Abstract
Mycobacterium avium subsp. paratuberculosis is the causative agent of Johne's disease, a chronic granulomatous enteritis of ruminants and other species. Detection of infection in animals is hampered by the lack of sensitive and specific diagnostic assays. We describe here an approach that utilizes translationally active PCR fragments for the rapid in vitro transcription and translation of recombinant proteins for antigen discovery in M. avium subsp. paratuberculosis. The investigations showed that the MAP1272c protein selectively reacts with sera from Johne's disease-positive cattle and represents an antigen of potential utility in M. avium subsp. paratuberculosis immunodiagnostics.
Johne's disease is a chronic gastrointestinal inflammatory disease caused by Mycobacterium avium subsp. paratuberculosis (reviewed in reference 5). The disease occurs in wild and domestic ruminants, including dairy cattle, and has considerable impact on the global agricultural economy. The slow growth of the organism in laboratory culture and its extensive genetic relatedness with Mycobacterium avium subsp. avium (4, 7) have hindered diagnosis of Johne's disease using methods such as bacterial isolation, genomic assays, and serology. Recent investigations showed that the current enzyme-linked immunosorbent assay (ELISA)-based immunoassays have poor sensitivity, detecting fewer than one-third of all infected cattle (6). Furthermore, the use of crude M. avium subsp. paratuberculosis protein mixtures as antigens compromises assay specificity due to conservation of proteins across Mycobacterium avium complex organisms. Hence, identification of suitable M. avium subsp. paratuberculosis antigens that could enable early, sensitive, and specific detection of M. avium subsp. paratuberculosis infection is critically needed to facilitate adequate disease control measures.
In order to capitalize on the availability of the complete genome sequence of M. avium subsp. paratuberculosis (10) for novel antigen discovery, we describe here the application of an in vitro transcription and translation system that enables expression of M. avium subsp. paratuberculosis recombinant proteins directly from transcriptionally active PCR (TAP) fragments (11). This approach obviates the need for cloning of individual genes and expression of proteins in a heterologous system. It is also amenable for adaptation to a high-throughput format and enables the expression of hundreds of genes in days versus the months needed for cloning-based expression. Hence, this method is labor-, time-, and cost-effective and is ideal for large-scale antigen discovery.
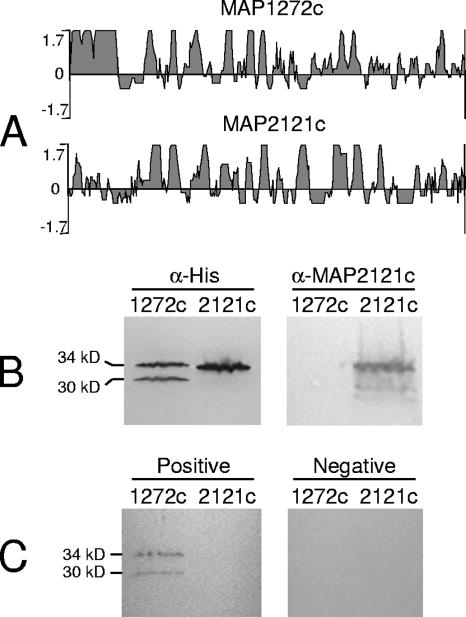
In order to evaluate the utility of this approach for rapid expression and screening of potential antigens for use in M. avium subsp. paratuberculosis immunodiagnostics, we chose two candidate M. avium subsp. paratuberculosis open reading frames (ORFs), MAP1272c and MAP2121c, that have not previously been characterized either functionally or immunologically. Preliminary computational and comparative genomic analyses suggested that the MAP1272c gene product is an ∼33.3-kDa protein that belongs to the NlpC/P60 superfamily. Orthologs of this protein are believed to function as putative secreted invasins and virulence factors in other mycobacteria, based on their homology with the p60 invasion protein of Listeria monocytogenes (9). The predicted MAP2121c gene product is an ∼33.5-kDa protein with homology to the 35-kDa major membrane protein 1 (MMP-1) of M. avium and Mycobacterium leprae. MMP-1 has been shown to be a surface protein that plays a role in virulence by mediating invasion of epithelial cells (3). Previous studies have also suggested that MMP-1 may be an important antigen and a target of the immune response to M. leprae (14). Computational analysis of antigenic profiles can be performed with several programs, including DNAStar, DS Gene, PEOPLE, CEP, BEPITOPE, and PREDITOP (1, 8, 12, 13). In the current investigation, the MAP1272c and MAP2121c proteins were analyzed by Protean (DNAStar, Madison, WI), using the Jameson-Wolf method. The computational analyses of MAP1272c and MAP2121c protein sequences showed the presence of a large number of antigenic regions (Fig. 1A), and hence these two ORFs represented attractive candidates for an initial screen of antigens in M. avium subsp. paratuberculosis.
FIG. 1.
Analysis and expression of MAP1272c and MAP2121c proteins. (A) Antigenic indexes of MAP1272c and MAP2121c proteins, determined by protein sequence analysis with Protean (DNAStar, Madison, WI), using the Jameson-Wolf method. (B) Western immunoblot of recombinant MAP1272c and MAP2121c proteins, using anti-His and anti-MAP2121c monoclonal antibodies. Proteins were separated in a 4 to 20% Tris-HCl ready gel (Bio-Rad, Hercules, CA) and electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were blocked overnight at 4°C in PBST-BSA (1× PBS, 0.1% Tween 20, 2% BSA), followed by incubation with anti-His or anti-MAP2121c monoclonal antibodies diluted 1:2,000 and 1:500, respectively, in PBST-BSA for 1 h at room temperature. Membranes were then washed three times for 10 min each in PBST and treated with anti-mouse IgG-horseradish peroxidase conjugate (Sigma, St. Louis, MO) at a 1:2,000 dilution in PBST-BSA for 1 h at room temperature. After being washed, the blots were developed with TMB solution (Sigma). (C) Reaction of recombinant proteins with M. avium subsp. paratuberculosis-positive and -negative bovine serum samples. Membranes were incubated with pooled positive or negative control sera diluted 1:500 in PBST-BSA for 1 h at room temperature. After being washed, membranes were incubated with peroxidase-labeled anti-goat IgG (Vector Laboratories, Burlingame, CA) diluted 1:10,000 in PBST-BSA for 2 h at room temperature. Membranes were then washed, and blots were developed in TMB solution.
TAP fragments were generated by two sequential PCR steps for MAP1272c (GenBank accession no. NP_960206) and MAP2121c (GenBank accession no. NP_961055).
In the first step, MAP1272c and -2121c were PCR amplified with custom oligonucleotides, with the 5′ primer comprising a universal overlapping sequence and 18 nucleotides from the specific gene, beginning after the start codon, as described below. The 3′-end primer contained a C-terminal His tag overlapping sequence and 18 nucleotides of the specific gene before the stop codon. The 5′ primer for MAP1272c had the sequence 5′-AGAAGGAGATATACCATGGTGCGATCCCAGCGTGGG-3′ (a total of 36 nucleotides, with the start codon shown in bold). The 3′ primer for MAP1272c had the sequence 5′-TTAATGATGATGATGATGATGCCGGGTGAGTCCGGCGCC-3′ (39 nucleotides, with the stop codon shown in bold). The sequences for the 5′ and 3′ primers for MAP2121c were 5′-AGAAGGAGATATACCATGACGTCGGCTCAAAATGAG and 5′-TTAATGATGATGATGATGATGCTTGTACTCATGGAACTG, respectively. The first-step 25-μl PCR amplification mixture contained 1× PCR buffer II, 2.0 mM MgCl2 (ABI), a 200 μM concentration of each deoxynucleoside triphosphate (Roche Diagnostic Co., Indianapolis, IN), a 0.6 μM concentration of each primer (Integrated DNA Technologies, Coralville, IA), 1.0 U of AmpliTaq Gold (ABI), 5% dimethyl sulfoxide (Sigma, St. Louis, MO), and 2 ng of genomic DNA from isolate MAP K-10. The amplification conditions consisted of an initial denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 60°C for 45 s, and 72°C for 2.5 min, with a final extension step at 72°C for 10 min. PCR amplicons were purified with a MultiScreen PCR purification plate (Millipore, Bedford, MA). The TAP fragments were generated by a second-step PCR using a TAP Express T7 IVT kit (Genlantis, San Diego, CA). The 50-μl PCR amplification mixture contained 1× PCR buffer II, 2.0 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, 5% dimethyl sulfoxide, 2.0 μl (each) of TAP-T7 promoter mix and TAP-His terminator mix, 5.0 U of AmpliTaq Gold, and 5.0 μl of purified DNA template from the first-step PCR. The amplification conditions consisted of an initial denaturation at 95°C for 1 min, followed by 35 cycles of 95°C for 30 s, 55°C for 45 s, and 72°C for 2.5 min, with a final extension step at 72°C for 10 min. Verification of all PCR products was conducted by agarose gel electrophoresis. The PCR products were further purified with Microcon 100 concentrators (Millipore, Bedford, MA) and concentrated to a final volume of 25.0 μl.
In vitro transcription and translation of TAP fragments obtained from second-step PCRs were accomplished with a Rapid Translation System RTS 100 E. coli HY kit (Roche Applied Science, Indianapolis, IN). The reaction mix was prepared in a 50-μl volume per the manufacturer's instructions. Briefly, 12.0 μl Escherichia coli lysate, 10 μl reaction mix, 12 μl amino acids, 1 μl methionine, and 5 μl reconstitution buffer were mixed together, and then 10 μl DNA template from the second-step PCR (0.5 μg) was added to the mix. The reactions were incubated for 6 h at 30°C, and miniscale purification of recombinant proteins was performed with metal-affinity resins, using a BD TALON purification kit (BD Biosciences Clontech, Mountain View, CA) per the manufacturer's instructions. Western blotting was performed to verify the expression of recombinant proteins, using anti-His tag and anti-MAP2121c monoclonal antibodies as well as pooled sera from Johne's disease-positive (n = 3) and -negative (n = 4) dairy cows (Fig. 1B and C).
The results show that both MAP1272c and MAP2121c were successfully amplified by two-step PCR. As expected, the amplicons obtained by the second-step PCR were larger, by 180 bp (data not shown), than the first-step PCR products due to the addition of TAP-T7 promoter and TAP-His transcription terminator sequences at the 5′ and 3′ ends, respectively. Furthermore, the expression of MAP1272c and MAP2121c recombinant proteins from the TAP products was shown by the immunoblots developed with anti-His antibodies (Fig. 1B). Next, the recombinant proteins were analyzed for antigenicity by Western immunoblotting using serum samples obtained from known M. avium subsp. paratuberculosis-positive and -negative cattle, and the results showed that the recombinant MAP1272c protein was recognized by the pooled Johne's disease-positive sera but not by negative control sera (Fig. 1C). Note that immunoblotting with both anti-His antibodies and positive sera resulted in two protein bands for the recombinant MAP1272c protein (Fig. 1B and C). MAP1272c is a 951-bp ORF and possesses a second translation initiation codon at position 112 that is in frame with the full-length ORF. The conceptual translation of these two ORFs with the inclusion of the C-terminal six-His tag results in protein products of 34.2 kDa and 30 kDa that correspond well with the two protein bands observed on the immunoblots. Since both protein products react with anti-His antibodies as well as serum samples, the smaller band may well represent the product of the slightly smaller ORF. Regardless, the data show that the MAP1272c gene product is immunoreactive and represents a potential candidate for use in the development of the next generation of immunodiagnostic assays for Johne's disease. In contrast to the MAP1272c protein, the results show that although the recombinant MAP2121c-His fusion protein had the expected mass of ∼34.5 kDa (Fig. 1B), it did not react with the pooled positive or negative sera (Fig. 1C).
To further examine the MAP1272c and MAP2121c proteins as diagnostic antigens, both proteins were tested by ELISA. The TAP expression system is essentially a screening tool and allows expression of proteins in relatively small quantities. In order to test these antigens by ELISA, the MAP1272c and MAP2121c proteins were expressed in E. coli, using the pMAL-c2 vector (New England Biolabs, Ipswich, MA) as previously described (2). Seven positive and two negative individual serum samples obtained from cattle that were confirmed to be positive or negative for Johne's disease by bacterial isolation were tested by ELISA. ELISA plates were coated overnight with 100 ng of each of the recombinant proteins. The plates were blocked for 2 h with 2% bovine serum albumin-phosphate-buffered saline-Tween 20 (2% BSA-PBST), and 50 μl of each serum sample diluted 1:500 in BSA-PBST was added to individual wells in duplicate. After 2 h of incubation at room temperature, plates were washed nine times with distilled water. Next, horseradish peroxidase-labeled anti-goat immunoglobulin G (IgG; Vector Laboratories, Burlingame, CA) diluted 1:5,000 in 2% BSA-PBST was added and incubated for 2 h at room temperature. After a washing step, 200 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) solution (Sigma, St. Louis, MO) was added, and absorbance was measured at 655 nm. The two known Johne's disease-negative serum samples were found to be negative by both MAP1272c and MAP2121c antigen-based ELISAs (Table 1). Of the seven known positive sera, MAP1272c and MAP2121c antigen ELISAs were able to detect MAP antibodies in seven and five serum samples, respectively (Table 1). Furthermore, the mean positive-to-negative ratio of the optical density values obtained for MAP1272c antigen ELISA was higher than that obtained for MAP2121c antigen ELISA. Thus, more sensitive detection of M. avium subsp. paratuberculosis antibodies by the MAP1272c antigen than by the MAP2121c antigen in ELISA correlates well with the immunoblotting results, where MAP2121c antigen could not detect M. avium subsp. paratuberculosis antibodies in pooled positive serum samples. Several reasons could account for this relatively poor immune recognition by the MAP2121c protein. First, the most plausible explanation is that the MAP2121c protein is either not expressed during infection or is poorly antigenic. Second, since the incubation period of Johne's disease can extend to several years, it is also possible that the bacterium may express proteins such as the MAP2121c protein only during, for instance, early subclinical stages of infection rather than during clinical disease. This hypothesis should be tested by further screening of the MAP2121c protein, using a larger number of serum samples, preferably from animals during various phases of disease. Third, it is possible that the MAP2121c protein is posttranslationally modified in M. avium subsp. paratuberculosis in a manner that is different from the protein produced through the in vitro approach so as to alter its antigenicity. Furthermore, the improper folding of the MAP2121c protein may also contribute to a weak or diminished antibody response. At the present time, it is unclear which, if any, of these hypotheses for the lack of seroreactivity of the MAP2121c protein are true, and further investigations will be needed to address this issue.
TABLE 1.
Detection of M. avium subsp. paratuberculosis antibodies in serum samples by recombinant MAP1272c and MAP2121c antigen ELISAs
| Antigen | ELISA result for serum samplesa
|
||
|---|---|---|---|
| Negative | Positive | Strongly positive | |
| MAP1272c protein | 0.09 ± 0.01 | 0.61 ± 0.43 | 0.46 ± 0.23 |
| MAP2121c protein | 0.09 ± 0.02 | 0.20 ± 0.08 | 0.33 ± 0.07 |
Values shown are average optical density values ± standard deviations for known negative (n = 2), Johne's disease-positive (n = 5), and strongly Johne's disease-positive (n = 2) serum samples.
Conclusion.
Taken together, the results of our investigation provide compelling evidence that in vitro expression of proteins directly from linear TAP fragments is an efficient time- and cost-effective technology for M. avium subsp. paratuberculosis recombinant protein production and screening. The protocols can be adapted to run in a high-throughput format to rapidly screen for potential antigens and are currently being used in our laboratory to express more M. avium subsp. paratuberculosis proteins. However, it must be noted that TAP protein expression is a screening tool that enables initial screening of proteins on a large scale for antigen identification and expresses proteins in relatively small quantities. Once identified, the selected antigens have to be cloned and expressed in larger quantities, using a suitable system, for further characterization. Future studies for M. avium subsp. paratuberculosis antigen discovery will entail large-scale screening of proteins by the TAP system, evaluation of their cross-reactivities with other mycobacteria, and determination of their ability to detect M. avium subsp. paratuberculosis antibodies in serum samples from cattle shedding low, medium, and high levels of organisms. Based on the results of our current study, the identification of the MAP1272c protein as a potential M. avium subsp. paratuberculosis antigen for use as an immunodiagnostic reagent may have important implications in the development of improved Johne's disease immunological assays.
Acknowledgments
This research was supported, in part, by competitive research grants from the U.S. Department of Agriculture-CSREES-NRI-CAP JDIP program.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Alix, A. J. 1999. Predictive estimation of protein linear epitopes by using the program PEOPLE. Vaccine 18:311-314. [DOI] [PubMed] [Google Scholar]
- 2.Bannantine, J. P., J. K. Hansen, M. L. Paustian, A. Amonsin, L. L. Li, J. R. Stabel, and V. Kapur. 2004. Expression and immunogenicity of proteins encoded by sequences specific to Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 42:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannantine, J. P., J. F. Huntley, E. Miltner, J. R. Stabel, and L. E. Bermudez. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 149:2061-2069. [DOI] [PubMed] [Google Scholar]
- 4.Bannantine, J. P., Q. Zhang, L. L. Li, and V. Kapur. 2003. Genomic homogeneity between Mycobacterium avium subsp. avium and Mycobacterium avium subsp. paratuberculosis belies their divergent growth rates. BMC Microbiol. 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chacon, O., L. E. Bermudez, and R. G. Barletta. 2004. Johne's disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu. Rev. Microbiol. 58:329-363. [DOI] [PubMed] [Google Scholar]
- 6.Collins, M. T., S. J. Wells, K. R. Petrini, J. E. Collins, R. D. Schultz, and R. H. Whitlock. 2005. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin. Diagn. Lab. Immunol. 12:685-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilbink, F., D. M. West, G. W. de Lisle, R. Kittelberger, B. D. Hosie, J. Hutton, M. M. Cooke, and M. Penrose. 1994. Comparison of a complement fixation test, a gel diffusion test and two absorbed and unabsorbed ELISAs for the diagnosis of paratuberculosis in sheep. Vet. Microbiol. 41:107-116. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni-Kale, U., S. Bhosle, and A. S. Kolaskar. 2005. CEP: a conformational epitope prediction server. Nucleic Acids Res. 33:W168-W171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labo, M., L. Gusberti, E. D. Rossi, P. Speziale, and G. Riccardi. 1998. Determination of a 15437 bp nucleotide sequence around the inhA gene of Mycobacterium avium and similarity analysis of the products of putative ORFs. Microbiology 144:807-814. [DOI] [PubMed] [Google Scholar]
- 10.Li, L., J. P. Bannantine, Q. Zhang, A. Amonsin, B. J. May, D. Alt, N. Banerji, S. Kanjilal, and V. Kapur. 2005. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc. Natl. Acad. Sci. USA 102:12344-12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang, X., A. Teng, D. M. Braun, J. Felgner, Y. Wang, S. I. Baker, S. Chen, O. Zelphati, and P. L. Felgner. 2002. Transcriptionally active polymerase chain reaction (TAP): high throughput gene expression using genome sequence data. J. Biol. Chem. 277:3593-3598. [DOI] [PubMed] [Google Scholar]
- 12.Odorico, M., and J. L. Pellequer. 2003. BEPITOPE: predicting the location of continuous epitopes and patterns in proteins. J. Mol. Recognit. 16:20-22. [DOI] [PubMed] [Google Scholar]
- 13.Pellequer, J. L., and E. Westhof. 1993. PREDITOP: a program for antigenicity prediction. J. Mol. Graph. 11:191-192, 204-210. [DOI] [PubMed] [Google Scholar]
- 14.Triccas, J. A., P. W. Roche, N. Winter, C. G. Feng, C. R. Butlin, and W. J. Britton. 1996. A 35-kilodalton protein is a major target of the human immune response to Mycobacterium leprae. Infect. Immun. 64:5171-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]