Abstract
Glucuronoxylomannan (GXM) is the major capsular polysaccharide of Cryptococcus neoformans. GXM receptors have been characterized in phagocytes and endothelial cells, but epithelial molecules recognizing the polysaccharide remain unknown. In the current study, we demonstrate that GXM binds to the CD14 receptor in human type II alveolar epithelial cells, resulting in the production of the proinflammatory chemokine interleukin-8.
Cryptococcus neoformans is an encapsulated fungal pathogen infecting mainly immunosuppressed patients. Infections are acquired by inhalation of desiccated cells, which are available in the environment as basidiospores or poorly encapsulated yeasts. Inhaled cells are deposited in the alveolar space, where they are ingested by macrophages (24) and interact with epithelial cells (2, 7, 8). An effective interaction of C. neoformans with epithelial alveolar cells is probably essential for the establishment of pulmonary infection. Microscopic studies of pulmonary cryptococcal infection reveal that yeast cells are in close apposition to lung epithelial cells (7). Once C. neoformans becomes established in the lung, it proliferates locally and causes a primary lesion that is usually contained by granuloma formation (10, 24). Two components of C. neoformans that are important for adhesion to human type II alveolar epithelial cells are phospholipase B (8) and glucuronoxylomannan (GXM) (2).
GXM is a virulence factor (4) that represents a potential vaccine component and is the target of therapeutic antibodies (3, 11, 17, 19). As the major constituent of the capsule, it is the primary component of a structure that is antiphagocytic and thus protects the fungal cell from immune cells. However, cryptococcal infections are also associated with the release of large amounts of GXM into host tissues, where they have many deleterious effects on the host immune response through multiple mechanisms (15). GXM has been reported to interact with numerous cellular receptors. Receptors for GXM in macrophages, neutrophils, and endothelial cells include Toll-like receptor 2 (TLR2), TLR4, CD14, and CD18 (12, 13, 16, 25). The receptors for GXM in epithelial cells, however, remain to be characterized.
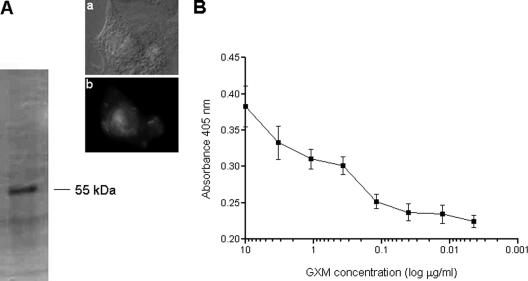
GXM was purified from strain T1-444 of C. neoformans (serotype A) by following standard methods (5) and incubated with A549 cells (100 μg/ml) for 1 h at 37°C (2). Binding of GXM to host cells was confirmed by immunofluorescence with the GXM-binding monoclonal antibody (MAb) 18B7 (3) (Fig. 1, inset). Lysates were obtained as described previously (21) and immunoprecipitated by sequential incubation with MAb 18B7 (3) and Sepharose-bound protein G. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the immunoprecipitated material indicated the presence of a major band with a molecular mass corresponding to 55 kDa (Fig. 1A). The same mixture analyzed by SDS-PAGE was boiled for 5 min to disintegrate protein-polysaccharide conjugates. GXM was then removed from this preparation by ultrafiltration (cutoff, 100 kDa), and the filtrate was incubated with Sepharose-bound protein G for antibody depletion. The purified fraction containing the GXM-binding protein, but no polysaccharide or antibodies, was concentrated to dryness and used to coat polystyrene 96-well plates (5 μg/ml, 100 μl/well). After the addition of GXM in serial dilutions, the plates were sequentially incubated for 1 h with MAb 12A1, a mouse immunoglobulin M (IgM) MAb with specificity for GXM (6), and a phosphatase-labeled goat anti-mouse antibody with specificity for IgM. Reactions were developed after the addition of p-nitrophenyl phosphate, followed by a reading at 405 nm. As demonstrated in Fig. 1B, the purified fraction binds GXM in a dose-dependent fashion.
FIG. 1.
Purification of a GXM-binding molecule from A549 cells by immunoprecipitation. (A) Protein extracts from GXM-treated human cells were sequentially incubated with MAb 18B7 and Sepharose-bound protein G for further analysis by SDS-PAGE. GXM binding to epithelial cells was confirmed by immunofluorescence with MAb 18B7 (inset). An A59 cell observed under differential interferential contrast (a) and fluorescence (b) modes is shown. A single major protein with a molecular mass corresponding to 55 kDa was detected. The purified fraction was used in GXM-binding assays in 96-well plates. (B) Dose-dependent binding of the 55-kDa protein to GXM detected by an IgM MAb to GXM.
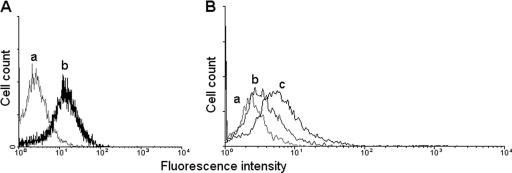
CD14 is a 55-kDa glycosylphosphatidylinositol-anchored membrane protein found mainly on cells derived from the monocyte/macrophage lineage, as well as neutrophils and B lymphocytes (8, 18). This molecule, which is a member of the heteromeric lipopolysaccharide (LPS) receptor complex that also contains TLR4 and MD2 (17), is a GXM receptor in endocytic cells (12, 16, 25). This information and the molecular mass of the purified GXM-binding protein presented in Fig. 1A led us to investigate whether CD14 could be the GXM receptor expressed by A549 cells. Human cells were detached from culture plates using 1 mM EDTA and fixed in 4% paraformaldehyde. After blocking with Tris-buffered saline containing bovine serum albumin, A549 cells were incubated with a phycoerythrin (PE)-labeled mouse MAb to CD14 (BD Biosciences, San Jose, CA). Analysis in a FACSCalibur (BD Biosciences, San Jose, CA) flow cytometer revealed that A549 cells express CD14 (Fig. 2A), confirming previous reports (9, 20, 23). Control cells, incubated with an irrelevant PE-labeled antibody, presented low levels of fluorescence.
FIG. 2.
Expression of CD14 by A549 cells. (A) Flow cytometric analysis of human cells incubated with a PE-labeled MAb to CD14 demonstrating that the receptor is expressed on the surface of A549 cells. Unstained (a) and antibody-treated (b) cells are shown. (B) Pretreatment of human cells with LPS, a CD14-binding compound, decreases their association with cryptococci. The increase in fluorescence after incubation of infected cells with MAb 18B7 was used to identify A549 cells in association with cryptococci. Infected cells that were pretreated with LPS (b) presented a reduced reaction with MAb 18B7, in comparison with untreated cells (c). The fluorescence levels of uninfected cells is shown in panel a.
Given our recent observations that C. neoformans efficiently infects A549 cells (2), we evaluated whether a CD14-binding molecule would interfere with this process. Untreated human cells or an A549 population that was pretreated with LPS (10 μg/ml) for 1 h was incubated with C. neoformans under previously established conditions (2). The index of association between cryptococci and alveolar cells was measured as the reactivity of A549 cells with MAb 18B7 in flow cytometry assays (2), which showed that the efficacy of the interaction of cryptococci with untreated cells was higher than that observed with LPS-treated epithelia (Fig. 2B). Controls consisted of similar preparations that were not infected with C. neoformans. Pretreatment of A549 cells with anti-CD14 antibodies also resulted in a lower association between C. neoformans and host cells (data not shown).
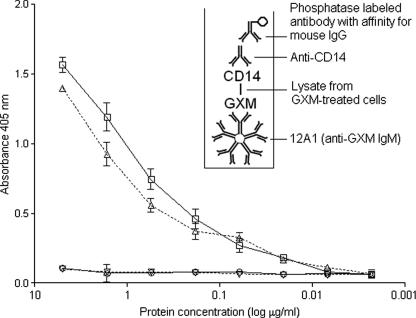
To confirm that CD14 is indeed a GXM-binding molecule in human alveolar cells, a modification of classical immunoprecipitation methods was used. Lysates of GXM-treated cells, presumably containing polysaccharide-CD14 complexes, were added to the wells of a 96-well plate previously coated with the anti-GXM IgM 12A1. After successive blocking and washing, the plate was incubated with a mouse MAb (IgG) to CD14 (BD Biosciences, San Jose, CA) and then with a phosphatase-labeled goat antibody with specificity for mouse IgG. CD14 was coprecipitated with GXM, as suggested by the dose-dependent recognition of complexes by the anti-CD14 antibody (Fig. 3). Controls consisted of similar assays in which lysates from GXM-treated cells were replaced by extracts from untreated alveolar cells. Additional controls included enzyme-linked immunosorbent assays (ELISAs) in which A549 lysates (positive control) and protein extracts from Chinese hamster ovary cells (negative control) were used to coat the plates, followed by blocking and sequential incubation with the anti-CD14 MAb and phosphatase-labeled secondary antibodies.
FIG. 3.
Identification of CD14 as the epithelial receptor for GXM in A549 cells. CD14-GXM complexes (triangles) from polysaccharide-treated cells were captured by IgM antibodies in ELISA. An anti-CD14 was used as the detection probe, as demonstrated in the schematic presentation of the capture ELISA (inset). No significant reactions with the anti-CD14 antibody were observed when protein extracts from untreated cells (inverted triangles) were used. Additional controls included an ELISA in which A549 lysates (positive control; squares) and protein extracts from Chinese hamster ovary cells (negative control; circles) were used to coat the plates, followed by sequential incubation with the anti-CD14 MAb and phosphatase-labeled secondary antibodies.
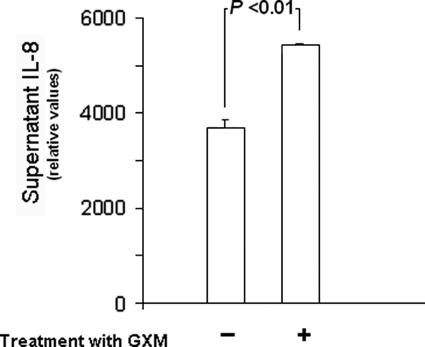
The association of microbial compounds with CD14 in alveolar cells can elicit the secretion of proinflammatory cytokines (20). We therefore speculated whether A549 cells could produce a cytokine response activated by GXM. For cytokine determinations, the culture medium was replaced by fresh medium containing no serum but supplemented with 10 μg/ml GXM. After 4 h at 37°C in a 5% CO2 atmosphere, culture supernatants were collected and assayed for cytokines by using a RayBio human cytokine antibody array (RayBiotech, Inc.). Procedures followed the manufacturer's protocol. Cytokine production was quantified by using Scion Image 2000 software (Scion Corporation, NIH). Twenty cytokines were assayed, and under our experimental conditions, the production of interleukin-8 (IL-8) by GXM-treated cells was considered to be significantly different from that detected in nonstimulated supernatants (Fig. 4). GXM induced the secretion of growth-related oncogene α and IL-3 by human cells (data not shown), although the results obtained with the control and GXM-treated cells were not significantly different.
FIG. 4.
Production of IL-8 by GXM-treated cells. Supernatants of untreated (−) or polysaccharide-treated (+) cells were collected for cytokine detection. An increased production of IL-8 was observed in supernatants of GXM-treated cells.
Since the CD14-LPS association resulted in IL-8 production by epithelial cells in previous studies (20), we investigated whether binding of GXM to CD14 molecules would have the same effect. Supernatants of nonstimulated A549 cells or of human cells after treatment for 1 h with LPS (positive control, 10 μg/ml), C. neoformans (2), or GXM (100 μg/ml) were collected, and the presence of IL-8 was assayed by ELISA-based techniques (human CXCL8/IL-8 detection kit; R&D Systems, Minneapolis, MN). Under any of the conditions used for chemokine determination, the viability of the A549 population was significantly affected (data not shown). A significant increase in IL-8 production was observed when fungal cells, GXM, or LPS was incubated with A549 cells (Table 1) . The levels of IL-8 produced under our experimental conditions were very similar to those observed in a previous study using LPS-treated A549 cells (14). Supplementation of the polysaccharide solution with polymyxin B produced similar (P = 0.18) levels of IL-8 (data not shown), indicating that the results obtained were not due to LPS contamination. When human cells had been previously incubated with antibodies to CD14, however, GXM-treated and nonstimulated cells expressed similar levels of IL-8 (Table 1).
TABLE 1.
Stimulation of A549 cells with GXM results in CD14-mediated production of IL-8a
| Treatment of A549 cellsb | Supernatant IL-8 (ng/ml) (mean ± SD) | Pc | Statistical significance |
|---|---|---|---|
| None | 0.82 ± 0.06 | ||
| LPS (10 μg/ml, 1 h) | 1.33 ± 0.01 | 0.007 | Yes |
| C. neoformans (10 yeast cells/host cell) | 1.06 ± 0.01 | 0.030 | Yes |
| GXM (100 μg/ml, 1 h)d | 1.18 ± 0.03 | 0.017 | Yes |
| Anti-CD14 (10 μg/ml), then GXM (100 μg/ml) | 0.54 ± 0.12 | 0.085 | No |
Results are representative of three different assays producing similar results. The error bars were generated from multiple trial wells (n = 3) of each single sample.
A549 cells were treated with LPS, fungal cells, GXM, or anti-CD14 antibodies for 1 h at 37°C.
P values were obtained by the use of Student's t test, after comparison with results of control systems (no stimulation). Differences between results with stimulated systems and control cells were considered statistically significant when P values were smaller than 0.05.
Results obtained for A549 cells treated with GXM alone were statistically compared to the data obtained for alveolar cells sequentially treated with the antibody to CD14 and GXM. IL-8 levels detected in cells after treatment with GXM alone were significantly higher (P < 0.01) than those obtained in A549 cells that were first treated with the antibody to CD14.
In microbial infections, a primary function of the airway epithelium is to act as a physical barrier for the exclusion of inhaled infectious propagules. However, there is increasing evidence that lung cells can induce a localized immune response through the production of a variety of mediators, including proinflammatory cytokines and chemokines (1). These mediators could act, for example, to recruit polymorphonuclear leukocytes from the pulmonary vasculature into the alveolar space. With fungi, it has been demonstrated that Aspergillus fumigatus proteases elicit IL-6 and IL-8 production in lung epithelial cells in vivo and in vitro (26). In cases of cryptococcosis, the production of these proinflammatory mediators is associated with the survival of human patients (22). The production of such molecules by the airway epithelium could therefore represent an effective mechanism for the establishment of a local immune response controlling microbial lung infections.
GXM receptors have been characterized in a number of cells (12, 13, 16, 25). This polysaccharide mediates the interaction of C. neoformans with different host cells and induces a deleterious effect on the immune system (15). Interactions of cryptococci with epithelial cells are influenced by GXM (2), but the host receptors involved in this process are unknown. The identification of CD14 as an epithelial molecule interacting with GXM with the consequent production of IL-8 brings to light the potential role of epithelial respiratory cells in immunity against C. neoformans.
Acknowledgments
The present work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação Universitária José Bonifácio (FUJB, Brazil), and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil). M.L.R. was the recipient of an International Fellowship for Latin America, provided by the American Society for Microbiology. A.C. is supported by NIH grants AI033142, AI033774, AI052733, and HL059842.
We thank Johanna Rivera and Antonio Nakouzi for helpful discussions, Venicio F. da Veiga for help with fluorescence microscopy, and Geralda A. Rodrigues for technical assistance.
Footnotes
Published ahead of print on 8 November 2006.
REFERENCES
- 1.Bals, R., and P. S. Hiemstra. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23:327-333. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa, F. M., F. L. Fonseca, C. Holandino, C. S. Alviano, L. Nimrichter, and M. L. Rodrigues. 2006. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect. 8:493-502. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L.-A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherniak, R., L. C. Morris, B. C. Anderson, and S. A. Meyer. 1991. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 59:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleare, W., and A. Casadevall. 1998. The different binding patterns of two immunoglobulin M monoclonal antibodies to Cryptococcus neoformans serotype A and D strains correlate with serotype classification and differences in functional assays. Clin. Diagn. Lab. Immunol. 5:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganendren, R., E. Carter, T. Sorrell, F. Widmer, and L. Wright. 2006. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 8:1006-1015. [DOI] [PubMed] [Google Scholar]
- 9.Jersmann, H. P. 2005. Time to abandon dogma: CD14 is expressed by non-myeloid lineage cells. Immunol. Cell Biol. 83:462-467. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami, K. 2004. Regulation by innate immune T lymphocytes in the host defense against pulmonary infection with Cryptococcus neoformans. Jpn. J. Infect. Dis. 57:137-145. [PubMed] [Google Scholar]
- 11.Larsen, R. A., P. G. Pappas, J. Perfect, J. A. Aberg, A. Casadevall, G. A. Cloud, R. James, S. Filler, and W. E. Dismukes. 2005. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 49:952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levitz, S. M. 2002. Receptor-mediated recognition of Cryptococcus neoformans. Nippon Ishinkin Gakkai Zasshi 43:133-136. [DOI] [PubMed] [Google Scholar]
- 13.Levitz, S. M., and A. Tabuni. 1991. Binding of Cryptococcus neoformans by human cultured macrophages. requirements for multiple complement receptors and actin. J. Clin. Investig. 87:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacRedmond, R., C. Greene, C. C. Taggart, N. McElvaney, and S. O'Neill. 2005. Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir. Res. 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monari, C., F. Bistoni, and A. Vecchiarelli. 2006. Glucuronoxylomannan exhibits potent immunosuppressive properties. FEMS Yeast Res. 6:537-542. [DOI] [PubMed] [Google Scholar]
- 16.Monari, C., E. Pericolini, G. Bistoni, A. Casadevall, T. R. Kozel, and A. Vecchiarelli. 2005. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of fas ligand in macrophages. J. Immunol. 174:3461-3468. [DOI] [PubMed] [Google Scholar]
- 17.Oscarson, S., M. Alpe, P. Svahnberg, A. Nakouzi, and A. Casadevall. 2005. Synthesis and immunological studies of glycoconjugates of Cryptococcus neoformans capsular glucuronoxylomannan oligosaccharide structures. Vaccine 23:3961-3972. [DOI] [PubMed] [Google Scholar]
- 18.Palsson-McDermott, E. M., and L. A. O'Neill. 2004. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirofski, L., R. Lui, M. DeShaw, A. B. Kressel, and Z. Zhong. 1995. Analysis of human monoclonal antibodies elicited by vaccination with a Cryptococcus neoformans glucuronoxylomannan capsular polysaccharide vaccine. Infect. Immun. 63:3005-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddi, K., S. B. Phagoo, K. D. Anderson, and D. Warburton. 2003. Burkholderia cepacia-induced IL-8 gene expression in an alveolar epithelial cell line: signaling through CD14 and mitogen-activated protein kinase. Pediatr. Res. 54:297-305. [DOI] [PubMed] [Google Scholar]
- 21.Riederer, I., S. D. Silva-Barbosa, M. L. Rodrigues, and W. Savino. 2002. Local antilaminin antibody treatment alters the rejection pattern of murine cardiac allografts: correlation between cellular infiltration and extracellular matrix. Transplantation 74:1515-1522. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui, A. A., A. E. Brouwer, V. Wuthiekanun, S. Jaffar, R. Shattock, D. Irving, J. Sheldon, W. Chierakul, S. Peacock, N. Day, N. J. White, and T. S. Harrison. 2005. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J. Immunol. 174:1746-1750. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsumi-Ishii, Y., and I. Nagaoka. 2003. Modulation of human beta-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J. Immunol. 170:4226-4236. [DOI] [PubMed] [Google Scholar]
- 24.Wormley, F. L., Jr., and J. R. Perfect. 2005. Immunology of infection caused by Cryptococcus neoformans. Methods Mol. Med. 118:193-198. [DOI] [PubMed] [Google Scholar]
- 25.Yauch, L. E., M. K. Mansour, S. Shoham, J. B. Rottman, and S. M. Levitz. 2004. Involvement of CD14, Toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect. Immun. 72:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, Z., R. Liu, J. A. Noordhoek, and H. F. Kauffman. 2005. Interaction of airway epithelial cells (A549) with spores and mycelium of Aspergillus fumigatus. J. Infect. 51:375-382. [DOI] [PubMed] [Google Scholar]