Abstract
Antibodies to a modified group B meningococcal polysaccharide vaccine were examined for antigenic and functional specificities. Bactericidal determinants were investigated by using immunoaffinity columns and competitive inhibition of bactericidal activity in an in vitro killing assay. We conclude that nearly all of the vaccine-induced bactericidal activity is specific for the native polysaccharide.
Neisseria meningitidis has emerged as an important cause of meningitis and septicemia throughout the world (8, 23, 24). The capsular polysaccharide of group B N. meningitidis, a homopolymer of α(2→8)-linked sialic acid, is chemically and physically identical to the long-chain polysialic acid (PSA) expressed abundantly in the brain, heart, and kidneys during fetal development (4, 21). Likely because of this identity with PSA, group B meningococcal polysaccharide (GBMP) is poorly immunogenic (25) even when complexed with (26) or conjugated to (11) protein. Serum antibodies to GBMP elicited by vaccination or invasive meningococcal disease are reported to be predominantly immunoglobulin M (IgM) (1, 15, 16, 26) and of low avidity (16).
To make the GBMP more immunogenic, the PSA has been chemically modified by replacing the N-acetyl group of the polysaccharide with an N-propionyl (NPr) group at the C-5 position (9, 22). This chemical modification has been shown to make the conjugate more immunogenic (2, 3, 5, 6, 7, 12, 14), but the epitope(s) on the bacterial surface recognized by the protective anti-NPrGBMP antibodies is not known (19, 20).
We examined the nature of the immune responses of mice and baboons to NPrGBMP-rPorB conjugate vaccine. Antisera produced from these animal models were subject to various affinity purification protocols and further examined by enzyme-linked immunosorbent assay (ELISA) and serum bactericidal assay (SBA). Reactivity of the antisera to NPrGBMP and GBMP, as well as cross-reactivity to group C meningococcal polysaccharide (GCMP), was examined.
(Portions of this work were presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October, 1997, abstr. G-2, p. 192.)
Immunoaffinity purification of mouse anti-NPrGBMP-rPorB antisera.
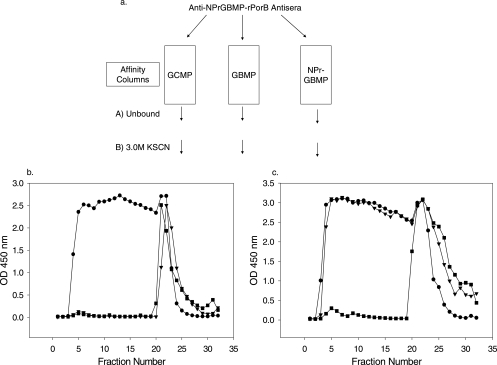
Mouse anti-NPrGBMP-rPorB antisera, raised as previously described (6), were pooled and subjected to affinity purification as characterized in Fig. 1a. Pooled antisera were diluted with an equal volume of phosphate-buffered saline (pH = 7.4) and applied to either GCMP-human serum albumin (HSA), GBMP-HSA, or NPrGBMP-HSA immunoaffinity resin constructed on an Affi-Gel 15 support according to the manufacturer's (Bio-Rad, Hercules, CA) instructions. The HSA conjugates used for ELISA and affinity chromatography were as previously described (6, 10, 17). Following the collection of the unbound effluent from each of the columns, bound antibodies were eluted with 3.0 M KSCN. Fractions collected from the columns were analyzed by ELISA (18). Representative ELISAs of column profiles eluted with 3.0 M KSCN following loading are shown in Fig. 1. Column fractions were analyzed on microtiter plates coated with native GBMP-HSA conjugate (Fig 1b). Both the GBMP-HSA and NPrGBMP-HSA columns retained all of the GBMP-specific Ig from the mouse antisera, while the GCMP-HSA column did not adsorb much GBMP-specific Ig. The same column fractions were also analyzed on microtiter plates coated with NPrGBMP-HSA (Fig 1c). The NPrGBMP-HSA column captured nearly all of the NPrGBMP-specific Ig; most of the NPrGBMP-specific Ig was not retained by either the native GBMP-HSA or the GCMP-HSA support.
FIG. 1.
(a) Overview of affinity column purification scheme. Mouse anti-NPrGBMP-rPorB antisera were pooled and run over affinity matrices as shown. Bound antibodies were eluted from each resin with 3.0 M KSCN. (b) ELISA of affinity column fractions on a microtiter plate coated with native GBMP-HSA. (c) ELISA of the same affinity column fractions on a microtiter plate coated with modified NPrGBMP-HSA. Symbols: •, GCMP column; ▾, GBMP column; ▪, NPrGBMP column. OD 450 nm, optical density at 450 nm.
The affinity purification results indicated that immunization of mice with NPrGBMP-rPorB induced a major antibody response specific for NPrGBMP but not highly cross-reactive with either native GBMP or GCMP. These results also demonstrated the effectiveness of the GBMP-HSA and NPrGBMP-HSA columns at capturing most of the specifically reactive antibody.
SBA of immunoaffinity column pools.
Mouse antisera were immunopurified as already described (Fig 1a), and unbound and 3.0 M KSCN pools from each column were normalized according to the initial volume of antisera applied and then used in an SBA (6). These results were compared to the original mouse antiserum pool (Table 1). Both the native GBMP-HSA and modified NPrGBMP-HSA columns retained virtually all of the serum bactericidal activity from the original mouse antisera while the GCMP-HSA column adsorbed no serum bactericidal activity. A portion of the serum bactericidal activity was recovered in the 3.0 M KSCN eluates of both the GBMP-HSA and NPrGBMP-HSA columns, whereas no bactericidal activity was recovered from the 3.0 M KSCN pool from the GCMP-HSA affinity resin. The lack of complete recovery of serum bactericidal activity from the columns is likely due to antibody denaturation.
TABLE 1.
Mouse anti-NPrGBMP-rPorB affinity purification
| Pool | Bactericidal titer | Bactericidal activity (%) |
|---|---|---|
| Mouse anti-NPrGBMP-rPorB | 1,437 | 100.0 |
| GCMP affinity, unbound | 1570 | 109.2 |
| GCMP affinity, 3.0 M KSCN | <10 | <0.7 |
| GBMP affinity, unbound | <24 | <1.7 |
| GBMP affinity, 3.0 M KSCN | 165 | 11.5 |
| NPrGBMP affinity, unbound | <25 | <1.7 |
| NPrGBMP affinity, 3.0 M KSCN | 350 | 24.3 |
Baboons were previously used as a nonhuman primate model to measure the response to NPrGBMP-rPorB conjugate vaccine (6). The baboon antisera were purified over the three immunoaffinity resins as described for the murine antisera. The results of these experiments (Table 2) were similar to those described for mice. The GBMP and NPrGBMP affinity columns retained most of the antibody responsible for serum bactericidal activity, while the GCMP column did not.
TABLE 2.
Baboon anti-NPrGBMP-rPorB affinity purification
| Pool | Bactericidal titer | Bactericidal activity (%) |
|---|---|---|
| Baboon anti-NPrGBMP-rPorB | 123,022 | 100.0 |
| GCMP affinity, unbound | 107,027 | 87.0 |
| GCMP affinity, 3.0 M KSCN | <41.1 | <0.04 |
| GBMP affinity, unbound | 12,859 | 10.2 |
| GBMP affinity, 3.0 M KSCN | 15,914 | 12.9 |
| NPrGBMP affinity, unbound | 3,906 | 3.2 |
| NPrGBMP affinity, 3.0 M KSCN | 20,275 | 16.5 |
It is notable that the native GBMP column captured less of the serum bactericidal activity-relevant antibody population than did the NPrGBMP column. Several explanations for this are possible. It may be that both of these columns were somewhat overloaded, that the baboon SBA was highly sensitive and therefore better at capturing minute active Ig populations, or that these unbound antibodies were representative of other bactericidal antibody populations not bound by either immunoaffinity resin. A fourth possibility has been described previously (6), where it was proposed that the increased efficiency of bactericidal inhibition by different forms of GBMP, whether conjugated or propionylated, might be due to a higher density of reactive epitopes, possibly related to some structural stabilization from the chemical process. It should also be noted that repeated immunization with NPrGBMP-protein conjugate vaccine has been observed to shift the immune response more to the chemically modified form of the polysaccharide and away from the native GBMP structure (6, 20). In fact, there may be a nonautoreactive epitope present in the native GBMP that is further expressed or exposed in the NPrGBMP (20). The higher density of reactive epitopes in NPrGBMP, coupled with the nonnative antibody population, might therefore account for the NPrGBMP-HSA affinity resin capturing more bactericidal activity than the native GBMP-HSA column (Table 2).
Inhibition of mouse anti-NPrGBMP-rPorB serum bactericidal activity.
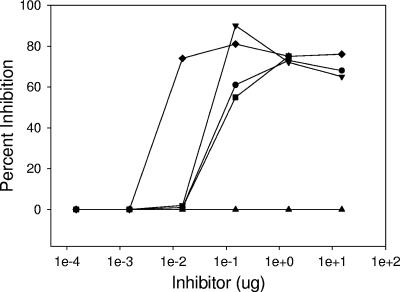
The specificity of mouse anti-NPrGBMP was further examined in an assay wherein the serum bactericidal activity of the antisera was inhibited with antigens related to those chosen to construct the immunoaffinity resins (Fig 2). Inhibition assays with the mouse antisera and the inhibitors were carried out overnight at 4°C to maximize antigen-antibody interactions (16). The most efficient inhibitor of serum bactericidal activity was the NPrGBMP-HSA conjugate, which was approximately 5-fold more efficient than unconjugated NPrGBMP and 8- and 15-fold more efficient than native GBMP and GBMP-HSA conjugate, respectively. Notably, both GBMP-HSA and unconjugated GBMP were effective inhibitors in this assay; GCMP-HSA conjugate, by contrast, was not at all effective.
FIG. 2.
Inhibition of serum bactericidal activity of mouse anti-NPrGBMP-rPorB pooled antisera. Symbols: •, GBMP; ▾, NPrGBMP; ▪, GBMP-HSA; ⧫, NPrGBMP-HSA; ▴, GCMP-HSA.
These results argue against the existence of a unique bactericidal epitope contained in the NPrGBMP-rPorB conjugate as previously described (9, 10). It was previously noted that larger-molecular-size GBMP was able to precipitate more antibody from a group B horse antiserum than a smaller counterpart (13). The GBMP used to construct our affinity matrix was approximately 55 to 60 monosaccharide repeats, compared with the 20 repeats described previously (10), and this may account for the improved capture of bactericidal antibody on our resin compared to the results described in the past. In addition, we used an HSA protein spacer between the resin and the polysaccharide, thereby possibly enabling greater access to the polysaccharide for antibody populations that might otherwise be sterically hindered.
ELISA of immunoaffinity column pools.
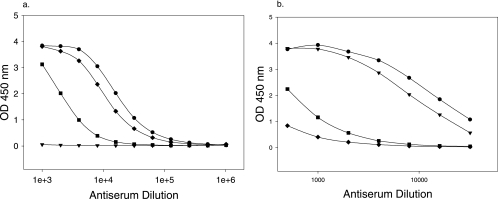
Pooled fractions from GBMP-HSA and NPrGBMP-HSA immunoaffinity column purifications of mouse antisera sequentially eluted with 1.0 M NaCl, followed by 3.0 M KSCN, were dialyzed against phosphate-buffered saline, normalized according to the original volume of antisera applied to the columns, and analyzed by ELISA (Fig. 3). The intent of the sequential elution protocol was to remove first weakly binding antibody from the columns with 1.0 M NaCl and then higher-affinity antibody with 3.0 M KSCN.
FIG. 3.
ELISA of affinity-purified pooled column fractions. Affinity-purified antiserum pools were analyzed on NPrGBMP-HSA-coated microtiter plates. (a) Pools isolated from a modified NPrGBMP-HSA affinity column. (b) Pools isolated from a native GBMP-HSA affinity column. Symbols: •, mouse anti-NPrGBMP pool; ▾, unbound; ▪, 1.0 M NaCl eluate; ⧫, 3.0 M KSCN eluate. OD 450 nm, optical density at 450 nm.
Pools recovered from the NPrGBMP-HSA column were examined on a microtiter plate coated with NPrGBMP-HSA (Fig. 3a). Unbound fractions displayed no anti-NPrGBMP activity. Bound Ig eluted from the NPrGBMP-HSA column with 3.0 M KSCN had higher specific activity to NPrGBMP than did Ig eluted from the same column with 1.0 M NaCl. This could be indicative of either a greater number of Ig molecules in the 3.0 M KSCN pool or an antibody population displaying greater avidity for the solid phase.
Pooled column fractions recovered from the GBMP-HSA column were similarly analyzed (Fig. 3b). Most of the NPrGBMP-specific Ig passed through the native PS column, while most of the Ig that bound to the resin was eluted with 1.0 M NaCl, indicating an antibody population of low avidity to native GBMP. The higher-avidity antibody population, eluted with 3.0 M KSCN, was a very minor component of the antibody population bound to the native GBMP column. The antibody populations from the GBMP column were in stark contrast to those eluted from the NPrGBMP affinity column. Most of the anti-NPrGBMP activity of the antiserum was not retained by the GBMP affinity matrix, and most of the antibody bound was gently eluted with 1.0 M NaCl. There was an antibody population that bound with higher apparent avidity to GBMP that was eluted with 3.0 M KSCN, but that was a small fraction of the initial antibody population specific for native GBMP and an even smaller fraction of the total antibody specific for NPrGBMP.
A valid area of concern that needs to be addressed is that chemical modification of the polysaccharide may result in high levels of antibodies that are not necessarily bactericidal or otherwise functional. Nonetheless, the modification appears to enhance the presence of the bactericidal epitopes which are identical to those contained within the native structure.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Andersen, J., L. Berthelsen, and I. Lind. 1997. Measurement of antibodies against meningococcal capsular polysaccharides B and C in enzyme-linked immunosorbent assays: towards an improved surveillance of meningococcal disease. Clin. Diagn. Lab. Immunol. 4:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashton, F. E., J. A. Ryan, F. Michon, and H. J. Jennings. 1989. Protective efficacy of mouse serum to the N-propionyl derivative of meningococcal group B polysaccharide. Microb. Pathog. 6:455-458. [DOI] [PubMed] [Google Scholar]
- 3.Coquillat, D., J. Bruge, B. Danve, M. Latour, C. Hurpin, D. Schulz, P. Durbec, and G. Rougon. 2001. Activity and cross-reactivity of antibodies induced in mice by immunization with a group B meningococcal conjugate. Infect. Immun. 69:7130-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finne, J., M. Leinonen, and P. H. Mäkelä. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355-357. [DOI] [PubMed] [Google Scholar]
- 5.Fusco, P. C., M. S. Blake, and F. Michon. 1998. Meningococcal vaccine development: a novel approach. Expert Opin. Investig. Drugs 7:245-252. [DOI] [PubMed] [Google Scholar]
- 6.Fusco, P. C., F. Michon, J. Y. Tai, and M. S. Blake. 1997. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and nonhuman primates. J. Infect. Dis. 175:364-372. [DOI] [PubMed] [Google Scholar]
- 7.Granoff, D. M., A. Bartoloni, S. Ricci, E. Gallo, D. Rosa, N. Ravenscoft, V. Guarnieri, R. C. Seid, A. Shan, W. R. Usinger, S. Tan, Y. E. McHugh, and G. R. Moe. 1998. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J. Immunol. 160:5028-5036. [PubMed] [Google Scholar]
- 8.Jafari, H. S., B. A. Perkins, and J. D. Wenger. 1997. Control and prevention of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 46:1-51. [PubMed] [Google Scholar]
- 9.Jennings, H. J., A. Gamian, and F. E. Ashton. 1987. N-propionylated group B meningococcal polysaccharide mimics a unique epitope on group B Neisseria meningitidis. J. Exp. Med. 165:1207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings, H. J., A. Gamian, F. Michon, and F. E. Ashton. 1989. Unique intermolecular bactericidal epitope involving the homosialopolysaccharide capsule on the cell surface of group B Neisseria meningitidis and Escherichia coli K1. J. Immunol. 142:3585-3591. [PubMed] [Google Scholar]
- 11.Jennings, H. J., and C. Lugowski. 1981. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 127:1011-1018. [PubMed] [Google Scholar]
- 12.Jennings, H. J., R. Roy, and A. Gamian. 1986. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J. Immunol. 137:1708-1713. [PubMed] [Google Scholar]
- 13.Jennings, H. J., R. Roy, and F. Michon. 1985. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J. Immunol. 134:2651-2657. [PubMed] [Google Scholar]
- 14.Jennings, H. J. 1997. N-Propionylated group B meningococcal polysaccharide glycoconjugate vaccine against group B meningococcal meningitis. Int. J. Infect. Dis. 1:158-164. [Google Scholar]
- 15.Leinonen, M., and C. E. Frasch. 1982. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect. Immun. 38:1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandrell, R. E., and W. D. Zollinger. 1982. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J. Immunol. 129:2172-2178. [PubMed] [Google Scholar]
- 17.Michon, F., C. H. Huang, E. K. Farley, L. Hronowski, J. Di, and P. C. Fusco. 2000. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev. Biol. (Basel) 103:151-160. [PubMed] [Google Scholar]
- 18.Michon, F., C. Uitz, A. Sarkar, A. J. D'Ambra, M. Laude-Sharpe, S. Moore, and P. C. Fusco. 2006. Group B streptococcal type II and III conjugate vaccines: physicochemical properties that influence immunogenicity. Clin. Vaccine Immunol. 13:936-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moe, G. R., A. Dave, and D. M. Granoff. 2005. Epitopes recognized by a nonautoreactive murine anti-N-propionyl meningococcal group B polysaccharide monoclonal antibody. Infect. Immun. 73:2123-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moe, G. R., A. Dave, and D. M. Granoff. 2006. Molecular analysis of anti-N-propionyl Neisseria meningitidis group B polysaccharide monoclonal antibodies. Mol. Immunol. 43:1424-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mühlenhoff, M., M. Eckhardt, and R. Gerardy-Schahn. 1998. Polysialic acid: three-dimensional structure, biosynthesis and function. Curr. Opin. Struct. Biol. 8:558-564. [DOI] [PubMed] [Google Scholar]
- 22.Pon, R. A., M. Lussier, Q. L. Yang, and H. J. Jennings. 1997. N-propionylated group B meningococcal polysaccharide mimics a unique bactericidal capsular epitope in group B Neisseria meningitidis. J. Exp. Med. 185:1929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsay, M., E. Kaczmarski, M. Rush, R. Mallard, P. Farrington, and J. White. 1997. Changing patterns of case ascertainment and trends in meningococcal disease in England and Wales. Commun. Dis. Rep. CDR Rev. 7:R49-R54. [PubMed] [Google Scholar]
- 24.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 25.Wyle, F. A., M. S. Artenstein, B. L. Brandt, E. C. Tramont, D. L. Kasper, P. L. Altieri, S. L. Berman, and J. P. Lowenthal. 1972. Immunologic response of man to group B meningococcal polysaccharide vaccines. J. Infect. Dis. 126:514-521. [DOI] [PubMed] [Google Scholar]
- 26.Zollinger, W. D., R. E. Mandrell, J. M. Griffiss, P. Altieri, and S. Berman. 1979. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J. Clin. Investig. 63:836-848. [DOI] [PMC free article] [PubMed] [Google Scholar]