Abstract
The failure of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells to control chronic HIV-1 infection could be due to the progressive loss of their capacities to undergo normal memory effector differentiation. We characterized and compared the expressions of CD27, CD28, CD57, and CD62L by Epstein-Barr virus (EBV)-, cytomegalovirus (CMV)-, and HIV-1-specific CD8+ T cells by six-color, eight-parameter flow cytometry. In contrast to the maturation of EBV- and CMV-specific memory CD8+ T cells, we found that HIV-1-specific CD8+ T cells did not display coordinated down-regulation of CD27 and up-regulation of CD57 and accumulated in an atypical CD27high CD57low subset. Moreover, the accumulation of CD27high CD57low HIV-1-specific CD8+ T cells was positively correlated with HIV-1 plasma viremia. The differentiation of HIV-1-specific CD8+ T cells to an effector subset is therefore impaired during chronic HIV-1 infection. This lack of normal CD8+ T-cell differentiation could contribute to the failure of cellular immune control of HIV-1 infection.
Understanding CD8+ T-cell memory effector differentiation is essential for studying how virus-specific CD8+ T cells control viral infection. Distinct stages of virus-specific CD8+ T-cell memory effector differentiation have been extensively characterized by phenotypic and functional analyses. Primed virus-specific CD8+ T cells typically differentiate from the least mature memory stage (CD27+ CD28+ CD45RO+) to the most mature effector stage (CD27− CD28− CD45RA+) (3, 13). Terminally differentiated effector cells can be further defined by CD57 expression (4).
The association of phenotypes of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells with disease progression remains controversial. While HIV-1-specific CD8+ T cells in long-term nonprogressors have been reported to be phenotypically enriched for an effector subset (CD27− CD45RA+) (12), others have reported that HIV-1-specific CD8+ T cells remain enriched in an intermediate stage (CD27+ CD28−) regardless of clinical status (9) and duration of infection (2). It is not clear from these latter studies, however, whether the differentiation of HIV-1-specific CD8+ T cells during chronic HIV-1 infection is impaired prior to the effector stage. Moreover, since continuous replication of HIV-1 during chronic persistent infection provides a constant supply of HIV-1 antigens, long-term HIV-1 infection should theoretically drive HIV-1-specific CD8+ T cells to the effector stage. Phenotypic enrichment observed in these studies (2, 9), therefore, could be a consequence of a blockade in CD8+ T-cell differentiation prior to the effector stage. Thus, HIV-1-specific CD8+ T-cell memory effector differentiation needs to be clarified in relation to the natural course of memory CD8+ T-cell differentiation.
In the present study, we assessed the differentiation of HIV-1-specific CD8+ T cells by phenotypic analysis based on CD27, CD28, CD57, and CD62L expression in a cohort of HIV-1-infected subjects with a range of T-cell counts and viral loads and uninfected participants. We found that, in contrast to Epstein-Barr virus (EBV)- and cytomegalovirus (CMV)-specific CD8+ T cells, the majority of HIV-1-specific CD8+ T cells were phenotypically enriched in CD27high CD57− and CD27high CD57low subsets and showed no evidence of progression to the effector subset (CD27− CD57high). These results suggest that the phenotypic enrichment of HIV-1-specific CD8+ T cells that persists in spite of the continuous presence of HIV-1 antigen is due to impaired CD8+ T-cell effector differentiation.
(This work was in partial fulfillment of the requirements for a Ph.D. for A.H. from the University of Pittsburgh.)
MATERIALS AND METHODS
Study population.
The study population consisted of 11 HIV-1-uninfected participants (median age, 52 years; range, 43 to 69 years; 1 female and 10 male Caucasians) and 7 HIV-1-infected participants (median age, 48 years; range, 25 to 70 years; all male Caucasians) from the Pittsburgh portion of the Multicenter AIDS Cohort Study with a range of HLA types, CD4+ T-cell counts (median, 453; range, 129 to 1,029 cells/mm3), and viral loads (median, 22,580; range, 101 to 66,750) (Table 1). None of the HIV-1-infected subjects were on antiretroviral therapy at the time of this study. The HIV-1 plasma viral load was measured by a quantitative reverse transcription-PCR assay (Amplicor; Roche Diagnostics, Alameda, CA). HLA typing was performed at the Laboratory of Genomic Diversity, National Cancer Institute, and the laboratory of M. Trucco, University of Pittsburgh.
TABLE 1.
HLA types, tetramer reactivity, and clinical data of study participants
| Identification no. | HLA type | Tetramer reactivitya | Clinical statusb | CD4 countc | Viral loadd |
|---|---|---|---|---|---|
| 1 | A1, A3, B8, B53 | B8p24, B8nef | HIV-1+ | 270 | 18,646 |
| 2 | A2, A3, B57, B40 | A2p17, A2pol, A3p17, A2EBV, A2CMV | HIV-1+ | 129 | 66,752 |
| 3 | A2, A3, B27, B37 | A2p17, A2pol, A2EBV, A2CMV | HIV-1+ | 459 | 939 |
| 4 | A2, A33, B15, B42 | A2p17 | HIV-1+ | 1,029 | 101 |
| 5 | A2, B27, B13 | A2p17, A2pol, A2EBV, A2CMV | HIV-1+ | 990 | 23,737 |
| 6 | A3, A31, B13, B51 | A3p17, A3pol | HIV-1+ | 453 | 22,580 |
| 7 | A3, A24, B27, B15 | A3p17, A3pol | HIV-1+ | 385 | 24,968 |
| 8 | A2 | A2CMV | HIV-1− | ||
| 9 | A2, B8 | A2EBV, B8EBV | HIV-1− | ||
| 10 | A2, A31, B15, B44 | A2CMV | HIV-1− | ||
| 11 | A2, A24, B44, B7 | A2EBV, A2CMV | HIV-1− | ||
| 12 | A2 | A2CMV | HIV-1− | ||
| 13 | A1, A2, B8, B56 | A2CMV | HIV-1− | ||
| 14 | A1, A11, B8, B15 | B8EBV | HIV-1− | ||
| 15 | A1, A2, B18, B51 | A2EBV, A2CMV | HIV-1− | ||
| 16 | A2, A23, B13, B44 | A2CMV | HIV-1− | ||
| 17 | A2, A23, A39, B41 | A2CMV | HIV-1− | ||
| 18 | A2, A24, A44, A15 | A2CMV | HIV-1− |
Each tetramer is designated by the HLA type and the antigen.
Clinical status of study participants at the time of their blood draw. HIV-1+ and HIV-1− indicate HIV-1-infected and uninfected individuals, respectively.
CD4 count indicates a number of CD4+ T cells per milliliter of blood.
Viral load indicates a number of HIV-1 viral genomes per milliliter of a serum sample.
Antibodies and major histocompatibility complex class I (MHC-I) tetramers.
The following monoclonal antibodies (MAbs) were used: fluorescein isothiocyanate-conjugated mouse anti-human CD57 and phycoerythrin (PE)-Cy5-conjugated mouse anti-human CD27 (BD Biosciences, San Jose, CA); ECD (PE-Texas Red)-conjugated mouse anti-human CD28 and PE-Cy7-conjugated mouse anti-human CD8 (Coulter-Immunotech, Miami, FL); and allophycocyanin-Cy7-conjugated mouse anti-human CD62L (Caltag, Burlingame, CA). Appropriate isotype-matched MAbs were obtained from the same companies and used throughout the course of the study.
MHC-I tetrameric reagents used in the study are listed in Table 2. All of the tetrameric agents except A2pp65 CMV and A2BMLF1 EBV were obtained from the NIH Tetramer Synthesis Facility. A2pp65 CMV and A2BMLF1 EBV tetramers were obtained from Coulter Immunomics (San Diego, CA). All of the tetramers were labeled with PE and used at a 1/50 to 1/200 dilution for staining 2 × 106 to 5 × 106 peripheral blood mononuclear cells (PBMCs).
TABLE 2.
List of epitopes used for MHC class I tetramer analysis
| HLA allele | Epitope amino acid sequence | Protein | Epitope locationa |
|---|---|---|---|
| A2 | SLYNTVATL | HIV-1 p17Gag | 77-85 |
| A2 | ILKEPVHGV | HIV-1 Pol | 309-317 |
| A2 | GLCTLVAML | EBV BMLF1 | 280-288 |
| A2 | NLVPMVATV | CMV pp65 | 495-503 |
| A3 | RLRPGGKKK | HIV-1 p17Gag | 20-28 |
| A3 | AIFQSSMTK | HIV-1 Pol | 158-166 |
| A3 | QVPLRPMTYK | HIV-1 Nef | 73-82 |
| B8 | EIYKRWII | HIV-1 p24Gag | 128-135 |
| B8 | FLKEKGGL | HIV-1 Nef | 90-97 |
| B8 | RAKFKQLL | EBV BZLF1 | 190-197 |
Epitope location is indicated by number of amino acids spanning the epitope from N terminus of the corresponding protein.
Isolation and preparation of stained PBMCs.
Blood specimens were collected in BD Vacutainer cell preparation tubes with sodium heparin (BD Biosciences, San Jose, CA), and PBMCs were isolated according to the manufacturer's protocol. PBMCs were stained as described previously (8), with modifications. Briefly, 2 × 106 to 5 × 106 fresh PBMCs were resuspended in RPMI 1640 medium (GIBCO, Grand Island, NY) with 10% heat-inactivated fetal calf serum (HyClone, Logan, UT) and stained with a 1/50 to 1/200 dilution of the appropriate tetramer at 37°C for 25 min. The cells were then washed and resuspended in phosphate-buffered saline with 4% fetal calf serum and 0.1% sodium azide and incubated with a cocktail of MAbs for 30 min at 4°C. After the final wash, cells were fixed with 1% paraformaldehyde and analyzed with a MoFlo flow cytometer (DakoCytomation, Boulder, CO).
Flow cytometry.
The MoFlo flow cytometer was calibrated for laser fluctuation/alignment and photomultiplier tube voltage adjustment by using FlowCheck beads (Coulter, Miami, FL) and Ultra Rainbow Calibration beads (Spherotech, Inc., Libertyville, IL) prior to the sample acquisition. A six-color compensation matrix was created by FlowJo (TreeStar, Cupertino, CA), which was based on six singly stained PBMC samples from actual donors. A PE-conjugated mouse anti-human CD8 MAb was used for PE compensation in place of the MHC-I tetramer.
We used a flow cytometric analysis described previously (8) with a few modifications. A minimum of 500 total tetrameter-positive CD8high events were collected for the fully stained sample and isotype control (the same number of events were collected for both samples), and this resulted in a collection of approximately 1 × 106 to 3 × 106 total events. We used a compounded gating scheme as previously described (7, 8), with necessary modifications. Briefly, cells were first gated on the CD8high population on a CD8 and side scatter (SS)-log plot, followed by a lymphocyte gate on an forward scatter (FS)- and SS-log plot. Potential doublets were excluded based on an FS-area and FS-height plot. The resulting tetrameter-positive population was visualized on a tetramer-PE versus SS-log plot for determining phenotypic analysis and the frequency of tetrameter-positive CD8+ T cells. Proper gating was established by using tetramer and CD8-PECy7 doubly stained cells in the presence of fluorescent-labeled isotype controls. Data analysis and graphic representations were performed with FlowJo software.
Statistical analysis.
We used analysis of variance and the Tukey-Kramer honest significant difference test for determining statistical significance between and among group means. We used the two-tailed Spearman correlation test for determining P values and a standard least square method for determining R2 correlation coefficients. A P value of <0.05 was considered statistically significant. Statistical analysis and graphical representation were performed using JMP IN, version 5.1 (JMP Sales, Cary, NC), and Aabel software (Gigawiz Ltd. Co., Tulsa, OK).
RESULTS
EBV-, CMV-, and HIV-1-specific CD8+ T cells show distinct levels of CD27, CD28, CD57, and CD62L expression.
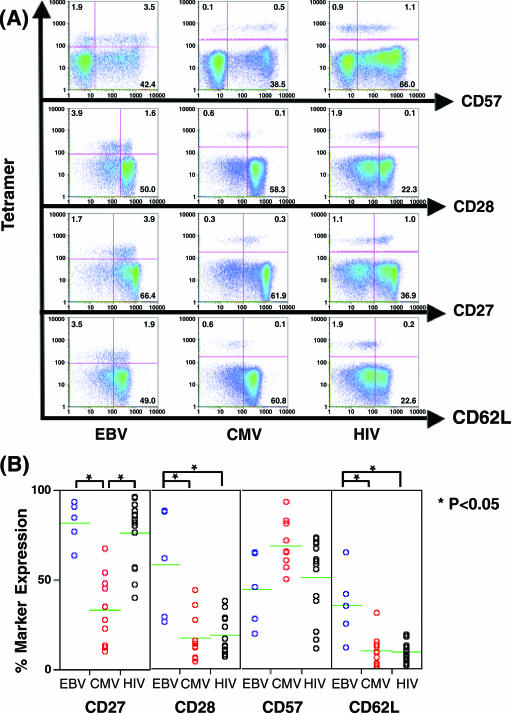
We first used a battery of phenotypic markers and MHC-I tetramers to assess virus-specific T-cell phenotypes comparable to those previously related to a unique virus-specific memory CD8+ T cell during persistent viral infections (1). Virus-specific CD8+ T cells were visualized by a host of MHC-I tetramers, and the phenotypes of virus-specific CD8+ T cells were assessed by costaining with antibodies against CD27, CD28, CD57, and CD62L (Fig. 1A).
FIG. 1.
Visualization of virus-specific CD8+ T cells by staining ex vivo with MHC-I tetramers and surface expression of memory/effector phenotypic markers. All cells shown are a result of compound gating; an initial gate was set on the CD8high subset on CD8 and SS-log, followed by a lymph-gate on FS-area and SS-log. (A) Representative figure showing surface expression of CD27, CD28, CD57, and CD62L on EBV-, CMV-, and HIV-1-specific CD8+ T cells. Numbers in the quadrants indicate percentages of cells. (B) Comparison of percentages of CD27, CD28, CD57, and CD62L expressing EBV-, CMV-, and HIV-1-specific CD8+ T cells. Data are from the participants with EBV tetramer reactivity (n = 5), CMV tetramer reactivity (n = 9), and HIV-1 tetramer reactivity (n = 14).
Comparison of percent surface marker expression revealed phenotypic subsets unique to each type of virus-specific CD8+ T cell (Fig. 1B). The proportion of the CD27+ CMV-specific CD8+ T cell (median 31%; range 10 to 67%) significantly differed from the proportions of the CD27+ HIV-1-specific (median, 83%; range, 40 to 96%) and the CD27+ EBV-specific CD8+ T cells (median, 85%; range, 63 to 93%) (P < 0.05). There were also significant differences in the proportion of CD28+ and CD62L+ subsets between HIV-1-specific (for CD28, median, 15%, and range, 6 to 38%; for CD62L, median, 9%, and range, 2 to 19%) or CMV-specific (for CD28, median, 13%, and range, 4 to 44%; for CD62L, median, 9%, and range, 1 to 31%) CD8+ T cells, and EBV-specific CD8+ T cells (for CD28, median, 61%, and range, 26 to 88%; for CD62L, median, 35%, and range, 12 to 65%) (P < 0.05). There were no significant differences in the proportions of the CD57+ subset, although the percentages of CD8+ CD57+ T cells specific for CMV-specific CD8+ T cells were slightly higher than the CD57+ EBV- or HIV-1-specific CD8+ T cells. Based on these results, the major phenotypic subsets of EBV-, HIV-1-, and CMV-specific CD8+ T cells appeared to be CD27+ CD28+ CD57− CD62L+/−, CD27+ CD28−, CD57+CD62L− and CD27− CD28− CD57+ CD62L−, respectively. Thus, HIV-1-specific CD8+ T cells from a cohort of HIV-1-infected participants were phenotypically in an intermediate stage of CD8+ T-cell differentiation. This is in agreement with observations made by Appay et al. (1).
Correlation between proportions of CD27+ CD57+ HIV-1-specific CD8+ T cells and plasma viral load.
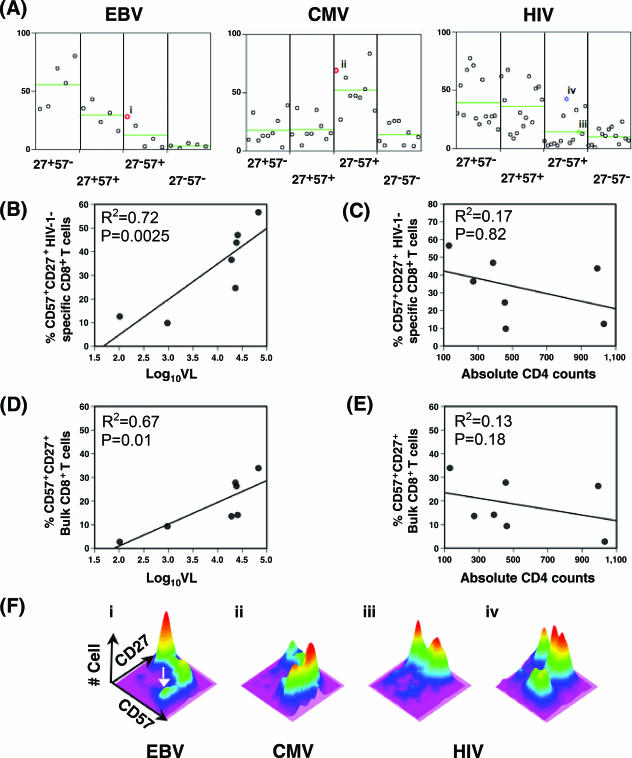
We assessed CD57 expression as a surrogate marker of terminal differentiation. The majority of HIV-1-specific CD8+ T cells were enriched evenly (P > 0.05) in CD27+ CD57− (median, 28%; range, 16 to 77%) and CD27+ CD57+ (median, 36%; range, 8 to 67%) subsets (Fig. 2A). In contrast, CMV-specific CD8+ T cells were enriched predominantly in the CD27− CD57+ (median, 47%; range, 17 to 84%) subset (P < 0.05), and EBV-specific CD8+ T cells were relatively more enriched in the CD27+ CD57− (median, 56%; range, 35 to 80%) subset.
FIG. 2.
HIV-1-specific CD8+ T cells do not show effector maturation-associated progression from the CD27high CD57− subset to the CD27− CD57high subset. (A) Proportions of four binary CD27 CD57 subsets of EBV-, CMV-, and HIV-1-specific CD8+ T cells are compared. The green line indicates the mean of each subset. Data are from the participants with EBV tetramer reactivity (n = 5), CMV tetramer reactivity (n = 9), and HIV-1 tetramer reactivity (n = 14). (B to E) Proportions of the CD27+ CD57+ (CD27high CD57low) subsets of HIV-1-specific and bulk CD8+ T cells positively correlated with HIV-1 plasma viral load (B and D) but not with absolute CD4 counts (C and E). HIV-specific CD8+ T cells from HIV-infected individuals (n = 7) are plotted against HIV-1 plasma viral load (log10VL). Mean values of corresponding percent CD27+ CD57+ of HIV-specific and bulk CD8+ T cells are used for those with multiple HIV-1-specific tetramer reactivity. (F) Representative three-dimensional topographical plots of CD27 and CD57 expression on EBV (panel i)-, CMV (panel ii)-, and HIV-1 (panels iii and iv)-specific CD8+ T cells are shown. Color variation indicates a number of cells from low (purple) to high (red) on a z axis. The white arrow indicates the CD27− CD57high subset. Percent CD27− CD57+ of representative results (colored circles) are shown in Fig. 2A. Panels i and ii represent EBV- and CMV-specific CD8+ T cells from HIV-1 uninfected (red circles, participants 9 and 8, respectively), while panels iii (green circle, B8nef from participant 1), and iv (blue circle, A3pol from participant 7) represent HIV-1-infected participants.
We found that the percentage of CD27+ CD57+ HIV-1-specific CD8+ T cells strongly correlated with the plasma viral load (R2 = 0.72; P = 0.0025) (Fig. 2B), while proportions of CD27+ CD57− (R2 = 0.66; P = 0.18), CD27− CD57+ (R2 = 0.15; P = 0.88), and CD27− CD57− other subsets (R2 = 0.09; P = 0.11) did not (data not shown). Moreover, bulk CD8+ T cells from the same HIV-1-infected participants showed a positive correlation (Fig. 2D) (R2 = 0.67; P = 0.01), suggesting that progression to the CD27high CD57low subset is not strictly limited to HIV-1-specific CD8+ T cells, whereas other subsets, CD27+ CD57− (R2 = 0.66; P = 0.21), CD27− CD57+ (R2 = 0.15; P = 0.45), and CD27− CD57− subsets (R2 = 0.09; P = 1.00), did not (data not shown). This suggests that progression to the CD27high CD57low subset is not strictly limited to HIV-1-specific CD8+ T cells. There was no correlation between absolute CD4+ T-cell counts and the proportion of CD27+ CD57+ HIV-1-specific CD8+ T cells or the bulk CD27+ CD57+ CD8+ T cell (Fig. 2C and E, respectively) or other T-cell subsets (data not shown).
Progression of the HIV-1-specific CD8+ T cell to the atypical CD27high CD57low subset.
Notably, the pattern of CD27 and CD57 expression revealed an additional aspect of virus-specific memory CD8+ T-cell differentiation. EBV- and CMV-specific CD8+ T cells appeared to uninterruptedly progress from CD27high CD57− (undifferentiated memory stage) to CD27− CD57high (effector stage) (Fig. 2F). This pattern of progression from an immature to mature stage was apparent in the relatively more differentiated EBV-specific CD8+ T cells (Fig. 2F, panel i) and in most of the CMV-specific CD8+ T cells (Fig. 2F, panel ii). In contrast, the CD57+ subset of HIV-1-specific CD8+ T cells typically appeared in the absence of CD27 down-regulation (Fig. 2F, panel iii). This pattern persisted even in HIV-1-specific CD8+ T cells with the highest proportion of the CD27− CD57+ subset (Fig. 2F, panel iv). Also, virtually all the CD27+ CD57+ HIV-1-specific CD8+ T cells were CD27high CD57low (Fig. 3).
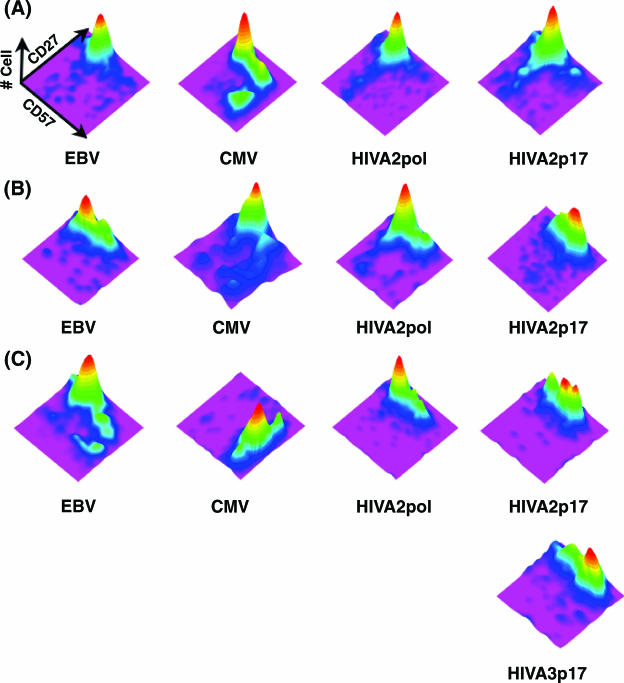
FIG. 3.
Pattern of CD27 and CD57 expression on EBV-, CMV-, and HIV-specific CD8+ T cells from three HIV-1-infected participants with a triple tetramer reactivity. Shown are three-dimensional topographical plots of CD27 and CD57 expression on tetrameter-positive CD8+ T cells from participant 3 (A), participant 5 (B), and participant 2 (C). Refer to the legend for Fig. 2F for a description of the topographical plot.
Three HIV-1-infected participants (Table 1) had EBV- and/or CMV-specific CD8+ T cells, making it possible to compare the phenotypes of these virus-specific CD8+ T cells from the same immunological background. EBV-specific CD8+ T cells from these HIV-1-infected participants appeared to be phenotypically enriched in the immature stage (CD27high CD57−), similar to the same cells from HIV-1-uninfected participants. Similarly, CMV-specific CD8+ T cells did not appear to accumulate the atypical CD27high CD57low phenotype and showed the expected pattern of CD27 and CD57 expression (Fig. 3). In contrast, CD8+ T cells specific for the HIV-1 p17 epitope (SLYNVATL) from two participants with high viral load (Table 1) accumulated in the atypical CD27high CD57low subset (Fig. 3B and C), whereas the same cell from a participant with a relatively lower viral load and higher CD4 counts rarely accumulated in the atypical CD27high CD57low subset (Fig. 3A). Interestingly, CD8+ T cells specific for HIV-1 polymerase epitope (ILKEPVHGV) did not show a similar level of CD27high CD57low accumulation. In fact, there was no statistically significant difference in the proportion of the CD27+(high) CD57+(low) subset between CD8+ T cells specific for HIV Gag and HIV Pol epitopes (P > 0.05) (data not shown). However, the development of the atypical CD27high CD57low subset from the CD27high CD57− subset (undifferentiated) was clearly demonstrated (Fig. 3B and 3C).
Based on these results, HIV-1-specific CD8+ T cells appear to differentiate to an atypical subset (CD27high CD57low) rather than to the typical effector subset (CD27− CD57high), even in the continuous presence of HIV-1 antigens. Furthermore, progression to the CD27high CD57low subset is positively correlated with HIV-1 viremia but not with absolute CD4+ T-cell counts.
DISCUSSION
We show that the majority of HIV-1-specific CD8+ T cells in participants with chronic, untreated HIV-1 infection displayed the phenotype CD27+ CD28− CD57−/low CD62L−, suggestive of the advanced intermediate stage of CD8+ T-cell differentiation. Previous studies have shown that HIV-1-specific CD8+ T cells lack CD62L expression (5) and are phenotypically enriched in the intermediate stage of CD8+ T-cell differentiation (1, 2, 9, 12). More recent studies have found a correlation between an increasing proportion of CD57+ HIV-specific CD8+ T cells and disease progression (4, 10).
A novel finding of our study is the development and accumulation of the previously uncharacterized CD27high CD57low subset of HIV-1-specific CD8+ T cells, which is significantly correlated with plasma viral load. Since the functional significance of the CD27high CD57low subset is unknown at present, it is difficult to address the cause-and-effect relationships between disease progression and the development of the CD27high CD57low subset. This question is best addressed by a longitudinal study to determine whether the CD27high CD57low subset arises prior to the loss of immune control of HIV-1 chronic infection. Clearly, further elucidation of the functional significance of the CD27high CD57low subset is warranted.
Our hypothesis as to how the CD27high CD57low subset may develop during chronic HIV-1 infection is that HIV-1-specific CD8+ T cells lose the capacity to undergo differentiation from the early stage (CD27high CD57−) to the effector stage (CD27− CD57high). They instead accumulate as an atypical CD27high CD57low subset, potentially due to the inability for coordinated down-regulation of CD27 and up-regulation of CD57. Recently it has been shown that CD27 down-regulation on CMV-specific CD8+ T cells requires CD70 expression induced by antigen-specific activation and the presence of interleukin-2 (6). Thus, long-term continuous activation of HIV-1-specific CD8+ T cells in the absence of IL-2 due to CD4+ T-cell loss could lead to impaired CD70 expression by activated HIV-1-specific CD8+ T cells.
Alternatively, the effector subset (CD27− CD57high) of HIV-1-specific CD8+ T cells is depleted in tissues and circulation during chronic HIV-1 infection. Interestingly, CD57+ CD8+ T cells are highly susceptible to activation-induced apoptosis (4). Also, chronic antigenic stimulation could deliver an excessive CD27-CD70-mediated costimulatory signal, ultimately leading to the exhaustion of antigen-specific CD8+ T cells (11). Thus, excessive and prolonged CD27-CD70 interaction during chronic HIV-1 infection may cause the deletion of CD27− CD57high HIV-1-specific CD8+ T cells.
Despite a large number of recent phenotypic and functional T-cell studies, it is still not entirely clear whether a particular phenotypic enrichment or differentiation stage of the virus-specific CD8+ T cells can provide necessary and sufficient protection from chronic viral infection. Intuitively, having a large proportion of the effector subset of virus-specific CD8+ T cells seems to be advantageous because of their capacities to mount immediate protection with direct cytotoxic functions. However, having a large proportion of effector cells may not be suitable for long-term protection if effectors readily undergo activation-induced apoptosis. This issue has remained controversial in the context of assessing phenotypic correlates of long-term survival in HIV-1 infection (3, 13). The mode of long-term nonprogression can be multifaceted, with HIV-1-infected individuals, including long-term survivors, being heterogeneous in their pathogenesis and clinical conditions (e.g., viral load, CD4+ T-cell counts, duration of infection, and types of medications) and bases of immune control of HIV-1 infection.
In conclusion, we assessed the state of differentiation of HIV-1-specific CD8+ T cells by characterizing and comparing phenotypes of HIV-1-, EBV-, and CMV-specific CD8+ T cells based on multiparameter flow cytometry. We found that HIV-1-specific CD8+ T cells predominantly displayed the phenotype CD27high CD28− CD57low CD62L−. Moreover, our novel finding was that some EBV-specific and a majority of CMV-specific CD8+ T cells displayed coordinated modulation of CD27, CD28, and CD57 expression during T-cell differentiation, while a majority of HIV-1-specific CD8+ T cells did not. Particularly, HIV-1-specific CD8+ T cells did not appear to differentiate to the effector CD27− CD57high subset but were enriched in the atypical CD27high CD57low subset. There was a marked increase in enrichment of the CD27high CD57lowsubset with chronic disease. Based on these results, we propose a model wherein HIV-1-specific CD8+ T cells sustain impaired effector differentiation during the course of chronic HIV-1 infection.
Acknowledgments
We are grateful to the Pitt Men's Study participants for their blood donations, Angela M. Alexander and Massimo Trucco for HLA typing, Albert Donnenberg for assistance on data analysis, and Pawel Kalinski and Otto Yang for critical review of the manuscript.
This work was supported by NIH grants R37-AI41870, U01-AI35041, and P01-AI055794 and the AIDS Clinical Trials Group subcontract 204IC006.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Appay, V., P. R. Dunbar, M. Callan, et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 2.Appay, V., L. Papagno, C. A. Spina, et al. 2002. Dynamics of T cell responses in HIV infection. J. Immunol. 168:3660-3666. [DOI] [PubMed] [Google Scholar]
- 3.Appay, V., and S. L. Rowland-Jones. 2002. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 23:580-585. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley, J. M., N. J. Karandikar, M. R. Betts, et al. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711-2720. [DOI] [PubMed] [Google Scholar]
- 5.Chen, G., P. Shankar, C. Lange, et al. 2001. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood 98:156-164. [DOI] [PubMed] [Google Scholar]
- 6.Gamadia, L. E., E. M. van Leeuwen, E. B. Remmerswaal, et al. 2004. The size and phenotype of virus-specific T cell populations is determined by repetitive antigenic stimulation and environmental cytokines. J. Immunol. 172:6107-6114. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann, T. K., V. S. Donnenberg, U. Friebe-Hoffmann, et al. 2000. Competition of peptide-MHC class I tetrameric complexes with anti-CD3 provides evidence for specificity of peptide binding to the TCR complex. Cytometry 41:321-328. [PubMed] [Google Scholar]
- 8.Hoji, A., and C. R. Rinaldo. 2005. Human CD8+ T cells specific for influenza A virus M1 display broad expression of maturation-associated phenotypic markers and chemokine receptors. Immunology 115:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papagno, L., V. Appay, J. Sutton, et al. 2002. Comparison between HIV- and CMV-specific T cell responses in long-term HIV infected donors. Clin. Exp. Immunol. 130:509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papagno, L., C. A. Spina, A. Marchant, et al. 2004. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesselaar, K., R. Arens, G. M. van Schijndel, et al. 2003. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat. Immunol. 4:49-54. [DOI] [PubMed] [Google Scholar]
- 12.van Baarle, D., S. Kostense, E. Hovenkamp, et al. 2002. Lack of Epstein-Barr virus- and HIV-specific CD27− CD8+ T cells is associated with progression to viral disease in HIV-infection. AIDS 16:2001-2011. [DOI] [PubMed] [Google Scholar]
- 13.van Baarle, D., S. Kostense, M. H. van Oers, D. Hamann, and F. Miedema. 2002. Failing immune control as a result of impaired CD8+ T-cell maturation: CD27 might provide a clue. Trends Immunol. 23:586-591. [DOI] [PubMed] [Google Scholar]