in viability (P = 0.002) and yield (P = 0.001) over the course of the QAP.
DISCUSSION
The objective of this QAP for PBMC was to provide accreditation to Australian laboratories for PBMC cryopreservation conforming to a quality standard and to raise performance to this standard across all sites. To help achieve this standard, a consensus laboratory protocol was implemented. In addition to the distribution of blood specimens from single HIV-positive and HIV-negative donors, a locally sourced fresh blood specimen was also included in the QAP. This sample was included to provide a backup in case of poor results from the single-donor specimens attributed to the time elapsed from blood collection or to effects of shipment on the specimen (1, 12, 14).
A common finding across all QAP rounds was that a low PBMC yield or viability was closely associated with clumping of the thawed PBMC sample. In earlier rounds, this was attributed to poor handling, which may have caused cell activation and cell-cell adherence. We noted from these early QAP rounds that PBMC samples with lower viabilities produced higher background counts in the LPA. Clumps of cells upon thawing were also the result of granulocyte contamination, which when mistakenly identified as PBMC during manual counting produced artificially poor yield and viability measurements. Since these were the major issues impacting on performance in the QAP, interventions to limit cell clumping were emphasized during the teleconference sessions and annual workshops. The following recommendations, based on experience and published studies, were made to address this issue: use a centrifugation speed appropriate for the density gradient product used, select a batch of Ficoll with high PBMC purification efficiency and low granulocyte contamination specifications, avoid harvesting the platelet aggregate that forms on the tube wall adjacent to the PBMC layer during the Ficoll step, minimize the amount of the Ficoll layer collected (as this may contain suspended granulocytes), resuspend cell pellets immediately after centrifugation, do not leave PBMC chilled for extended periods before freezing them, use cell culture-grade DMSO at 10% in the freezing medium, replace DMSO within 6 months of opening the container, select a serum batch for freezing medium that returns high cell viability (4), do not prechill PBMC before adding freezing medium (8), add cold DMSO medium (13) to the PBMC pellet immediately before freezing it, and commence the temperature-controlled freezing process from 4°C, not room temperature. Implementation of these recommendations was associated with improved performance. Based on specimens sent from laboratories without liquid nitrogen storage, short-term storage at noncryogenic temperatures (higher than −150°C) was acceptable, as other studies have similarly shown no loss in cell quality after short-term (10 weeks) storage of cryopreserved PBMC at −80°C (4) or as a result of shipment on dry ice for at least 3 days (4, 6).
Our results are in agreement with published QAP results showing that functional integrity of PBMC was more associated with viability (15) than with yield. Improved specimen handling in the recent QAP rounds produced PBMC samples with high viability and good functional readings, although cell clumping is an ongoing problem in some specimens. Further improvements in specimen quality may be achieved by selecting specific batches of Ficoll medium with high performance specifications (13). However, given the design of this QAP (shipped specimens processed at >24 h postcollection), a certain level of clumping in the thawed PBMC may be unavoidable (12).
The inclusion of a second measure of cell function (ELISPOT assay) in recent QAP rounds has provided useful information on cell quality that is relevant to many immunological substudies of clinical trials and cohort studies with HIV and hepatitis C virus. The ELISPOT assay is generally considered the gold standard for quantification of viral antigen-specific T-cell responses in stored PBMC specimens. Studies comparing the effects of different specimen handling protocols have based functional outcomes on either the LPA (4, 14, 15) or the ELISPOT assay (8, 12). It is important that the LPA and the ELISPOT assay measure immune responses from different cell populations (CD4 and CD8 T cells, respectively). In our experience of using both assay systems to assess PBMC function, the ELISPOT assay is a more reproducible assay than the LPA, whereas the LPA may be more informative in that results are more likely to decrease with reductions in cell quality. Increased background responses in both assay systems which are associated with reduced PBMC quality have also been reported in other studies (13). Hence, both assays have merit in a QAP to monitor operator effects on overall immune function of cryopreserved PBMC. Measurements of apoptosis have also been used to monitor the quality of cryopreserved PBMC (4, 6). High frequencies of PBMC undergoing apoptotic death have been observed in ex vivo blood samples from patients during primary HIV infection (11), in association with disease progression (10), and during other causes of excessive immune activation, and apoptotic markers increase upon delay in processing. Therefore, this assay would not be appropriate for a QAP based on overnight shipment of blood from HIV-positive donors. Flow cytometric determinations of immune function, including tetramer and intracellular cytokine staining, have proven reliable for analysis of cryopreserved PBMC (2) and may also be considered an assessment tool. Whichever functional readout is chosen to assess PBMC quality, it should be appropriate for the specimens provided and representative of the immune function assays for which PBMC are being stored.
This QAP for Australian laboratories demonstrates the value of careful monitoring of the cryopreservation skills of laboratory staff members who process PBMC for clinical trials and cohort studies. While large variations in expertise have also been observed in other QAPs (15), the interventions described here to improve PBMC handling skills resulted in substantial increases in viability and yield, along with cell quality, as determined by functional parameters. Based on data from the Multicenter AIDS Cohort Study specimens, showing an average viability of >90% after 12 years of storage (7), an expectation of high-quality PBMC preparations from our QAP is not unreasonable. The remaining issues to be addressed by the QAP therefore include increasing the acceptable standard for the yield of viable PBMC to higher levels (75% is the benchmark for the ACTG program) and maintaining a high standard of technical expertise at each laboratory site, despite the inevitable staff turnover. Given the need for high-quality cryopreserved PBMC for immune function studies, it is essential that PBMC QAPs continue to monitor laboratory performance and provide ongoing training and accreditation to such laboratories can demonstrate expertise in PBMC fractionation and cryopreservation. In summary, although improvements are still needed at some participating sites, we demonstrate that following several simple interventions, this QAP raised the performance of PBMC preparation and cryopreservation to a uniform and acceptable standard across diverse geographical sites in Australia.
Acknowledgments
We gratefully acknowledge the volunteers who donated blood for the QAP and thank the QAP participants at each laboratory, as follows: Jie Liu (Australian Red Cross Blood Service), Jacqueline Flynn and Rosemary Ffrench (Burnet Institute), Kate Merlin and Julie Yeung (CFI, St. Vincent's Hospital), Lina Papalia and Megan Barnden (CSL Ltd.), Sharon Dal-Cin and Peter Grantham (Gold Coast Hospital), Tina Mifsud and Sue Weir (Institute of Medical and Veterinary Science), Ajantha Solomon and Fiona Wightman (Monash University), Yong Pan and William Rawlinson (Prince of Wales Hospital), Gregory Bryson and Flavia Battistutta (Royal Brisbane Hospital), Steven Roberts and Martyn French (Royal Perth Hospital), Kerri Gallagher and Renfen Chen (Royal Prince Alfred Hospital), and Emma Jagger and Hui Li (University of New South Wales). We extend special thanks also to Matthew Law, National Centre in HIV Epidemiology and Clinical Research, for statistical advice; Karen MacRae and Helen Fraser, Immunology and Infectious Diseases Unit, St. Vincent's Hospital, for assistance with donor specimens; Alan Landay and Thomas Denny for advice on the ACTG QAP; and Emma Jagger and Suzy Teutsch, University of NSW, for administrative assistance.
This study was performed on behalf of the Immune-Based Therapies Working Group of the National Centre in HIV Epidemiology and Clinical Research and the Steering Committee of the Immunovirology Research Network (IVRN) of the Australian Centre for HIV and Hepatitis Virology Research (ACH2). The IVRN is funded by the Population Health Division of the Australian Commonwealth Department of Health and Aging via an operating grant to ACH2.
Footnotes
Published ahead of print on 18 October 2006.
REFERENCES
- 1.Betensky, R. A., E. Connick, J. Devers, A. L. Landay, M. Nokta, S. Plaeger, H. Rosenblatt, J. L. Schmitz, F. Valentine, D. Wara, A. Weinberg, and H. M. Lederman. 2000. Shipment impairs lymphocyte proliferative responses to microbial antigens. Clin. Diagn. Lab. Immunol. 7:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betts, M. R., J. P. Casazza, and R. A. Koup. 2001. Monitoring HIV-specific CD8+ T cell responses by intracellular cytokine production. Immunol. Lett. 79:117-125. [DOI] [PubMed] [Google Scholar]
- 3.Currier, J. R., E. G. Kuta, E. Turk, L. B. Earhart, L. Loomis-Price, S. Janetzki, G. Ferrari, D. L. Birx, and J. H. Cox. 2002. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 260:157-172. [DOI] [PubMed] [Google Scholar]
- 4.Disis, M. L., C. dela Rosa, V. Goodell, L. Y. Kuan, J. C. Chang, K. Kuus-Reichel, T. M. Clay, H. K. Lyerly, S. Bhatia, S. A. Ghanekar, V. C. Maino, and H. T. Maecker. 2006. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J. Immunol. Methods 308:13-18. [DOI] [PubMed] [Google Scholar]
- 5.Dyer, W. B., H. Kuipers, M. W. Coolen, A. F. Geczy, J. Forrester, C. Workman, and J. S. Sullivan. 2002. Correlates of antiviral immune restoration in acute and chronic HIV-1 infection: sustained viral suppression and normalisation of T cell subsets. AIDS Res. Hum. Retrovir. 18:999-1010. [DOI] [PubMed] [Google Scholar]
- 6.Fowke, K. R., J. Behnke, C. Hanson, K. Shea, and L. M. Cosentino. 2000. Apoptosis: a method for evaluating the cryopreservation of whole blood and peripheral blood mononuclear cells. J. Immunol. Methods 244:139-144. [DOI] [PubMed] [Google Scholar]
- 7.Kleeberger, C. A., R. H. Lyles, J. B. Margolick, C. R. Rinaldo, J. P. Phair, and J. V. Giorgi. 1999. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin. Diagn. Lab. Immunol. 6:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreher, C. R., M. T. Dittrich, R. Guerkov, B. O. Boehm, and M. Tary-Lehmann. 2003. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J. Immunol. Methods 278:79-93. [DOI] [PubMed] [Google Scholar]
- 9.Matthews, J. N. S., D. G. Altman, M. J. Campbell, and P. Royston. 1990. Analysis of serial measurements in medical research. BMJ 300:230-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandolfi, F., M. Pierdominici, A. Oliva, G. D'Offizi, I. Mezzaroma, B. Mollicone, A. Giovannetti, L. Rainaldi, I. Quinti, and F. Aiuti. 1995. Apoptosis-related mortality in vitro of mononuclear cells from patients with HIV infection correlates with disease severity and progression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9:450-458. [PubMed] [Google Scholar]
- 11.Roos, M. T., N. A. de Leeuw, F. A. Claessen, H. G. Huisman, N. A. Kootstra, L. Meyaard, P. T. Schellekens, H. Schuitemaker, and F. Miedema. 1994. Viro-immunological studies in acute HIV-1 infection. AIDS 8:1533-1538. [DOI] [PubMed] [Google Scholar]
- 12.Smith, J. G., X. Liu, R. M. Kaufhold, J. Clair, and M. J. Caulfield. 2001. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella-zoster virus. Clin. Diagn. Lab. Immunol. 8:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tree, T. I., B. O. Roep, and M. Peakman. 2004. Enhancing the sensitivity of assays to detect T cell reactivity: the effect of cell separation and cryopreservation media. Ann. N. Y. Acad. Sci. 1037:26-32. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg, A., R. A. Betensky, L. Zhang, and G. Ray. 1998. Effect of shipment, storage, anticoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin. Diagn. Lab. Immunol. 5:804-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg, A., L. Zhang, D. Brown, A. Erice, B. Polsky, M. S. Hirsch, S. Owens, and K. Lamb. 2000. Viability and functional activity of cryopreserved mononuclear cells. Clin. Diagn. Lab. Immunol. 7:714-716. [DOI] [PMC free article] [PubMed] [Google Scholar]