Abstract
The DNA sequences of two plasmids carrying vanA, pVEF1 (39,626 bp) and pVEF2 (39,714 bp), were determined. Forty-three shared coding sequences were identified, and the only nucleotide difference was an 88-bp indel. A postsegregational killing system was identified. This system possibly explains the persistence of the vanA gene cluster in Norwegian poultry farms.
Glycopeptide-resistant Enterococcus faecium (GREF) strains in which the resistance is plasmid encoded persist on Norwegian poultry farms, despite the ban on the use of avoparcin in 1995 (11, 18). Glycopeptide R plasmids were isolated from two genomically different GREF strains sampled from a chicken and a farmer to determine the basis for their long-term persistence in glycopeptide-free environments. Plasmids pVEF1 and pVEF2 are vanA-containing plasmids that express high-level glycopeptide resistance (vancomycin MICs, ≥64 mg/liter; teicoplanin MICs, >4 mg/liter). The plasmids were isolated in 1999 from two genomically different E. faecium strains, strains 399/F99/H8 and 399/F99/A9, from a farmer and his poultry, respectively. Both originated from a single Norwegian farm previously exposed to the animal growth promoter avoparcin (11).
Bacterial and plasmid isolation, species identification, and antimicrobial susceptibility testing were performed as previously described (11, 18). The plasmid DNA was randomly sheared and cloned into pCR4Blunt-TOPO. Plasmid DNA from approximately 450 subclones was purified, and the insert was sequenced by using ABI BigDye chemistry. Custom primers were used in the PCRs to fill gaps and to ensure the recovery of double-strand data for the complete sequences of the plasmids. The sequence data were assembled by use of the Staden package (19) and Phrap software (http://www.phrap.org/) and were completed by using Gap4 software (4). The final assemblies were verified by restriction map comparisons with BamHI, ClaI, EcoRI, HindIII, and PstI digests of the plasmids. The sequences were annotated by using the Artemis program (16), and the predicted coding sequences (CDSs) were identified by the use of GLIMMER software (7) and manually by correlation scores of the open reading frames (ORFs) with ≥50 amino acids. Sequence similarity was identified by using the FASTA (14) and the BLASTP (1) programs as well as the Pfam (3) and the ProSite (10) databases for protein domain prediction.
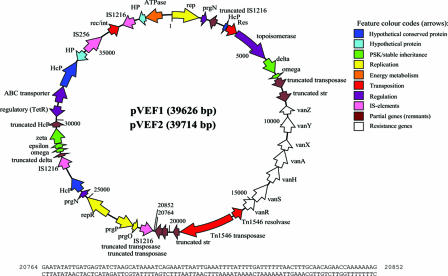
Sequence analysis of plasmids pVEF1 and pVEF2 revealed that they consist of 39,626 and 39,714 bp, respectively. Annotation revealed an identical gene composition expressed through 43 CDSs. The only difference identified between the two plasmids, an 88-bp indel, did not influence the gene composition but caused differences in the last six amino acids of the truncated transposase (CDS22). The functions of 37 CDSs were predicted on the basis of their similarities to previously characterized proteins (Table 1). A circular genetic map of the gene synteny is given in Fig. 1. The vanA genes expressing glycopeptide resistance are present on transposon Tn1546 on both plasmids, and the transposons show 100% identity to the first published sequence (2). Tn1546 is flanked by a truncated streptomycin resistance gene (str) with identity to plasmid pS194 from a clinical strain of Staphylococcus aureus (15). The G+C content of the two plasmids is 36.2%, which is similar to that of enterococcal genomic DNA (13). The G+C contents of individual CDSs ranged from 25.5% to 44.7%, consistent with the hypothesis that pVEF1 and pVEF2 are composed of DNA regions from multiple sources. The flanking sequences of Tn1546 identified in pVEF1 and pVEF2 are identical to the junction sequences (determined by inverse PCR) of the first described GREF plasmid (pIP816) isolated from a clinical strain in France in 1986 (2). The nonconjugative plasmids pVEF1 and pVEF2 have several insertion sequence (IS) elements that are likely facilitators of intra- or interplasmid recombinations (Table 1).
TABLE 1.
CDSs of the vanA plasmids pVEF1 and pVEF2
| CDS no. | CDSa | Nucleotide position (5′→3′)
|
Protein length (aac) | Database match (accession no.) | aa identity (%) | Alignment region of the match | |
|---|---|---|---|---|---|---|---|
| pVEF1 | pVEF2b | ||||||
| 1 | rep | 1-1500 | = | 499 | Plasmid replication protein in E. faecium DO (ZP_00602529) | 99 | 1-499/499 |
| 2 | prgN | 1636-1923 | = | 95 | pRUM prgN, E. faecium (Q848V1) | 57 | 1-96/96 |
| 3 | TIS1216 | 2427-2089 | = | 112 | IS1216 on pRE25, pRUM, pTEF1, pTEF3, pUW786, and pE418 (e.g., CAC29206) | 98 | 117-228/228 |
| 4 | HcP | 2946-3116 | = | 56 | pRE25 orf7, hypothetical conserved protein (CAC29163) | 100 | 1-56/56 |
| 5 | resIP | 3130-3747 | = | 205 | pGB354 resolvase, Streptococcus agalactiae (AAB48454) | 100 | 1-205/205 |
| 6 | top beta | 3747-5891 | = | 714 | pAMbeta1 type 1 topoisomerase E. faecalis (AAC38606) | 97 | 1-714/714 |
| 7 | δ | 5994-6890 | = | 298 | pIlo8 delta protein, Oenococcus oeni (CAD70616)/pSM19035 active partitioning δ protein (YP_232765) | 100/98 | 1-298/298 |
| 8 | ω | 6982-7197 | = | 71 | pRE25 orf17 (CAC29172)/pSM19035 transcriptional repressor ω (YP_232757) | 100/100 | 1-71/71 |
| 9 | Ttransposase | 7272-7802 | = | 176 | pLI100 transposase, Listeria innocua (CAC42047) | 98 | 51-226/226 |
| 10 | Tstr | 8084-8647 | = | 188 | N-terminal part of pS194 streptomycin resistance protein, S. aureus (P12055) | 95 | 1-188/282 |
| 11 | vanZ | 9383-8898 | = | 161 | pIP816 vanZ (Q06242) | 100 | 1-161/161 |
| 12 | vanY | 10447-9536 | = | 303 | pIP816 vanY (P37711) | 100 | 1-303/303 |
| 13 | vanX | 11483-10875 | = | 202 | pIP816 vanX (AAA65957) | 100 | 1-202/202 |
| 14 | vanA | 12520-11489 | = | 343 | pIP816 vanA (P25051) | 100 | 1-343/343 |
| 15 | vanH | 13481-12513 | = | 322 | pIP816 vanH (Q05709) | 100 | 1-322/322 |
| 16 | vanS | 14850-13696 | = | 384 | pIP816 vanS (Q06240) | 100 | 1-384/384 |
| 17 | vanR | 15523-14828 | = | 231 | pIP816 vanR (Q06239) | 100 | 1-231/231 |
| 18 | resTn1546 | 16312-15737 | = | 191 | pIP816 resolvase (Q06237) | 100 | 1-191/191 |
| 19 | Tn1546 transposase | 16458-19424 | = | 988 | pIP816 Tn1546 transposase (Q06238) | 100 | 1-988/988 |
| 20 | Tstr | 19504-19788 | = | 94 | C-terminal part of pS194 streptomycin resistance protein, S. aureus (P12055) | 95 | 189-282/282 |
| 21 | Ttransposase | 20102-20443 | = | 114 | pLI100 pLI0020 protein, putative transposase of L. innocua (Q926N5) | 87 | 1-114/160 |
| 22 | Ttransposase | 20430-20786 | = | 118 | pLI100 pLI0071 protein, putative transposase of L. innocua (Q925W6) | 85 | 117-224/226 |
| 23 | IS1216 | 21506-20820 | 21594-20908 | 228 | IS1216 on pRE25, pRUM, pTEF1, pTEF3, pUW786, and pE418 (e.g., CAC29206) | 100 | 1-228/228 |
| 24 | prgO | 21951-21676 | 22039-21764 | 91 | pRE25 prgO (CAC29214) | 100 | 1-91/91 |
| 25 | prgP | 22876-21923 | 22964-22011 | 317 | pRE25 prgP (CAC29215) | 100 | 1-317/317 |
| 26 | repR | 23488-24981 | 23576-25069 | 497 | pRE25 repR, orf1 (Q9AL28) | 100 | 1-497/497 |
| 27 | prgN | 25023-25412 | 25204-25500 | 98 | pRE25 prgN (Q9AL27) | 100 | 1-98/98 |
| 28 | HcP | 25513-26364 | 25601-26452 | 283 | Hypothetical conserved protein of E. faecium DO (EAN10371)/pRE25 hypothetical protein orf4 (Q9AL25) | 100/99 | 1-283/283 |
| 29 | IS1216 | 27024-27710 | 27112-27798 | 228 | IS1216, E. faecium (Q9KI43)/IS1216 on pRE25, pRUM, pTEF1, pTEF3, pUW786, pUW1965, and pE418 (e.g., CAC29206) | 100/99 | 1-228/228 |
| 30 | Tδ | 27752-27940 | 27840-28028 | 62 | pIlo1 delta protein, O. oeni (CAD70608) | 91 | 238-298/298 |
| 31 | ω | 28032-28247 | 28120-28335 | 71 | pRE25 orf17 (CAC29172)/pSM19035 transcriptional repressor ω (YP_232757) | 100/100 | 1-71/71 |
| 32 | ε | 28265-28537 | 28353-28625 | 90 | pRE25 orf18 epsilon antitoxin 2 (Q9AL19)/pSM19035 ɛ antitoxin (Q57231) | 100/78 | 1-90/90 |
| 33 | ξ | 28539-29402 | 28627-29490 | 287 | pRE25 orf19 ζ toxin (P0A4M1)/pSM19035 ζ toxin (Q54944) | 100/91 | 1-287/287 |
| 34 | THcP | 29524-29823 | 29579-29911 | 99 | pRE25 orf21 hypothetical conserved protein (CAC29176) | 97 | 1-99/168 |
| 35 | Transcriptional regulator | 30702-30106 | 30790-30194 | 198 | Regulatory protein, TetR-family, E. faecium DO (ZP_00602897) | 67 | 1-195/195 |
| 36 | ABC transporter | 31014-31895 | 31102-31983 | 293 | ABC transporter ATP-binding protein Oceanobacillus iheyensis (Q8ESC1) | 83 | 1-293/293 |
| 37 | HcP | 31919-33532 | 32007-33620 | 537 | Hypothetical conserved protein, O. iheyensis (Q8ESC0) | 71 | 1-537/537 |
| 38 | HP | 34194-33604 | 34282-33692 | 196 | Hypothetical protein | ||
| 39 | IS256 | 34341-35531 | 34429-35619 | 396 | Transposase, IS256 family, E. faecalis V583 (AA081627) | 95 | 1-396/396 |
| 40 | rec/int | 36026-36625 | 36114-36713 | 199 | pE88 recombinase/integrase, Clostridium tetani (AAO37444) | 40 | 1-190:1-192/202d |
| 41 | IS1216 | 37535-36849 | 37623-36937 | 228 | IS1216 on pRE25, pRUM, pTEF1, pTEF3, pUW786, and pE418 (e.g., CAC29206) | 100 | 1-228/228 |
| 42 | HP | 38163-37792 | 38251-37880 | 123 | Hypothetical protein | ||
| 43 | ATPase | 39076-38165 | 39164-38253 | 303 | pX02 ATPase, ParA family, CapR protein, Bacillus anthracis (NP_653010) | 53 | 19-300:4-283/288d |
T, truncated CDS; HcP, hypothetical conserved protein; HP, hypothetical protein.
=, identical to the nucleotide positions in pVEF1.
aa, amino acids.
Query alignment region:match alignment region/total aa of the match.
FIG. 1.
Genetic map of pVEF1 and pVEF2. The numbering of the plasmids commences at the first nucleotide of the ATG start codon of CDS1 of pVEF1 and pVEF2, which are predicted to encode replication proteins. Coding regions are represented by arrows indicating the direction of transcription. The coordinates of the indel are indicated on the map by positional marks.
Comparisons of the sequences of the vanA plasmids in this report and that of the only other sequenced enterococcal vanA plasmid, pHTβ (20), revealed exceptionally low levels of identity, in which the only shared CDS is the pheromone-responsive gene prgN. Plasmid pHTβ also has a Tn1546-like fragment, but direct comparative analysis is currently not possible since the pHTβ Tn1546-like sequence is not available in the databases.
Genes responsible for stable replication and plasmid persistence were found. The amino acid sequence of CDS1 has a high degree of identity to that of a replication protein found on the draft genome of E. faecium DO, while the sequence of CDS26 is identical to that of a repR gene (orf1) found on pRE25 from Enterococcus faecalis (17). Upstream of repR are two CDSs, prgO and prgP, previously described in pCF10 and pRE25 as putatively involved in plasmid replication (9, 17). Interestingly, several CDSs with high sequence similarity to a plasmid maintenance system previously described in pSM19035 and pRE25 (5, 12, 17) and encoded by the δ-ω-ɛ-ζ genes were identified. Such toxin/antitoxin systems secure the stable inheritance of plasmids during cell division by killing or impairing the growth of cells that have lost the plasmids and are also called postsegregational killing (PSK) systems (8). CDS32 and CDS33 of pVEF1 and pVEF2 are identical to ORF18 (ɛ gene) and ORF19 (ζ gene) in pRE25 and have 78% and 91% amino acid sequence identities to the ɛ antitoxin and ζ toxin of pSM19035, respectively. The ω protein regulates the expression of the ɛ and ζ genes (5, 6), and at the amino acid level CDS8 and CDS31 are identical to the ω gene of pSM19035 and ORF17 of pRE25, respectively. A δ gene is present on both pVEF1 and pVEF2 (CDS7), while a truncated δ gene (CDS30) is found upstream of the ω-ɛ-ζ genes. The high degree of identity of the amino acids to those of the ω-ɛ-ζ PSK system suggests the killing of plasmid-free cells as a major contributor to the long-term persistence of GREF in glycopeptide-free environments. A genetic linkage of Tn1546 to other known antimicrobial or heavy metal resistance determinants was not found.
Nucleotide sequence accession numbers.
Plasmids pVEF1 and pVEF2 have the following GenBank accession numbers: AM296544 and AM410096, respectively.
Acknowledgments
This work was supported by EC contract QLK2-CT-2002-00843 and the Medical Research Foundation, North Norway.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, M., C. Molinas, F. Depardieu, and P. Courvalin. 1993. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 175:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman, A., E. Birney, L. Cerruti, R. Durbin, L. Etwiller, S. R. Eddy, S. Griffiths-Jones, K. L. Howe, M. Marshall, and E. L. Sonnhammer. 2002. The Pfam protein families database. Nucleic Acids Res. 30:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonfield, J. K., K. Smith, and R. Staden. 1995. A new DNA sequence assembly program. Nucleic Acids Res. 23:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Hoz, A. B., S. Ayora, I. Sitkiewicz, S. Fernandez, R. Pankiewicz, J. C. Alonso, and P. Ceglowski. 2000. Plasmid copy-number control and better-than-random segregation genes of pSM19035 share a common regulator. Proc. Natl. Acad. Sci. USA 97:728-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Hoz, A. B., F. Pratto, R. Misselwitz, C. Speck, W. Weihofen, K. Welfle, W. Saenger, H. Welfle, and J. C. Alonso. 2004. Recognition of DNA by omega protein from the broad-host range Streptococcus pyogenes plasmid pSM19035: analysis of binding to operator DNA with one to four heptad repeats. Nucleic Acids Res. 32:3136-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delcher, A. L., D. Harmon, S. Kasif, O. White, and S. L. Salzberg. 1999. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 27:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerdes, K., S. K. Christensen, and A. Lobner-Olesen. 2005. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 9.Hirt, H., D. A. Manias, E. M. Bryan, J. R. Klein, J. K. Marklund, J. H. Staddon, M. L. Paustian, V. Kapur, and G. M. Dunny. 2005. Characterization of the pheromone response of the Enterococcus faecalis conjugative plasmid pCF10: complete sequence and comparative analysis of the transcriptional and phenotypic responses of pCF10-containing cells to pheromone induction. J. Bacteriol. 187:1044-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulo, N., C. J. Sigrist, V. Le Saux, P. S. Langendijk-Genevaux, L. Bordoli, A. Gattiker, E. De Castro, P. Bucher, and A. Bairoch. 2004. Recent improvements to the PROSITE database. Nucleic Acids Res. 32:D134-D137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnsen, P. J., J. I. Osterhus, H. Sletvold, M. Sorum, H. Kruse, K. Nielsen, G. S. Simonsen, and A. Sundsfjord. 2005. Persistence of animal and human glycopeptide-resistant enterococci on two Norwegian poultry farms formerly exposed to avoparcin is associated with a widespread plasmid-mediated vanA element within a polyclonal Enterococcus faecium population. Appl. Environ. Microbiol. 71:159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meinhart, A., J. C. Alonso, N. Strater, and W. Saenger. 2003. Crystal structure of the plasmid maintenance system epsilon/zeta: functional mechanism of toxin zeta and inactivation by epsilon 2 zeta 2 complex formation. Proc. Natl. Acad. Sci. USA 100:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 14.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Projan, S. J., S. Moghazeh, and R. P. Novick. 1988. Nucleotide sequence of pS194, a streptomycin-resistance plasmid from Staphylococcus aureus. Nucleic Acids Res. 16:2179-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz, F. V., V. Perreten, and M. Teuber. 2001. Sequence of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis RE25. Plasmid 46:170-187. [DOI] [PubMed] [Google Scholar]
- 18.Sorum, M., P. J. Johnsen, B. Aasnes, T. Rosvoll, H. Kruse, A. Sundsfjord, and G. S. Simonsen. 2006. Prevalence, persistence, and molecular characterization of glycopeptide-resistant enterococci in Norwegian poultry and poultry farmers 3 to 8 years after the ban on avoparcin. Appl. Environ. Microbiol. 72:516-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staden, R., K. F. Beal, and J. K. Bonfield. 2000. The Staden package, 1998. Methods Mol. Biol. 132:115-130. [DOI] [PubMed] [Google Scholar]
- 20.Tomita, H., and Y. Ike. 2005. Genetic analysis of transfer-related regions of the vancomycin resistance enterococcus conjugative plasmid pHTβ: identification of oriT and a putative relaxase gene. J. Bacteriol. 187:7727-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]