Abstract
An experimental bivalent meningococcal outer membrane vesicle (OMV) vaccine (B:4:P1.19,15 and B:4:P1.7-2,4) has been developed to provide wide vaccine coverage particularly of the circulating strains in Europe. A randomized, controlled phase II study (study identification number, 710158/002; ClinicalTrials.gov identifier number, NCT00137917) to evaluate the immunogenicity and safety of three doses of the OMV vaccine when given to healthy 12- to 18-year-olds on a 0-2-4 month (n = 162) or 0-1-6 month schedule (n = 159). A control group received two doses of hepatitis A and one of conjugated meningococcal serogroup C vaccine on a 0-1-6 month schedule (n = 157). Immune response, defined as a fourfold increase in serum bactericidal titer using a range of vaccine-homologous or PorA-related and heterologous strains, was determined for samples taken before and 1 month after vaccination; assays were performed at two laboratories. As measured at the GlaxoSmithKline (GSK) laboratory, the OMV vaccine induced an immune response against homologous or PorA-related strains (in at least 51% of subjects against strains of serosubtype P1.19,15 and at least 66% against strains of serosubtype P1.7-2,4) and against a set of three heterologous strains (in 28% to 46% of subjects). Both laboratories showed consistent results for immune response rates. The OMV vaccine had a similar reactogenicity profile for each schedule. Pain preventing normal activities occurred in approximately one-fifth of the subjects; this was significantly higher than in the control group. The immune responses induced by the bivalent OMV vaccine demonstrated the induction of bactericidal antibodies against the vaccine-homologous/PorA-related strains but also against heterologous strains, indicating the presence of protective antigens in OMVs and confirming the potential of clinical cross-protection.
Neisseria meningitidis serogroup B is a major cause of meningococcal disease worldwide. To date, most research into the development of vaccines against serogroup B meningococcal (MenB) disease has been directed towards outer membrane vesicle (OMV) vaccines that elicit serum bactericidal antibodies (SBA) against cell surface outer membrane proteins (OMPs) (10). Clinical trials have shown that OMV vaccines induce serum bactericidal antibodies against vaccine-homologous strains and (to a certain extent) against heterologous strains, that in different settings where meningococcal disease is endemic they are efficacious in subjects older than 4 years of age, and that three doses induce a better response than two doses (2, 5-7, 12, 14-16, 18-20, 22).
VA-MENGOC-BC is a vaccine consisting of OMVs (50 μg) from the epidemic strain B:4:P1.19,15 and the capsular polysaccharide of meningococcal serogroup C (50 μg) that was developed by the Finlay Institute in response to a serogroup B epidemic in Cuba. VA-MENGOC-BC was used effectively in a public health campaign in Cuba (9); it has also been shown to be efficacious in subjects of more than 4 years of age in settings in Brazil where heterologous meningococcal strains were circulating (7, 14). Recently, a new vaccine (MeNZB; Chiron) has been tailor-made to control the long-term epidemic of group B meningococcal disease in New Zealand, which has been dominated by subtype P1.7-2,4 (15). This new strain-specific vaccine is an OMV vaccine prepared from the B:4:P1.7-2,4 strain (New Zealand strain) and is licensed in New Zealand for use in all age groups from 6 weeks of age upwards (www.immunise.moh.govt.nz). No protective efficacy trials have been performed with the vaccine, but three vaccine doses given at 6-week intervals induced a seroresponse in approximately 75% of children and 96% of adults (15).
In contrast to N. meningitidis serogroups A, C, W135, and Y, for which immunity is related to the capsular polysaccharides, natural immunity against meningococcal serogroup B strains appears to be related mainly to the different serosubtype- and immunotype-specific protein and lipooligosaccharide (LOS) antigens, which vary from one geographical region to another. Although monovalent vaccines appear to offer some cross-protection against heterologous strains, bivalent or even multivalent vaccines would offer wider protection and would be more useful in routine vaccination programs (4).
The Finlay Institute, Havana, Cuba, in collaboration with GlaxoSmithKline (GSK) Biologicals, has developed an experimental bivalent meningococcal serogroup B vaccine containing OMVs from the B:4:P1.7-2,4 strain (from ST-44 complex/lineage 3) and B:4:P1.19,15 strain (from ST-32 complex/ET-5 complex). The experimental vaccine, which is derived from VA-MENGOC-BC with the addition of OMVs from the B:4:P1.7-2,4 strain (but without serogroup C polysaccharide), will provide wider vaccine coverage than the parent vaccine, particularly for the strains circulating in Europe: data report that the most numerous serogroup B meningococcal strains in 1999/2000 were B:4:P1.4, B:15:P1.7,16, and B:4:P1.15, representing, respectively, 59, 17, and 14% of typed serogroup B samples (13).
The primary objective of the current study was to evaluate the bactericidal immune response induced by the experimental OMV vaccine when it was given to healthy adolescents using two different vaccination schedules. As standardization of the serum bactericidal assay is known to be difficult (3, 11), the assay was performed at two different laboratories to allow interlaboratory comparisons. The secondary objective was to evaluate the safety of this vaccine.
MATERIALS AND METHODS
Subjects and ethical aspects.
Healthy adolescents 12 to 18 years of age were enrolled in the study in Spain (379 subjects in 22 centers) and Belgium (99 subjects in one center). Female subjects of childbearing potential had to agree to avoid pregnancy (by being abstinent or by using adequate contraceptive precautions); they also had to have a negative pregnancy test before each vaccination.
Adolescents were excluded from the study if they had been immunized with a meningococcal serogroup B vaccine or if they had a history of or exposure to meningococcal serogroup B infection. Acute disease or a temperature of ≥37.5°C at a vaccination visit resulted in postponement of vaccination or withdrawal from the study.
The study was approved by the ethics review committee of each of the study centers and conducted according to the Declaration of Helsinki and Good Clinical Practices. Written informed consent was obtained from all subjects and their parents or guardians.
Vaccines.
One 0.5-ml dose of the experimental vaccine contained purified OMVs of N. meningitidis strain B:4:P1.19,15 (CU385) and strain B:4:P1.7-2,4 (NZ228/98) (25 μg of each) and 0.69 mg of aluminum hydroxide. One lot of the vaccine was used.
Control vaccines were Wyeth Lederle's Meningitec (10 μg of capsular serogroup C polysaccharide conjugated to 15 μg of Corynebacterium diphtheriae CRM197 protein with 0.125 mg aluminum phosphate per 0.5-ml dose) and GSK Biologicals' Havrix (720 enzyme-linked immunosorbent assay units of hepatitis A virus, strain HM 175, with 0.05 mg aluminum hydroxide per 0.5 ml dose).
Study design.
The study was a randomized, partially unblinded study with three parallel groups. Two groups received three doses of the meningococcal OMV vaccine according to either a 0-2-4 month (MenB 0-2-4m group) or a 0-1-6 month schedule (MenB 0-1-6m group). The control group received Havrix at month 0 and month 6 and Meningitec at month 1 (control 0-1-6m group). The vaccines given to the control group and to the MenB 0-1-6m group were administered in an observer-blinded manner (i.e., subjects, parents/guardians, and personnel in charge of the reactogenicity assessment were all unaware of which vaccines were administered).
Adolescents at each study site were assigned to the three study groups using a computer-generated list of random numbers. Subjects were further stratified by age into two equal strata (12 to 15 years and 16 to 18 years).
All vaccines were administered intramuscularly into the (nondominant) deltoid. Venous blood samples were collected from each subject immediately prior to the first vaccination and at 1 month after the third vaccination. Sera were stored at temperatures between −20°C and −70°C until they were sent to GSK Biologicals (Rixensart, Belgium), where they were divided into aliquots for testing. Sera from all subjects in the MenB groups and from a randomized subset of half of the subjects in the control group were tested at GSK Biologicals. In addition, aliquots of serum from randomly selected subjects from the MenB 0-1-6m group (n = 80) and the control group (n = 40) were also sent to the Health Protection Agency (HPA) laboratory (Meningococcal Reference Unit, Manchester, United Kingdom) for testing.
Serum bactericidal assays.
Serum bactericidal antibody assays were performed using MenB wild-type clinical isolates (Table 1). All human sera to be tested were heat inactivated for 30 to 40 min at 56°C. Sera from humans with no SBA activity against the tested strains were used as the complement source.
TABLE 1.
Meningococcal strains used in serum bactericidal assays at the two laboratories
| Strain type | Laboratory | Strain | Typing | Epidemic clone | Country origin and yr |
|---|---|---|---|---|---|
| Homologous or PorA related | GSK Biologicals | M97250687a | B:4:P1.19,15:L3 | ST-32 complex | United Kingdom, 1997 |
| NZ124/98a | B:4:P1.7-2,4:L3 | ST-44 complex | New Zealand, 1998 | ||
| HPA | M01 240101b,c | B:NT:P1.19-1,15-11 | ST-269 complex | United Kingdom, 2001 | |
| M01 240149a,c | B:4:P1.7-2,4 | ST-44 complex | United Kingdom, 2001 | ||
| Heterologous | GSK Biologicals | H44/76 | B:15:P1.7,16:L3 | ST-32 complex | Norway, 1976 |
| BZ10 | B:2b:P1.2:L3 | ST-8 complex | Netherlands, 1967 | ||
| B16B6 | B:2a:P1.2:L2 | ST-11 complex | United States, mid-1960s | ||
| HPA | H44/76 | B:15:P1.7,16:L3 | ST-32 complex | Norway, 1976 | |
| M01 240355c | B:1:P1.22,14 | ST-213 complex | United Kingdom, 2001 | ||
| M01 240185c | B:2a:P1.15-1,10-8 | ST-11 complex | United Kingdom, 2001 | ||
| M01 240013c | B:NT:P1.22,9 | ST-269 complex | United Kingdom, 2001 |
Strain has PorA identical to that of the corresponding vaccine strain and is from the same MLST complex.
This strain has PorA similar but not identical to that of the corresponding vaccine strain and is from a different MLST complex.
LOS typing not available.
(i) GSK assays.
Working seed lots obtained from tryptic soy broth cultures were stored at −70°C. To eliminate phase variation, a fresh aliquot was plated on Columbia agar plate (with 5% horse serum) (Columbia horse serum agar [CHSA]) (Biomerieux) for each day's experiment.
The strains (50 μl from working seed) were spread on CHSA and incubated overnight at 37°C in 5% CO2. The following morning, bacteria from one plate were scraped with a sterile swab and streaked onto fresh CHSA. After 4 h of culture at 37°C in 5% CO2, the plates were swabbed and bacteria resuspended in phosphate-buffered saline (PBS) with 0.5 mM MgCl2-0.9 mM CaCl2 and 0.1% glucose in order to reach an A600 of 0.4 nm (bacterial suspension).
In wells of sterile flat-bottom 96-well microplates (Nunc), 25 μl of undiluted test serum was mixed with 12.5 μl of human complement and 12.5 μl of bacterial suspension. Serial twofold dilutions of test sera (in PBS-MgCl2-CaCl2-glucose) were treated similarly. Controls included bacteria plus complement, bacteria plus heat-inactivated complement, and test serum samples plus bacteria plus heat-inactivated complement.
The microplates were then sealed and incubated for 75 min at 37°C at 210 rpm without CO2. After this incubation, 50 μl of 0.9% Mueller-Hinton agar were added in each microwell, and a second layer of 50 μl of PBS-0.9% agar was added 30 min later. After an overnight incubation at 33°C in 5% CO2, the colonies were counted. The average number of CFU of the controls corresponding to bacteria plus complement was set at 100%. The lowest dilution of the test is 1:2 (seropositivity).
(ii) HPA assays.
Working cultures were prepared by streaking the mother cultures for single-colony isolation on Columbia horse blood agar (CHBA) plates (blood agar base no. 2 [Oxoid] and 10% defibrinated horse blood [Oxoid]) and culturing overnight at 37°C with 5% CO2. Following incubation, cells were harvested into brain heart infusion broth (Oxoid), aliquoted, and stored at −80°C.
Working cultures were streaked for single-colony isolation on CHBA and incubated overnight at 37°C with 5% CO2. The following morning, approximately 50 colonies were picked, streaked on fresh CHBA, and incubated for 4 h at 37°C with 5% CO2. After the incubation, colonies were suspended in bactericidal buffer (Gey's balanced salts solution [Gibco] containing 0.5% bovine serum albumin [Sigma]), and the A650 was adjusted to 0.1 nm. The number of CFU was adjusted to approximately 6 × 104 organisms/ml in bactericidal buffer.
U-bottom 96-well microtiter plates (Grenier) were used in the SBA assay. In wells, test sera (20 μl) were double diluted in bactericidal buffer, followed by the addition of 10 μl of the bacterial suspension (6 × 104 organisms/ml) and 10 μl human complement. The controls were identical to those used in the GSK procedure. Microtiter plates were sealed and incubated for 60 min at 37°C at 65 rpm without CO2. Following the incubation, 10 μl from each well was plated out using the tilt method onto CHBA to determine the number of CFU 60 min after the start (T60). After an overnight incubation at 37°C with 5% CO2, the colonies were counted. The lowest dilution of the test is 1:4 (seropositivity).
At both laboratories, a known positive serum sample was included (either on each plate or at least in each run) in addition to the controls. The acceptable limit of variability was 1 dilution in a twofold-dilution series from the assigned mean from a historical database. The reciprocal of the highest serum dilution yielding at least 50% killing at T60/T75 was reported as the titer.
Monitoring for adverse events.
All subjects were closely monitored for at least 30 min after each vaccination. Reactogenicity data were collected from diary cards completed by the subjects or their parents/guardians during the 15 days after each dose for pain, redness, and swelling at the injection site and for fever, fatigue, gastrointestinal symptoms, headache, and rash; other (unsolicited) symptoms were recorded during the 30 days after each dose. All serious adverse events occurring throughout the study period were reported by the investigators.
Statistical analysis.
Immunogenicity analyses were performed for the according-to-protocol (ATP) cohort, defined as vaccinated subjects who met all eligibility criteria, complied with protocol-defined procedures, and had pre- and postvaccination assay results available for at least one bactericidal assay. For each assay, seropositivity rates (defined as an SBA titer of ≥1:2 for GSK assays and an SBA titer of ≥1:4 for HPA assays) and response rates (defined at each laboratory as a ≥4-fold increase in prevaccination SBA titer; for GSK, this was 1 to ≥4, while for HPA, it was 2 to ≥8) each with their 95% confidence intervals (CI) and geometric mean titers (GMTs) and their 95% CI were calculated prior to the first vaccine dose and at 1 month after the third vaccine dose. GMTs were calculated by taking the antilog of the mean of the log10 titer transformations. Antibody titers below the cutoff of the assay were given an arbitrary value of half the cutoff value for the purpose of GMT calculation.
An exploratory evaluation of the coprimary endpoints (defined as the SBA-MenB response rates at 1 month after the third dose for each GSK assay) was carried out. The primary objective was considered reached if the immune response rates for each of the two homologous or PorA-related strains were statistically above 40% (one-sided P value < 0.0125 for null hypothesis; P = 40%) and if the immune response for the three heterologous strains was statistically above 20% (one-sided P value < 0.0125 for null hypothesis; P = 20%). Table 1 shows MenB strain characteristics.
The safety analysis was performed with the entire vaccinated cohort. The incidences of any adverse events within the 15 days following vaccination and their exact 95% CI were computed by group according to the type of adverse events, intensity, and relationship to vaccination.
The two-sided Fisher's exact test was used to explore differences between groups.
RESULTS
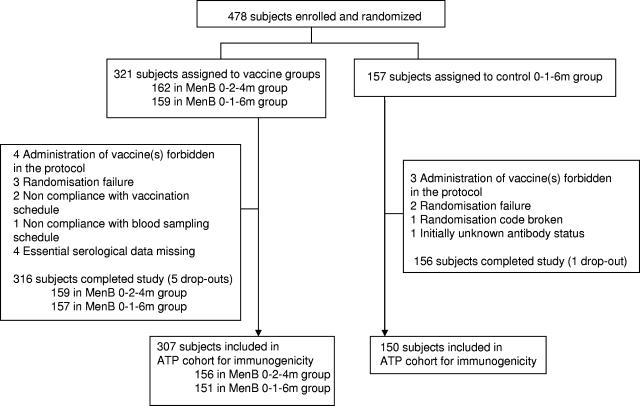
A total of 478 subjects were enrolled and were to receive three doses of the meningococcal OMV or control vaccines between 10 July 2002 and 10 June 2003. Of these, 472 completed the study and 457 were included in the ATP cohort (Fig. 1). The demographic profiles of the three groups were similar (Table 2).
FIG. 1.
Trial profile.
TABLE 2.
Demographics of subjects enrolled
| Group (n) | Mean age (yr) at the time of the first dose (range, 12-18 yr) | Sex ratio (% female/% male) |
|---|---|---|
| 0-2-4m MenB group (162) | 15.2 | 54.9/45.1 |
| 0-1-6m MenB group (159) | 15.1 | 55.3/44.7 |
| 0-1-6m control group (157) | 15.2 | 47.1/52.9 |
As the numbers of subjects in the two age strata were equal and there was no difference between the strata for the immunogenicity and reactogenicity analyses, only the results for the overall cohort are presented.
Immunogenicity.
Prevaccination seropositivity rates were high for most strains (Table 3): for GSK assays, rates ranged from 16.2% for strain B16B6 to 78.8% for strain NZ124/98; for HPA assays, rates ranged from 18.2% for M01 240185 to 90.5% for M01 240101.
TABLE 3.
Percentage of subjects with SBA titers of ≥1:4 pre- and postvaccinationa
| Laboratory and strain group | Strain | Subject group | Timing | n | % of subjects with SBA titer of ≥1:4 (95% CI) |
|---|---|---|---|---|---|
| GSK | |||||
| Vaccine homologous or PorA related strains | M97250687 | MenB 0-2-4m | Prevacc | 155 | 34.8 (27.4-42.9) |
| Postvacc | 153 | 71.2 (63.4-78.3) | |||
| MenB 0-1-6m | Prevacc | 151 | 35.8 (28.1-44.0) | ||
| Postvacc | 149 | 75.8 (68.2-82.5) | |||
| Control 0-1-6m | Prevacc | 76 | 28.9 (19.1-40.5) | ||
| Postvacc | 76 | 36.8 (26.1-48.7) | |||
| NZ124/98 | MenB 0-2-4m | Prevacc | 152 | 49.3 (41.1-57.6) | |
| Postvacc | 150 | 96.0 (91.5-98.5) | |||
| MenB 0-1-6m | Prevacc | 149 | 50.3 (42.0-58.6) | ||
| Postvacc | 149 | 95.3 (90.6-98.1) | |||
| Control 0-1-6m | Prevacc | 52 | 63.5 (49.0-76.4) | ||
| Postvacc | 53 | 56.6 (42.3-70.2) | |||
| Vaccine heterologous strains | B16B6 | MenB 0-2-4m | Prevacc | 154 | 9.7 (5.6-15.6) |
| Postvacc | 149 | 34.9 (27.3-43.1) | |||
| MenB 0-1-6m | Prevacc | 148 | 4.7 (1.9-9.5) | ||
| Postvacc | 148 | 36.5 (28.7-44.8) | |||
| Control 0-1-6m | Prevacc | 52 | 15.4 (6.9-28.1) | ||
| Postvacc | 51 | 19.6 (9.8-33.1) | |||
| BZ10 | MenB 0-2-4m | Prevacc | 152 | 38.2 (30.4-46.4) | |
| Postvacc | 150 | 62.0 (53.7-69.8) | |||
| MenB 0-1-6m | Prevacc | 150 | 38.7 (30.8-47.0) | ||
| Postvacc | 148 | 71.6 (63.6-78.7) | |||
| Control 0-1-6m | Prevacc | 54 | 50.0 (36.1-63.9) | ||
| Postvacc | 54 | 51.9 (37.8-65.7) | |||
| H44/76 | MenB 0-2-4m | Prevacc | 155 | 29.7 (22.6-37.5) | |
| Postvacc | 149 | 59.7 (51.4-67.7) | |||
| MenB 0-1-6m | Prevacc | 151 | 23.8 (17.3-31.4) | ||
| Postvacc | 149 | 55.7 (47.3-63.8) | |||
| Control 0-1-6m | Prevacc | 53 | 22.6 (12.3-36.2) | ||
| Postvacc | 53 | 30.2 (18.3-44.3) | |||
| HPA | |||||
| Vaccine homologous or PorA related strains | M02240149 | MenB 0-1-6m | Prevacc | 77 | 53.2 (41.5-64.7) |
| Postvacc | 76 | 94.7 (87.1-98.5) | |||
| Control 0-1-6m | Prevacc | 37 | 59.5 (42.1-75.2) | ||
| Postvacc | 38 | 52.6 (35.8-69.0) | |||
| M01240101 | MenB 0-1-6m | Prevacc | 74 | 90.5 (81.5-96.1) | |
| Postvacc | 75 | 100.0 (95.2-100.0) | |||
| Control 0-1-6m | Prevacc | 36 | 80.6 (64.0-91.8) | ||
| Postvacc | 35 | 85.7 (69.7-95.2) | |||
| Vaccine heterologous strains | M01240013 | MenB 0-1-6m | Prevacc | 76 | 64.5 (52.7-75.1) |
| Postvacc | 76 | 97.4 (90.8-99.7) | |||
| Control 0-1-6m | Prevacc | 37 | 73.0 (55.9-86.2) | ||
| Postvacc | 38 | 84.2 (68.7-94.0) | |||
| M01240355 | MenB 0-1-6m | Prevacc | 76 | 67.1 (55.4-77.5) | |
| Postvacc | 77 | 84.4 (74.4-91.7) | |||
| Control 0-1-6m | Prevacc | 37 | 59.5 (42.1-75.2) | ||
| Postvacc | 38 | 71.1 (54.1-84.6) | |||
| H44/76 | MenB 0-1-6m | Prevacc | 74 | 32.4 (22.0-44.3) | |
| Postvacc | 76 | 72.4 (60.9-82.0) | |||
| Control 0-1-6m | Prevacc | 36 | 33.3 (18.6-51.0) | ||
| Postvacc | 37 | 37.8 (22.5-55.2) | |||
| M01240185 | MenB 0-1-6m | Prevacc | 77 | 18.2 (10.3-28.6) | |
| Postvacc | 77 | 44.2 (32.8-55.9) | |||
| Control 0-1-6m | Prevacc | 37 | 32.4 (18.0-49.8) | ||
| Postvacc | 38 | 28.9 (15.4-45.9) |
See Table 1 for typing of strains. n, number of subjects with available results for specified assay. Prevacc, prevaccination blood sample at month 0; Postvacc, post-dose 3 vaccination blood sample at month 5 (MenB 0-2-4m) or month 7 (0-1-6m groups).
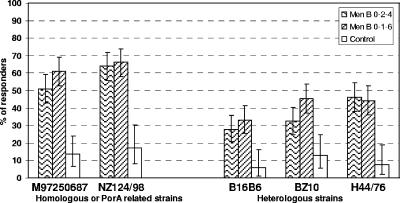
In GSK assays, the SBA-MenB response rates for the homologous or PorA-related strains ranged from 51.0% (M97250687 in the MenB 0-2-4m group) to 66.2% (NZ124/98 in MenB 0-1-6m group), and those for the heterologous strains ranged from 27.9% (B16B6 in the MenB 0-2-4m group) to 46.3% (H44/76 in the MenB 0-2-4m group) (Fig. 2).
FIG. 2.
Percentage of subjects with a ≥4-fold increase in serum bactericidal antibody to vaccine-homologous or PorA-related and heterologous strains (assays done at GSK Biologicals). All subjects in the ATP cohort for immunogenicity (156 in the MenB 0-2-4m group, 151 in the MenB 0-1-6m group, and 150 in the control group) with results available for a specific assay were included in the analysis for that assay. See Table 1 for strain typing details. The response rate was statistically higher for BZ10 for the MenB 0-1-6m versus the MenB 0-2-4m group and statistically lower for all strains for the control versus the MenB 0-1-6m group (P < 0.05; two-sided Fisher's exact test).
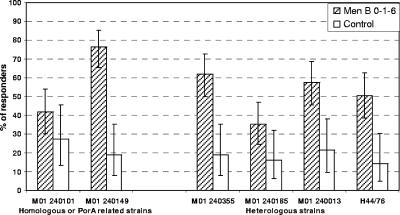
In HPA assays (MenB 0-1-6m group only), the response rate for homologous or PorA-related strains was higher for M01 240149 (76.3%) than for the vaccine-related M01 240101 strain (41.7%); the response rate for the heterologous strains ranged from 35.1% for M01 240185 to 61.8% for M01 240355 (Fig. 3).
FIG. 3.
Percentage of subjects with ≥4-fold increases in serum bactericidal antibody to vaccine-homologous or PorA-related and heterologous strains (assays done at HPA). A randomly selected subset of subjects from the MenB and control 0-1-6m groups in the ATP cohort for immunogenicity (80 in the MenB group, 40 in control group) with results available for a specific assay were included in the analysis for that assay. See Table 1 for strain typing details. The response rate was statistically lower for all strains for the control versus MenB 0-1-6m group (P < 0.05) except for M01240101 (P = 0.194; two-sided Fisher's exact test).
The primary objective of the study was met, as the SBA-MenB response rate (GSK assays) after the third dose of the meningococcal OMV vaccine was statistically above 40% (lower limit of 95% CI) for the two (M97250687 and NZ124/98) homologous or PorA-related strains and statistically above 20% (lower limit of 95% CI) for all three heterologous strains.
There was no difference between the two schedules for the two vaccine homologous or PorA-related strains or for two of the three heterologous strains; however, for bactericidal antibodies against the BZ10 heterologous strain, the 0-1-6 month schedule induced a higher response rate (45.3%) than the 0-2-4 month schedule (32.4%) (GSK assays; P value < 0.05).
One month after the third meningococcal OMV vaccine dose, the SBA-MenB GMTs for all strains were higher than before vaccination (range, 1.8-fold to 6.2-fold in GSK assays and 1.6-fold to 5.3-fold in HPA assays) (Table 4).
TABLE 4.
Pre- and postvaccination geometric mean serum bactericidal antibody titersa
| Laboratory and strain group | Strain | Subject group | Timing | n | GMT (95% CI) |
|---|---|---|---|---|---|
| GSK | |||||
| Vaccine homologous or PorA related strains | M97250687 | MenB 0-2-4m | Prevacc | 155 | 2.85 (2.27-3.57) |
| Postvacc | 153 | 10.94 (8.37-14.29) | |||
| MenB 0-1-6m | Prevacc | 151 | 2.68 (2.19-3.28) | ||
| Postvacc | 149 | 12.33 (9.45-16.08) | |||
| Control 0-1-6m | Prevacc | 76 | 2.44 (1.82-3.29) | ||
| Postvacc | 76 | 2.78 (2.05-3.77) | |||
| NZ124/98 | MenB 0-2-4m | Prevacc | 152 | 4.11 (3.20-5.28) | |
| Postvacc | 150 | 22.52 (18.41-27.56) | |||
| MenB 0-1-6m | Prevacc | 149 | 4.04 (3.16-5.16) | ||
| Postvacc | 149 | 25.01 (20.41-30.63) | |||
| Control 0-1-6m | Prevacc | 52 | 4.95 (3.48-7.04) | ||
| Postvacc | 53 | 4.16 (2.85-6.08) | |||
| Vaccine heterologous strains | B16B6 | MenB 0-2-4m | Prevacc | 154 | 1.38 (1.21-1.58) |
| Postvacc | 149 | 2.64 (2.11-3.31) | |||
| MenB 0-1-6m | Prevacc | 148 | 1.18 (1.10-1.27) | ||
| Postvac | 148 | 2.92 (2.34-3.65) | |||
| Prevacc | 52 | 1.73 (1.23-2.42) | |||
| Postvacc | 51 | 1.92 (1.34-2.75) | |||
| BZ10 | MenB 0-2-4m | Prevacc | 152 | 3.59 (2.75-4.68) | |
| Postvacc | 150 | 8.11 (6.03-10.90) | |||
| MenB 0-1-6m | Prevacc | 150 | 2.99 (2.35-3.81) | ||
| Postvacc | 148 | 9.60 (7.32-12.59) | |||
| Control 0-1-6m | Prevacc | 54 | 5.04 (3.09-8.22) | ||
| Postvacc | 54 | 5.37 (3.28-8.79) | |||
| H44/76 | MenB 0-2-4m | prevacc | 155 | 2.27 (1.85-2.78) | |
| Postvacc | 149 | 8.82 (6.41-12.14) | |||
| MenB 0-1-6m | Prevacc | 151 | 1.88 (1.57-2.24) | ||
| Postvacc | 149 | 6.64 (5.00-8.82) | |||
| Control 0-1-6m | Prevacc | 53 | 2.63 (1.70-4.08) | ||
| Postvacc | 53 | 2.14 (1.52-2.99) | |||
| HPA | |||||
| Vaccine homologous or PorA related strains | M02240149 | MenB 0-1-6m | Prevacc | 77 | 4.46 (3.58-5.55) |
| Postvacc | 76 | 23.47 (17.51-31.46) | |||
| Control 0-1-6m | Prevacc | 37 | 5.93 (3.81-9.21) | ||
| Postvacc | 38 | 5.66 (3.65-8.78) | |||
| M01240101 | MenB 0-1-6m | Prevacc | 74 | 16.61 (12.65-21.81) | |
| Postvacc | 75 | 34.46 (25.51-46.54) | |||
| Control 0-1-6m | Prevacc | 36 | 14.53 (9.06-23.32) | ||
| Postvacc | 35 | 18.75 (11.48-30.61) | |||
| Vaccine heterologous strains | M01240013 | MenB 0-1-6m | Prevacc | 76 | 7.78 (5.72-10.60) |
| Postvacc | 76 | 29.75 (22.55-39.24) | |||
| Control 0-1-6m | Prevacc | 37 | 8.95 (5.86-13.68) | ||
| Postvacc | 38 | 8.15 (5.61-11.8) | |||
| M01240355 | MenB 0-1-6m | Prevacc | 76 | 7.57 (5.58-10.28) | |
| Postvacc | 77 | 28.72 (19.93-41.40) | |||
| Control 0-1-6m | Prevacc | 37 | 8.31 (4.98-13.84) | ||
| Postvacc | 38 | 8.76 (5.64-13.62) | |||
| H44/76 | MenB 0-1-6m | Prevacc | 74 | 3.89 (2.93-5.16) | |
| Postvacc | 76 | 14.87 (10.27-21.55) | |||
| Control 0-1-6m | Prevacc | 36 | 3.92 (2.69-5.73) | ||
| Postvacc | 37 | 4.73 (3.07-7.31) | |||
| M01240185 | MenB 0-1-6m | Prevacc | 77 | 2.50 (2.19-2.87) | |
| Postvacc | 77 | 4.00 (3.21-4.99) | |||
| Control 0-1-6m | Prevacc | 37 | 3.02 (2.36-3.86) | ||
| Postvacc | 38 | 3.33 (2.36-4.71) |
See Table 1 for typing of strains. n, number of subjects with available results for specified assay. Prevacc, prevaccination blood sample at month 0; Postvacc, post-dose 3 vaccination blood sample at month 5 (MenB 0-2-4m) or month 7 (0-1-6m groups).
SBA-MenB assays using the H44/76 strain were done at both the HPA and GSK laboratories: the immune response results from both laboratories were in the same range (response rates for MenB 0-1-6m group, 50.7% with the GSK assay and 44.3% with HPA assay) (Fig. 2 and 3).
One month after the three vaccine doses, SBA-MenB response rates were lower (P < 0.05) in the control group than in the MenB 0-1-6m groups for each of the strains tested in both GSK and HPA assays, except for M01 240101 (P = 0.194) (Fig. 2 and 3). GMTs in the control group were either lower or very slightly higher (maximum, 1.3-fold increase) after vaccination than before vaccination for all strains (Table 4).
Safety.
There were no serious adverse reactions that were related to the administration of the meningococcal OMV vaccine. The safety and reactogenicity profiles of the OMV vaccine were similar for both schedules (Table 5). The incidences of each solicited local symptom and of those graded 3 were higher in the MenB 0-1-6m group than in the control group (P < 0.05 for each symptom).
TABLE 5.
Incidence of local and general solicited symptoms over the three-dose vaccination coursea
| Symptom | Typeb | MenB 0-2-4m group (n = 481)
|
MenB 0-1-6m group (n = 476)
|
Control 0-1-6m group (n = 469)
|
P valuec for difference in incidence between MenB and control 0-1-6m groups | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| Pain | Any | 85.0 | 81.5-88.1 | 84. | 80.4-87.2 | 42.9 | 38.3-47.5 | <0.001 |
| Grade 3 | 11.0 | 8.4-14.2 | 10.3 | 7.7-13.4 | 0.9 | 0.2-2.2 | <0.001 | |
| Redness | Any | 26.8 | 22.9-31.0 | 30.0 | 26.0-34.4 | 8.3 | 6.0-11.2 | <0.001 |
| >50 mm | 1.5 | 0.6-3.0 | 1.7 | 0.7-3.3 | 0.0 | 0.0-0.8 | <0.001 | |
| Swelling | Any | 22.5 | 18.8-26.4 | 27.9 | 24.0-32.2 | 6.2 | 4.2-8.8 | <0.001 |
| >50 mm | 2.1 | 1.0-3.8 | 2.7 | 1.5-4.6 | 0.4 | 0.1-1.5 | 0.007 | |
| Fatigue | Any | 28.5 | 24.5-32.7 | 30.3 | 26.2-34.6 | 25.4 | 21.5-29.6 | 0.096 |
| Grade 3 | 1.7 | 0.7-3.3 | 2.1 | 1.0-3.8 | 1.3 | 0.5-2.8 | 0.451 | |
| Gastrointestinal symptoms | Any | 14.3 | 11.3-17.8 | 13.2 | 10.3-16.6 | 10.4 | 7.8-13.6 | 0.192 |
| Grade 3 | 1.2 | 0.5-2.7 | 1.9 | 0.9-3.6 | 2.3 | 1.2-4.2 | 0.658 | |
| Headache | Any | 28.3 | 24.3-32.5 | 35.9 | 31.6-40.4 | 25.2 | 21.3-29.3 | <0.001 |
| Grade 3 | 2.5 | 1.3-4.3 | 3.4 | 1.9-5.4 | 2.1 | 1.0-3.9 | 0.321 | |
| Rash | Any | 0.2 | 0.0-1.2 | 0.2 | 0.0-1.2 | 0.6 | 0.1-1.9 | 0.368 |
| Grade 3 | 0.0 | 0.0-0.8 | 0.0 | 0.0-0.8 | 0.0 | 0.0-0.8 | ||
| Fever (axillary route) | ≥37.5°C | 5.0 | 3.2-7.3 | 5.0 | 3.3-7.4 | 4.3 | 2.6-6.5 | 0.644 |
| >39.5°C | 0.0 | 0.0-0.8 | 0.0 | 0.0-0.8 | 0.0 | 0.0-0.8 | ||
n, number of injected doses. %, percentage of doses followed by specified symptom.
Symptoms were graded 1 to 3 in intensity, with grade 3 symptoms defined as those preventing normal activity. At the time of analysis, injection site redness or swelling of >50 mm in diameter and fever of >39.5°C were graded 3.
Two-sided Fisher's exact test (assuming independence of doses within subject).
For each solicited general symptom there was no difference between the MenB 0-1-6m group and the control group except for headache, which was higher (P value < 0.01) in the MenB 0-1-6m group than in the control group. Rashes were reported in two subjects vaccinated with the meningococcal OMV vaccine; both resolved within a few days.
DISCUSSION
A three-dose vaccination schedule with the experimental bivalent meningococcal vaccine containing OMVs of N. meningitidis serogroup B strains B:4:P1.15,19 and B:4:P1.7-2,4 induced SBAs against both vaccine-homologous or PorA-related and heterologous strains.
An SBA titer of ≥1:4 has been proposed as protective (9). In this study, most subjects had a titer of SBA against strain B:4:P1.7-2,4 of at least 1:4 prior to vaccination, which is as described previously for a similar age group (8). A fourfold increase in SBA titer is therefore a more appropriate measure of vaccine response to a meningococcal B vaccine in this population (25). The meningococcal OMV vaccine induced immune response rates of 51% to 66% against the different vaccine-homologous or PorA-related strains tested in this study (GSK assays).
The rates of immune response to the vaccine-homologous or PorA-related strains of serosubtype B:4:P1.19,15 were similar in the European subjects in this study to those reported for the parent vaccine, VA-MENGOC-BC, in subjects in studies in Chile (20) and in Iceland (16).
Clinical studies have estimated the efficacy of VA-MENGOC-BC against the Cuban epidemic strain (from which the vaccine was derived) to be 83% (19) and the efficacy of the OMV vaccine developed at the National Institute of Public Health (Oslo, Norway) against the Norwegian epidemic strain to be 57% in children 12 to 16 years of age (2). In addition, case-control studies have shown VA-MENGOC-BC to be effective (>70%) in subjects older than 4 years of age in epidemiological settings where both vaccine-homologous and heterologous strains were circulating (5, 14); furthermore, no evidence for serotype-specific protection was found in these studies, suggesting that the protection was cross-reactive. From these results, it appears that the bactericidal immune response underestimates the true efficacy of meningococcal serogroup B vaccines for both homologous and heterologous strains. This underestimation is most likely explained by the fact that even though SBA activity is the major mechanism of protection, others, such as opsonic activity, may also confer protection in the absence of complement-mediated bactericidal activity, as observed in the infant rat challenge model (21, 23, 24) and in human volunteers (1).
A correlation between immune responses and protection rates has also been shown for other meningococcal OMV vaccines (5, 9). Furthermore, MeNZB has been licensed in New Zealand on the basis of immune response, as measured by SBA assay, on the grounds that this provides a good indication of a probable protection (15).
In this study, the vaccine response against heterologous strains was variable (28 to 62% for the six strains tested at the two laboratories), with the lowest response (28% and 33%) being against strain B16B6. As all the strains used in the SBA assays in our study express LOS immunotype 3 except B16B6, which expresses LOS immunotype 2, these results suggest that non-PorA-cross-reactive bactericidal antibodies are induced partly by the conserved OMP and partly by the LOS part of the OMVs. The demonstrated SBA response against strain B16B6 can best be explained via cross-reactive OMPs. The importance of LOS was also demonstrated in a recent study of a meningococcal B vaccine with LOS as its major component (W. D. Zollinger, J. G. Babcock, B. L. Brandt, E. E. Moran, N. M. Wassif, and C. R. Alving, poster no. 200, 14th International Pathogenic Neisseria Conference, Milwaukee, Wis., 5 to 10 September, 2004).
The SBA-MenB assays were performed at two different laboratories using different methods and different strains, although one strain (H44/76) was common to both laboratories. As expected, there were differences in the titers achieved at each laboratory.
Based on responses against the standard strain (H44/76) but also the other strains, HPA SBA titers are on average twice as high as GSK titers, and this was observed for both prevaccination and postvaccination sera. Despite these differences, the measured vaccine responses were similar between laboratories. Additionally, the overall response rates were comparable at both laboratories for the homologous or PorA-related and the heterologous strains, although in the vaccine response against the P1.19,15 strains, the result was slightly lower (41%) for M01 240101 (ST-269 complex) at HPA than that for M97250687 (ST-32 complex) at GSK (61.1%); interlaboratory variability and/or the use of a strain of a different epidemic clone may have played a role. The P1.19,15 strain used at GSK was similar to the Cuban P1.19,15 vaccine strain (both strains belong to the same clonal complex, ST-32), while the P1.19-1,15-11 strain used at HPA is from the ST-269 complex and can be considered to represent a variant from the vaccine strain. These results are similar to those already reported for another interlaboratory comparison of SBA-MenB assays (3, 11).
The relatively high proportion (about 20%) of subjects in the control group with at least a fourfold increase in SBA for half the strains tested by the HPA lab might be explained by the higher sensitivity of the assays developed by HPA than those developed by GSK, as observed by the overall higher GMT obtained by HPA (compared to GSK) for all time points and with all strains. Between the bleedings done before the vaccination and after vaccination, some subjects from the control group may have been exposed to N. meningitidis strains (carriage) and have produced low levels of bactericidal antibodies that were detected only by the HPA assays.
The bivalent meningococcal OMV vaccine was safe, with no serious adverse reactions related to vaccination reported. It has already been recognized that a high rate of local reactions occurs following the administration of meningococcal OMV vaccines containing aluminum hydroxide (17), as in this study. Although adverse reactions were reported frequently, few severe systemic reactions were related to vaccination. As reported for this age group with other meningococcal OMV vaccines and the parent vaccine, pain at the injection site was the most frequently reported adverse event (6, 15, 20). Indeed, the incidence of local pain in this study is similar to that seen in the older age group vaccinated with the new MeNZB vaccine (also aluminum hydroxide adsorbed), which has recently been licensed for use in New Zealand (15); the incidence is also similar to that reported following administration of the parent vaccine, VA-MENGOC-BC, which has now been administered with an acceptable safety profile to approximately 28 million subjects.
The immune responses induced by the bivalent OMV vaccine demonstrated the induction of bactericidal antibodies against both PorA-homologous strains but also against strains with different PorA proteins, indicating the presence of other protective antigens in OMVs and confirming the potential for clinical cross-protection.
Acknowledgments
This study (study identifier number 710158/02, ClinicalTrials.gov identifier number NCT00137917) was supported by GlaxoSmithKline Biologicals, Rixensart, Belgium, which covered all the costs of the study except for the bactericidal assays performed at the Health Protection Agency, which were supported by United Kingdom Meningitis Research Foundation grant number 16/00.
We are grateful to all the children and parents that participated in the study, to Sheila Woods for her assistance in preparation of the manuscript and to all those who helped carry out the study, including the collaborating physicians in the Spanish MenB-002 study group (Vicente Cabedo [Grupo de Investigación de Atención Primaria de Castellón {GIAP-CS group}], José Carvajal, Alberto Cortilla [GIAP-CS group], Manuel Enrubia, María Garcés [Vaccines Institute of Valencia {VIVA}], Luis García [VIVA], Mercedes García [VIVA], Josep Marés, Manuel Martínez-Pons [VIVA], Vicente Meneu [GIAP-CS group], Carmen Peidró [VIVA], Angels Ramón, Neus Rodríguez [GIAP-CS group], Nidia Ruiz [VIVA], Blanca Ruiz-Borau [GIAP-CS group], Vicente Santamaría [GIAP-CS group], Isabel Ubeda [VIVA], Pilar Garcia-Corbeira, Sylvie Vandendunghen, Marc Gillet, and Reyes Boceta-Munoz) and the personnel at the HPA laboratory (Rita Barchha, Ewan Harrison, and Ann Lowe).
Footnotes
Published ahead of print on 25 October 2006.
REFERENCES
- 1.Aase, A., T. K. Herstad, L. M. Naess, T. E. Michaelsen, and D. R. Martin. 2004. Opsonophagocytic activity of human sera after vaccination with two different group B meningococcal vaccines in a clinical trial in New Zealand, abstr. 63. Abstracts of the 14th International Pathogenic Neisseria Conference, Milwaukee, Wis.
- 2.Bjune, G., E. A. Holby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, and E. Rosenqvist. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, R., I. S. Aaberge, G. F. Santos, T. L. Eudey, P. Oster, A. Glennie, J. Findlow, E. A. Høiby, E. Rosenqvist, P. Balmer, and Martin. 2005. Interlaboratory standardization of the measurement of serum bactericidal activity using human complement against meningococcal serogroup B, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab. Immunol. 12:970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, J. M., H. C. Rümke, R. Welte, M. J. Postma, and J. C. Jager. 2001. Heath economics of an hexavalent meningococcal outer-membrane vesicle vaccine in children: potential impact of introduction in the Dutch vaccination programme. Vaccine 20:202-207. [DOI] [PubMed] [Google Scholar]
- 5.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, and W. Silva. 1995. Efficacy, safety and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 6.de Kleijn, E. D., R. de Groot, A. B. Lafeber, J. Labadie, K. C. J. P. van Limpt, J. Visser, G. A. M. Berbers, L. van Alphen, and H. C. Rümke. 2000. Immunogenicity and safety of monovalent P1.7h,4 meningococcal outer membrane vesicle vaccine in toddlers: comparison of two vaccination schedules and two formulations. Vaccine 19:1141-1148. [DOI] [PubMed] [Google Scholar]
- 7.de Moraes, J. C., B. A. Perkins, M. C. C. Camargo, N. T. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. Landgraf, V. L. Gattas, and H. G. Vasconcelos. 1992. Protective efficacy of a serogroup B meningococcal vaccine in São Paulo, Brazil. Lancet 340:1074-1078. [DOI] [PubMed] [Google Scholar]
- 8.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of natural humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Hoiby, H. Nokleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccine against Neisseria meningitidis serogroup B disease. Vaccine 21:734-737. [DOI] [PubMed] [Google Scholar]
- 10.Jódar, L., I. M. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 11.Martin, D. R., L. McCallum, A. Gennie, N. Ruijne, P. Blatchford, J. O'Hallahan, and P. Oster. 2005. Validation of the serum bactericidal assay for measurement of functional antibodies against group B meningococci associated with vaccine trials. Vaccine 23:2218-2221. [DOI] [PubMed] [Google Scholar]
- 12.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. Melles, V. S. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noah, N., and B. Henderson. 2002. Surveillance of bacterial meningitis in Europe 1999/2000. Abridged version. Public Health Laboratory Service. Consulted at www.hpa.org.uk/hpa/inter/m_surveillance9900.pdf, 23 August 2006.
- 14.Noronha, C. P., C. J. Struchiner, and M. E. Halloran. 1995. Assessment of the direct effectiveness of BC meningococcal vaccine in Rio de Janeiro, Brazil: a case-control study. Int. J. Epidemiol. 24:1050-1057. [DOI] [PubMed] [Google Scholar]
- 15.Oster, P., D. Lennon, J. O'Hallahan, K. Mulholland, S. Reid, and D. Martin. 2005. MeNZB™: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23:2191-2196. [DOI] [PubMed] [Google Scholar]
- 16.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 17.Rosenqvist, E., E. A. Hoiby, G. Bjune, A. Aase, A. Halstensen, A. Lehmann, J. Paulssen, J. Holst, T. Michaelsen, H. Nokleby, L. Froholm, and O. Closs. 1998. Effect of aluminium hydroxide and meningococcal serogroup C capsular polysaccharide on the immunogenicity and reactogenicity of a group B Neisseria meningitidis outer membrane vesicle vaccine. Dev. Biol. Stand. 92:323-333. [PubMed] [Google Scholar]
- 18.Rosenqvist, E., E. A. Høiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sierra, G. V. G., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Annals 14:195-210. [PubMed] [Google Scholar]
- 20.Tappero, J. W., R. Lagos A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines. A randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 21.Toropainen, M., L. Saarinen, E. Wedege, et al. 2005. Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis. Infect. Immun. 73:4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermont, C. L., H. H. van Dijken, A. J. Kuipers, et al. 2003. Cross-reactivity of antibodies against PorA after vaccination with a meningococcal B outer membrane vesicle vaccine. Infect. Immun. 71:1650-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsch, J. A., G. R. Moe, R. Rossi, et al. 2003. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J. Infect. Dis. 188:1730-1740. [DOI] [PubMed] [Google Scholar]
- 24.Welsch, J. A., R. Rossi, M. Comanducci, and D. M. Granoff. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 172:5606-5615. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 1981. Thirty-first report of the WHO Expert Committee on Biological Standardization: WHO technical report series, no. 658. Annex 5. Amendment. WHO Tech. Rep. Ser. 1981:183-184. [PubMed] [Google Scholar]