Abstract
We evaluated the efficacy of bacteriophage (phage) therapy by using a murine model of gut-derived sepsis caused by Pseudomonas aeruginosa that closely resembles the clinical pathophysiology of septicemia in humans. Oral administration of a newly isolated lytic phage strain (KPP10) significantly protected mice against mortality (survival rates, 66.7% for the phage-treated group versus 0% for the saline-treated control group; P < 0.01). Mice treated with phage also had lower numbers of viable P. aeruginosa cells in their blood, liver, and spleen. The levels of inflammatory cytokines (tumor necrosis factor alpha TNF-α, interleukin-1β [IL-1β], and IL-6) in blood and liver were significantly lower in phage-treated mice than in phage-untreated mice. The number of viable P. aeruginosa cells in fecal matter in the gastrointestinal tract was significantly lower in phage-treated mice than in the saline-treated control mice. We also studied the efficacy of phage treatment for intraperitoneal infection caused by P. aeruginosa and found that phage treatment significantly improved the survival of mice, but only under limited experimental conditions. In conclusion, our findings suggest that oral administration of phage may be effective against gut-derived sepsis caused by P. aeruginosa.
Fatality rates among patients with infections caused by Pseudomonas aeruginosa, a common pathogen that causes septicemia in immunocompromised hosts, are higher than those among patients with infections caused by any other gram-negative bacterium. P. aeruginosa is also a common pathogen in patients with cystic fibrosis and burn wound infections. P. aeruginosa colonization, generally with environmental isolates, occurs in a large number of patients before the age of 3 years (38), and colonization with this organism clearly has a negative effect on pulmonary function (31). Although antibiotic therapy is thought to be the most effective therapy against infections caused by this microorganism, such therapy is frequently ineffective due to bacterial resistance. Selection and dissemination of intrinsic and acquired resistance mechanisms of P. aeruginosa increase the probability of burn wound infections (29), and multidrug-resistant P. aeruginosa causes wound infections, which are associated with high rates of mortality and morbidity in burn patients (39).
Phage therapy is a method of harnessing phages as bioagents for the treatment of bacterial infectious diseases and was first introduced more than 80 years ago (15). Phages continue to be used in place of antibiotics for the treatment of bacterial infections in the former Soviet Union and Eastern Europe (1, 15, 32, 42). The ongoing active bacterial evolution of multidrug resistance has recently motivated the Western scientific community to reevaluate the therapeutic potential of phages for diverse bacterial infections that are often incurable with conventional chemotherapy (1, 15, 25, 40). Phage therapy may be a viable alternative to antibiotics because it has already been proven to be medically superior to antibiotic therapy for certain uses (2, 4, 17, 28, 36, 37).
The therapeutic efficacy of phage therapy against infectious diseases caused by P. aeruginosa (12), Staphylococcus aureus (including methicillin-resistant S. aureus) (24), Escherichia coli (3), Enterococcus faecium (including vancomycin-resistant Enterococcus) (6), and Streptococcus pneumoniae (16) has been shown in experimental animal models. However, the models used in those studies were simple models of infection that did not closely resemble the pathophysiologies of diseases in humans. We induced gut-derived sepsis caused by P. aeruginosa by administering cyclophosphamide and ampicillin to specific-pathogen-free mice fed P. aeruginosa (13, 14, 19-23). In this model, the inoculated P. aeruginosa organisms crossed the gastrointestinal mucosal barrier after overgrowth in the intestinal tract and spread systemically by a process termed bacterial translocation (5, 9). By using this model, we were able to examine the therapeutic efficacy of phage strain KPP10 against a P. aeruginosa-induced gut-derived sepsis model that closely mimics the clinical pathophysiology of septicemia in humans (13).
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female ICR mice (weight, 21 to 25 g) were purchased from Charles River Co., Atsugi, Japan, and used in the experiments described here. All mice were maintained under specific-pathogen-free conditions, housed in sterile cages, and given sterile water, except during the period when the bacteria were being orally administered.
Bacterial strain and culture conditions.
P. aeruginosa strain D4 was isolated from the blood of a neutropenic mouse with bacteremia (13) and was stored at −80°C in heart-brain broth (Difco Laboratories, Detroit, MI) containing 15% glycerol.
Phage.
Phage strain KPP10 was isolated from a water sample collected from a highly polluted river in Kochi Prefecture, Japan, by using a P. aeruginosa strain (strain PA20) as the host. Strain PA20 was derived from a clinical specimen of a patient in the Kochi Medical School Hospital, Kochi, Japan. Restriction enzyme analysis revealed that the KPP10 genome is double-stranded DNA, with an expected size of about 48 kbp.
Strain KPP10 also showed strong lytic activity against the P. aeruginosa strain used in this study, strain D4. KPP10 is expected to be a virulent phage, because (i) all KPP10-insensitive strains derived from P. aeruginosa D4 examined, which were isolated at a low frequency, had a defective adsorption property, which is supposed to be caused by mutations in the receptor protein gene(s) and not by lysogenization, and (ii) no integrase gene, which is required for lysogenization, was detected on the KPP10 genome.
Electron microscopy.
Purified phage samples in AAS (0.1 M ammonium acetate, 10 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, pH 7.2) were fixed in 5% formalin and negatively stained with 2% uranyl acetate (pH 4.0). Electron micrographs were taken with a transmission electron microscope (H-7100; Hitachi Co. Ltd., Tokyo, Japan) at 75 kV.
Large-scale purification of phage particles.
Phage strain KPP10 was purified essentially by the procedure used for T4-type phages (24). P. aeruginosa strain D4 was used as the host, and the bacterial strain was suspended at 1 × 108 cells/ml in 50 ml LB broth. The cells were exposed to a crude preparation of KPP10 at a multiplicity of infection (MOI) of 0.01 and were vigorously shaken for less than 4 h at 37°C, resulting in complete lysis of the bacteria. The culture fluid was centrifuged at 8,000 × g for 10 min at 4°C to remove the cell debris, and the fluid was filtered (pore size, 0.45 μm). Polyethylene glycol (average molecular weight, 6,000) and NaCl were added to the supernatant to final concentrations of 10% and 0.5 M, respectively, and the mixture was kept at 4°C for 3 days. The resultant precipitate containing the phage particles was collected by centrifugation at 8,000 × g for 20 min at 4°C, resuspended in 10 mM Tris-HCl and 5 mM MgCl2 (pH 7.2) buffer, and treated with 200 μg/ml of DNase I (type II; Sigma Chemical Co. St. Louis, MO) and RNase A (type IA; Sigma) for 30 min at 37°C. The phage suspension then was placed on top of a discontinuous CsCl gradient (1.3, 1.5, and 1.7 g/cm3) diluted in AAS and centrifuged at 100,000 × g for 1 h at 4°C. The phage band was collected and dialyzed against AAS for 2 h at 4°C. CsCl gradient separation and dialysis (1 h) were repeated. The purified phage suspension was filtered, divided into aliquots, and stored at 4°C until use. The samples were appropriately diluted with SM buffer, which is used for the routine manipulation of phage suspensions (11), just before use for infections. The titers (PFU/ml) of the purified samples were determined by inoculating them into P. aeruginosa strain D4. In our preliminary study, we confirmed that intraperitoneal administration of KPP10 alone did not produce a rise in the concentrations of inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α), in mouse serum (data not shown). Therefore, we believe that the level of endotoxin in the KPP10 preparation that we used in our study may not have any influence on our animal studies.
Effect of phage strain KPP10 on P. aeruginosa strain D4 in vitro.
A bacterial suspension containing 2.5 × 107 CFU/ml of P. aeruginosa strain D4 in 5 ml of LB broth was incubated with phage strain KPP10 (MOI = 1) in shaking culture. To determine the numbers of viable bacteria, portions of the suspensions were serially diluted and plated onto nutrient agar (Difco Laboratories) at different time points. The agar plates were cultured at 37°C for 24 h.
Murine gut-derived sepsis caused by P. aeruginosa.
Murine gut-derived sepsis was produced as described previously (19, 21-23). Briefly, bacteria grown on nutrient agar plates (Difco Laboratories) at 37°C for 18 h were suspended in sterile 0.45% saline and adjusted to a concentration of 108 CFU/ml. The bacterial suspension was added to the drinking water bottle between days 1 and 3. To aid with P. aeruginosa colonization, the normal intestinal flora of the mice was disturbed by daily subcutaneous injection of 100 mg of ampicillin (Sigma) per kg of body weight between days 1 and 3. In this model, P. aeruginosa cells that colonize the intestinal tract can invade body tissues after the induction of immunosuppression or disruption of the mucosal barrier of the intestine by the administration of cyclophosphamide (CY). Therefore, after the administration of the bacteria, that mice were then given an intraperitoneal injection of 300 mg of CY (Shionogi & Co., Ltd., Tokyo, Japan) per kg on days 7 and 9 to induce the translocation of the P. aeruginosa bacteria from the intestine. A total of 0.1 ml of SM buffer with 1.0 × 1010 PFU of KPP10 was orally administered to each mouse. To evaluate the effect of the timing of phage treatment, the mice were given phage strain KPP10 orally either 1 day before (group 1), 1 day after (group 2), or 6 days after (group 3) the oral inoculation of P. aeruginosa (n = 18 mice in each group). The number of deaths was counted every 24 h. The experimental protocols were approved by the Committee for Institutional Animal Care and Use of the Toho University School of Medicine.
Determination of numbers of viable bacteria and phage in blood and organs.
Mice were euthanized by inhalation of ether on the second day after the second CY treatment. Cardiac blood, liver, spleen, and mesenteric lymph node (MLN) samples were obtained aseptically. The liver, spleen, and MLN samples (10 mice in each group) were homogenized with a tissue homogenizer (Yamato Scientific Co., Ltd., Tokyo, Japan) in sterile saline. Portions of the blood and homogenized tissue samples were plated onto nutrient agar and cultured at 37°C for 24 h to detect the challenge strain, P. aeruginosa strain D4. The remaining blood samples were allowed to clot at 4°C in sterile glass tubes and were then centrifuged at 10,000 × g for 5 min. Serum samples and the remaining liver tissue supernatants were preserved at −80°C until cytokine levels were measured. For determination of the number of viable phage in the organ tissues, serum samples and the remaining liver tissue supernatants were filtered by a 0.45-μm-pore-size filter (Kanto Chemical Co., Inc. Tokyo, Japan) and mixed with P. aeruginosa strain D4 in NZCYM top agarose (31a). Portions of the mixture were plated onto LB agar (Difco Laboratories) and cultured at 37°C for 24 h for the determination of the numbers of phage strain KPP10.
Clearance of P. aeruginosa from the gastrointestinal tract.
To evaluate the kinetics of viable P. aeruginosa in the gastrointestinal tract, we cultured feces from the mice by using media that were selective for the bacteria. Fecal samples were obtained 1 day after the second CY treatment and were weighed and homogenized. Portions of the serially diluted homogenized samples were inoculated onto medium selective for P. aeruginosa (NAC agar; Eiken Kizai Co., Ltd., Japan); the plates were incubated at 37°C under aerobic conditions.
Intraperitoneal infection.
To induce acute intraperitoneal infection, each mouse was intraperitoneally injected with from 2.4 × 106 to 300 × 106 CFU of P. aeruginosa strain D4 suspended in sterile saline on one side of the abdomen (n = 12 or 13 mice in each group). Purified phage strain KPP10 was suspended in SM buffer. A total of 0.1 ml of KPP10 suspension containing 1.0 × 1010 PFU of the phage was intraperitoneally injected into the contralateral side of the abdomen of each mouse. Mouse deaths were counted every 24 h after the bacterial challenge.
Measurement of cytokine levels.
Since inflammatory cytokines are thought to be good markers of the severity of bacterial infections (8, 20, 30), we measured the levels of IL-1β, IL-6, and TNF-α in the blood and liver tissues of the mice after the induction of gut-derived sepsis. Samples from each group of mice (n = 9) were collected 2 days after the second administration of cyclophosphamide. The concentrations of IL-1β, IL-6, and TNF-α in serum and liver tissue were measured by using commercial enzyme-linked immunosorbent assay kits (R&B Systems, Inc., Minneapolis, MN).
Statistical analysis.
The survival rate for each group of mice after the induction of gut-derived sepsis with P. aeruginosa strain D4 was analyzed by the chi-square test. The numbers of viable bacteria in blood and organs and cytokine levels were analyzed by the Mann-Whitney U test.
RESULTS
Electron micrographs of KPP10.
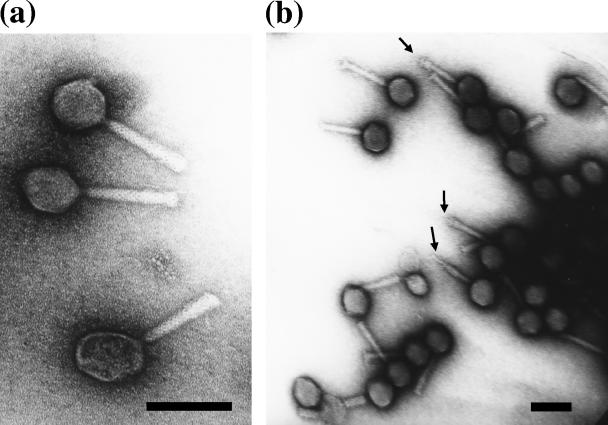
Electron microscopy revealed that strain KPP10 had an isometrically hexagonal head (diameter, 72 nm) and a 116-nm-long contractile tail. KPP10 evidently belongs to the family Myoviridae, morphotype A1, because the phage particles, which have a shrunken tail sheath and an exposed tail tube, were frequently observed during the electron microscopic examination of KPP10 (Fig. 1).
FIG. 1.
Electron micrographs of P. aeruginosa phage KPP10. Purified phage particles were negatively stained with 2% uranyl acetate and were observed at high (a) and low (b) magnifications with a transmission electron microscope. The arrows indicate examples of the tail tube protruding from the contracted tail sheath. Bars, 100 nm.
Lytic activity of phage strain KPP10 against P. aeruginosa in vitro.
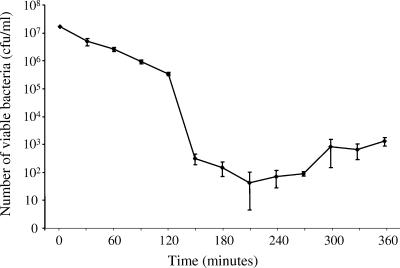
To evaluate the lytic activity of phage strain KPP10 against P. aeruginosa in vitro, P. aeruginosa strain D4 was incubated in medium containing phage strain KPP10 (MOI = 100). The number of viable bacteria gradually decreased from 1.8 × 107 CFU/ml at the start of the incubation to 3.7 × 105 ± 2.7 × 104 CFU/ml after 120 min of incubation (Fig. 2), demonstrating that phage strain KPP10 has potent lytic activity against P. aeruginosa strain D4. Phage KPP10 was seen to have rapid lytic activity after 150 min of incubation. We also found that the number of bacteria gradually increased after 210 min of incubation, and we speculate that these results may suggest the emergence of strains resistant to KPP10.
FIG. 2.
Lytic activity of phage KPP10 against P. aeruginosa in vitro. Bacterial suspensions of P. aeruginosa D4 and phage KPP10 were incubated together in LB broth (MOI = 1). The numbers of viable bacteria were determined as described in Materials and Methods. The data represent the means and standard deviations of the means (n = 3 at each time point).
Effect of KPP10 on survival of mice after induction of gut-derived sepsis.
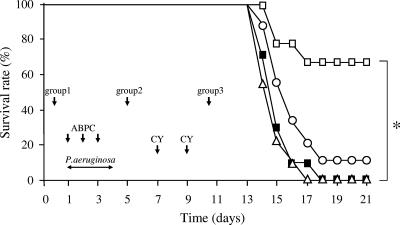
To evaluate whether phage strain KPP10 possesses a protective effect in vivo, we compared the rates of survival of phage-treated and phage-untreated mice with gut-derived sepsis caused by P. aeruginosa. As shown in Fig. 3, the survival of the mice in group 2, which were treated with KPP10 1 day after inoculation of P. aeruginosa, was significantly higher than that of the control group (66.7% versus 0%; P < 0.01). However, there was no significant difference between the survival rates for either group 1 or group 3 and the controls. However, there was only a small improvement in the survival rate among the mice in group 3 (10%) and no improvement among the mice in group 1 compared to that for the control mice. These results suggest that oral administration of phage protects mice against gut-derived sepsis caused by P. aeruginosa and that the effectiveness of phage treatment is highly dependent on the timing of phage administration.
FIG. 3.
Protective effect of phage KPP10 on the survival of mice with gut-derived sepsis caused by P. aeruginosa. Mice in group 1 (Δ; n = 18), group 2 (□; n = 18), and group 3 (○; n = 18) were treated with phage KPP10 1 day before, 1 day after, and 6 days after P. aeruginosa inoculation, respectively. Control mice (▪; n = 20) were treated with saline without phage according to the same schedule used for group 1. A significant protective effect on survival from KPP10 was noted only in mice in group 2 (*, P < 0.01). The results represent the averages of four independent experiments.
Effect of phage treatment on viable bacteria in blood and organs.
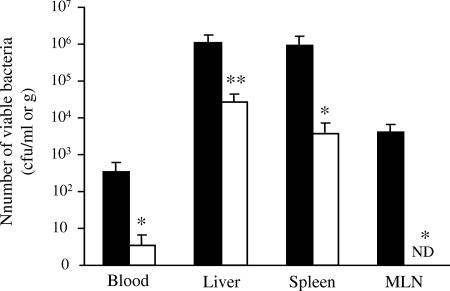
To confirm whether the administration of phage strain KPP10 combats infection, we counted the viable bacteria in mouse blood and various mouse organs. Each sample was collected 2 days after the second administration of cyclophosphamide. The average numbers of viable bacteria in the blood, liver, spleen, and MLN of phage-treated mice were significantly lower than those in the blood and organs of phage-untreated mice (Fig. 4). We also studied the kinetics of phage strain KPP10 in the blood and the same three organs 8 days after oral administration and found viable phage KPP10 in blood (9.2 × 103 ± 8.7 × 103 PFU/ml) and liver tissue (2.3 × 103 ± 9.1 × 102 PFU/ml). The counts of viable phage strain KPP10 were high, especially in mice that had high levels of viable bacteria in organs (data not shown).
FIG. 4.
Numbers of viable bacteria in blood, liver, spleen, and MLN in KPP10-treated and -untreated mice. We determined the viable bacterial counts in blood, liver, spleen, and MLN from mice treated with KPP10 1 day after P. aeruginosa inoculation (□; group 2) and saline-treated mice (▪; control group). Measurements are the means ± the standard errors of the means (10 mice in each group). Symbols and abbreviation: *, P < 0.05; **, P < 0.01; ND, not detectable.
Influence of phage on P. aeruginosa colonization of the intestinal tract.
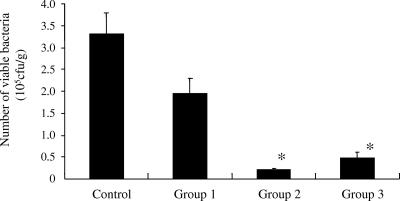
In the animal model used for the present study, P. aeruginosa is believed to reside mainly in the gastrointestinal tract. We therefore evaluated the effect of phage strain KPP10 on bacterial colonization of the intestine. Each mouse was inoculated with 1.0 × 1010 CFU of P. aeruginosa, and stool specimens were obtained 1 day after the second CY treatment. In mice given KPP10 1 day after (group 2) or 6 days after (group 3) the administration of P. aeruginosa, the number of viable bacteria in feces was significantly less than that in the feces of the control group (P < 0.01) (Fig. 5).
FIG. 5.
Influence of phage on colonization of P. aeruginosa in the intestinal tract. The phage-treated mice had significantly lower numbers of viable bacteria in feces than the saline-treated controls (*, P < 0.05).
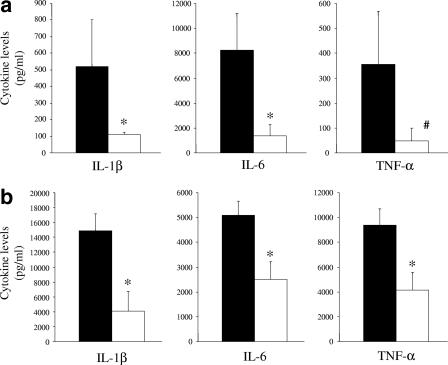
Cytokine levels in serum and liver during gut-derived sepsis.
The levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α in the blood (Fig. 6a) and livers (Fig. 6b) of mice were evaluated by the use of samples collected 2 days after the second administration of cyclophosphamide. As shown in these Fig. 6a and b, the cytokine levels were significantly lower in phage-treated mice than in phage-untreated mice.
FIG. 6.
Cytokine levels in serum and liver during gut-derived sepsis. The levels of IL-1β and IL-6 in serum (a) and liver (b) of phage-treated mice 1 day after P. aeruginosa inoculation (□; n = 9) were significantly lower than those in saline-treated control mice (▪; n = 9) (*, P < 0.05). The TNF-α level in the livers of phage-treated mice was significantly lower than that in the livers of the control mice (*, P < 0.05), and the results for TNF-α in the serum of phage-treated mice approached statistical significance (#, P = 0.0523) compared to the results for the control mice.
Effect of phage on intraperitoneal infection with P. aeruginosa.
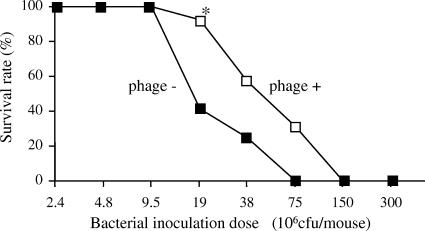
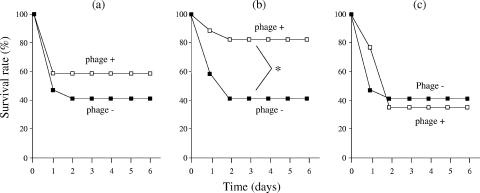
Phage strain KPP10 was simultaneously injected intraperitoneally with different doses of P. aeruginosa strain D4 (MOI = 100 to 10,000) (Fig. 7). We found that treatment with phage significantly protected the mice against mortality at a concentration of 19 × 106 CFU/mouse of P. aeruginosa strain D4. Finally, 12 of 13 (92.3%) phage-treated mice survived, whereas only 5 of 12 (41.7%) phage-untreated mice survived (Fig. 7). In addition, we studied the effect of the timing of phage administration on survival. Administration of phage 1 day prior to bacterial challenge had a minor effect (60% survival versus 40% survival for the controls); however, simultaneous inoculation of phage induced significant protection against intraperitoneal infection with P. aeruginosa (Fig. 8).
FIG. 7.
Survival rate for KPP10-treated and -untreated mice after intraperitoneal injection of P. aeruginosa. Each mouse was intraperitoneally injected with from 2.4 × 106 to 300 × 106 CFU of P. aeruginosa strain D4 and 2.0 × 1010 PFU of KPP10 (n = 12 or 13 in each group). A significant protective effect was detected only at a P. aeruginosa strain D4 concentration of 19 × 106 CFU/mouse (*, P < 0.05). The results represent the averages of three independent experiments.
FIG. 8.
Survival rate after KPP10 treatment for intraperitoneal infection with P. aeruginosa (19 × 106 CFU/mouse). Mice were intraperitoneally injected with KPP10 (2.0 × 1010 PFU/mouse) either (a) 24 h before, (b) concurrently with, or (c) 6 h after bacterial challenge (n = 12 or 13 in each group). Only mice simultaneously inoculated with P. aeruginosa and KPP10 showed significantly improved survival (*, P < 0.05). The results are representative of those from two independent experiments.
Stability of KPP10 in bile secretion, stomach acid, and serum.
To confirm whether bile secretion has any influence on phage survival, we examined the effect of sodium cholic acid, a component of bile, on the stability of KPP10. It was very stable in LB medium containing sodium cholic acid at concentrations from 0.01 to 1% for 30 min at 37°C (data not shown), suggesting that KPP10 may be relatively stable in bile. Furthermore, we also examined the effects of various pHs (1.8 to 11.2) on the stability of KPP10. The phage was relatively stable in LB medium with pHs ranging from 3.8 to 11.2 for 30 min at 37°C. On the other hand, at pH 2.8 or less, the survival rate of the phage decreased remarkably (data not shown). However, as is the case for KPP10, some phages (e.g., phage T4) have been reported to be able to pass the stomach without a remarkable decrease in viability. One of the reasons may be that the phages were given with a large amount of solution when they were administered orally.
We also examined the lytic activity of KPP10 on P. aeruginosa D4 in mouse serum. KPP10 at concentrations of 3 × 108/ml (MOI = 1.5), 1 × 108/ml (MOI = 0.5), and 1 ×107/ml (MOI = 0.05) was added to 2 × 108 bacteria/ml; and the mixtures were incubated at 37°C. In all cases, strong lytic activities against strain D4 were observed within 1 h, suggesting that serum did not affect phage multiplication (data not shown).
DISCUSSION
Despite advances in antimicrobial therapy and supportive care for critically ill patients, there are at least 500,000 cases of sepsis annually in the United States alone, with mortality rates ranging from 30% to 50% (26). Several treatments that have been developed to reduce sepsis-associated mortality have been unsuccessful; therefore, the need for an effective new therapy for sepsis remains urgent.
P. aeruginosa is one of the leading causes of hospital-acquired infections due to its ability to cause a variety of diseases and its high-level resistance to several antibiotics (7). The results of clinical studies that used surveillance cultures of fecal samples from immunocompromised patients suggest that the gastrointestinal tract is the primary reservoir for opportunistic bacteria (41). After bacterial translocation was demonstrated experimentally (5, 9), bacteria in the gastrointestinal tract came to be regarded as an important pathogenic factor in immunocompromised patients, such as those undergoing anticancer chemotherapy. We therefore developed a murine model of gut-derived sepsis caused by P. aeruginosa (13, 14, 19-23). This model incorporates the oral inoculation of bacteria, subsequent bacterial colonization, overgrowth in the intestinal tract, and invasion of the bloodstream. We believe that this animal model closely resembles the clinical pathophysiology of septicemia in humans (13).
After first confirming the lytic activity of phage KPP10 by culturing P. aeruginosa strain D4 with the phage, we then found that oral administration of KPP10 induced significant protection against gut-derived sepsis caused by P. aeruginosa. In addition, the numbers of viable P. aeruginosa organisms in the feces were significantly lower in the phage-treated mice than in the saline-treated control mice, a finding that suggests that the main mechanism responsible for this protective effect may be the significant reduction of P. aeruginosa in the gastrointestinal tract, i.e., the main source of the pathogen.
Our results also revealed that inflammatory cytokine (IL-1β, IL-6, and TNF-α) production in the blood and livers of mice treated with phage KPP10 was much lower than that in the blood and livers of phage-untreated mice, possibly because of a lower bacterial burden in the affected organs. Our results further suggest that systemic inflammation is lower in phage-treated mice than in phage-untreated mice.
We compared the efficacy of intraperitoneal administration of KPP10 with that of intravenous administration of the phage in our murine gut-derived sepsis model. Compared to oral administration, the rate of mortality was significantly lower after both intraperitoneal and intravenous administration of an identical amount of KPP10 (data not shown). These results revealed that, in addition to oral administration, intraperitoneal and intravenous administration of phage may protect against gut-derived sepsis caused by P. aeruginosa. However, intraperitoneal and intravenous administration of KPP10 may be more likely to result in adverse effects caused by increased levels of endotoxins. One study found that the levels of endotoxin released after the administration of phages were 30- to 60-fold higher in lytic phage-treated mice than in non-lytic phage-treated mice (12). Because of the possibility of adverse effects, we believe that oral administration of phage strain KPP10 is preferable.
Reports have revealed that a single treatment with phage leads to recovery in mice with infections caused by E. coli (34), P. aeruginosa (12), methicillin-resistant Staphylococcus aureus (24), and vancomycin-resistant Enterococcus faecium (6). In the present study, we also found that a single treatment with phage strain KPP10 prevented the death of mice after gut-derived sepsis and intraperitoneal infection with P. aeruginosa. In a preliminary study, we found that a regimen requiring multiple phage treatments was not more effective than a single-treatment regimen (data not shown).
Concerning the timing of phage treatment, our results showed that administration of phage 1 day before the bacterial challenge was not effective, perhaps because the phage does not remain confined in the gastrointestinal tract in the absence of P. aeruginosa. Our results also revealed that phage administration after CY treatment resulted in a significantly lower number of viable bacteria in feces. However, the rate of mortality was not improved, suggesting that phage treatment at a late stage of infection does not produce significant protection in this model of infection.
In our investigation of the efficacy of phage treatment in mice after intraperitoneal infection with P. aeruginosa, we found that only the simultaneous administration of KPP10 protected against intraperitoneal infection. This finding may raise certain questions, such as, “Is simultaneous inoculation of phage useful for control of the pathogen in a real clinical situation?” We believe that simultaneous injection is the easiest method for examination of the antibacterial effects of phages or drugs in vivo. Furthermore, the bacteria and the phage injected into the intraperitoneal cavity of the mouse were immediately transferred into blood. As this is thought to be the most suitable in vivo situation for the phage-bacterium interaction, if the phage cannot rescue the mice infected in this situation, it will be impossible to rescue the mice from any conditions of infection. For this reason, we first examined the effect of simultaneous injection of the phage and its host routinely.
The possibility of bacterial resistance to phage is unquestionably an obstacle in the development of an effective phage therapy system (18). Indeed, we noted the emergence of mutants of P. aeruginosa D4 that were resistant to phage strain KPP10 in vitro (data not shown). However, Smith and colleagues previously showed that infections produced by phage-resistant mutants of an enteropathogenic strain of E. coli and their parents could be successfully controlled with mutant phage derived from phage that had been active against the parent bacteria (33, 35). Even if the bacteria acquire phage resistance, new mutant phage that acts lytically against these bacteria can be isolated (24). It is already possible to prepare a mixture of different strains of phages that would prevent the emergence of a resistant strain during phage treatment. We are therefore optimistic that a solution to the problem of phage-resistant bacteria can be found.
There is also the undesirable possibility that phage particles may be removed from the circulatory system by host defense systems, perhaps by neutralization of the administered phages by antibodies. The preparation of several phage strains with different antigenicities may solve this problem. It is encouraging to note that Duckworth and Gulig have suggested that phage therapy is usually completed before specific immunity develops (10). Furthermore, Merril et al. (27) developed a technique of serial passage in mice to select for phage mutants able to remain in the circulatory system, and they isolated phage mutants that circulated for long periods. In our preliminary study, we found that smaller numbers of PFU of KPP10 were recovered from mice with low levels of P. aeruginosa isolation (data not shown). Therefore, we assume that KPP10 is easily eliminated by the host defense system and phage particles are removed from the circulatory system in a short period of time if only a few P. aeruginosa organisms are present in mice.
In conclusion, our results suggest that phage treatment is potentially useful for the treatment of P. aeruginosa infection, especially cases of gut-derived sepsis. We look forward to further studies and to the possible widespread use of phage treatment as a clinical therapy for humans.
Acknowledgments
This work was supported by a project research grant from the Toho University School of Medicine, Tokyo, Japan.
We thank R. Shibuya for technical assistance.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Alisky, J., K. Iczkowski, A. Rapoport, and N. Troitsky. 1998. Bacteriophages show promise as antimicrobial agents. J. Infect. 36:5-15. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1983. Phage therapy. Lancet ii:1287-1288. (Editorial.) [Google Scholar]
- 3.Barrow, P., M. Lovell, and A. Berchieri, Jr. 1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 5:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrow, P. A., and J. S. Soothill. 1997. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 5:268-271. [DOI] [PubMed] [Google Scholar]
- 5.Berg, R. D., and A. W. Garlington. 1980. Translocation of Escherichia coli from the gastrointestinal tract to the mesenteric lymph nodes in gnotobiotic mice receiving Escherichia coli vaccines before colonization. Infect. Immun. 30:894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas, B., S. Adhya, P. Washart, B. Paul, A. N. Trostel, B. Powell, R. Carlton, and C. R. Merril. 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 70:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodey, G. P., R. Bolivar, V. Fainstein, and L. Jadeja. 1983. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 5:279-313. [DOI] [PubMed] [Google Scholar]
- 8.Damas, P., D. Ledoux, M. Nys, Y. Vrindts, D. De Groote, P. Franchimont, and M. Lamy. 1992. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann. Surg. 215:356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deitch, E. A., J. Winterton, and R. Berg. 1986. Thermal injury promotes bacterial translocation from the gastrointestinal tract in mice with impaired T-cell-mediated immunity. Arch. Surg. 121:97-101. [DOI] [PubMed] [Google Scholar]
- 10.Duckworth, D. H., and P. A. Gulig. 2002. Bacteriophages: potential treatment for bacterial infections. BioDrugs 16:57-62. [DOI] [PubMed] [Google Scholar]
- 11.Elliott, R. M., and C. D. Green. 1989. A simple purification procedure for lambda gt bacteriophage DNA with hybridization size screening for isolation of longest length cDNA clones. Anal. Biochem. 183:89-93. [DOI] [PubMed] [Google Scholar]
- 12.Hagens, S., A. Habel, U. von Ahsen, A. von Gabain, and U. Blasi. 2004. Therapy of experimental Pseudomonas infections with a nonreplicating genetically modified phage. Antimicrob. Agents Chemother. 48:3817-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirakata, Y., M. Kaku, K. Tomono, K. Tateda, N. Furuya, T. Matsumoto, R. Araki, and K. Yamaguchi. 1992. Efficacy of erythromycin lactobionate for treating Pseudomonas aeruginosa bacteremia in mice. Antimicrob. Agents Chemother. 36:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirakata, Y., K. Tomono, K. Tateda, T. Matsumoto, N. Furuya, K. Shimoguchi, M. Kaku, and K. Yamaguchi. 1991. Role of bacterial association with Kupffer cells in occurrence of endogenous systemic bacteremia. Infect. Immun. 59:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho, K. 2001. Bacteriophage therapy for bacterial infections. Rekindling a memory from the pre-antibiotics era. Perspect. Biol. Med. 44:1-16. [DOI] [PubMed] [Google Scholar]
- 16.Jado, I., R. Lopez, E. Garcia, A. Fenoll, J. Casal, and P. Garcia. 2003. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 52:967-973. [DOI] [PubMed] [Google Scholar]
- 17.Lederberg, J. 1996. Smaller fleas ad infinitum: therapeutic bacteriophage redux. Proc. Natl. Acad. Sci. USA 93:3167-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowbury, E. J., and A. M. Hood. 1953. The acquired resistance of Staphylococcus aureus to bacteriophage. J. Gen. Microbiol. 9:524-535. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto, T., K. Tateda, N. Furuya, S. Miyazaki, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1998. Efficacies of alkaline protease, elastase and exotoxin A toxoid vaccines against gut-derived Pseudomonas aeruginosa sepsis in mice. J. Med. Microbiol. 47:303-308. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1997. Adverse effects of tumor necrosis factor in cyclophosphamide-treated mice subjected to gut-derived Pseudomonas aeruginosa sepsis. Cytokine 9:763-769. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1998. Effect of immunisation with Pseudomonas aeruginosa on gut-derived sepsis in mice. J. Med. Microbiol. 47:295-301. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1998. Effect of interleukin-10 on gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 42:2853-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1997. Immunomodulating effect of fosfomycin on gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 41:308-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaki, S., M. Yasuda, H. Nishikawa, M. Kuroda, T. Ujihara, T. Shuin, Y. Shen, Z. Jin, S. Fujimoto, M. D. Nasimuzzaman, H. Wakiguchi, S. Sugihara, T. Sugiura, S. Koda, A. Muraoka, and S. Imai. 2003. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J. Infect. Dis. 187:613-624. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki, S., M. Rashel, J. Uchiyama, S. Sakurai, T. Ujihara, M. Kuroda, M. Ikeuchi, T. Tani, M. Fujieda, H. Wakiguchi, and S. Imai. 2005. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J. Infect. Chemother. 11:211-219. [DOI] [PubMed] [Google Scholar]
- 26.Matthay, M. A. 2001. Severe sepsis—a new treatment with both anticoagulant and antiinflammatory properties. N. Engl. J. Med. 344:759-762. [DOI] [PubMed] [Google Scholar]
- 27.Merril, C. R., B. Biswas, R. Carlton, N. C. Jensen, G. J. Creed, S. Zullo, and S. Adhya. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 93:3188-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirisi, A. 2000. Phage therapy—advantages over antibiotics? Lancet 356:1418. [DOI] [PubMed] [Google Scholar]
- 29.Pruitt, B. A., Jr., A. T. McManus, S. H. Kim, and C. W. Goodwin. 1998. Burn wound infections: current status. World J. Surg. 22:135-145. [DOI] [PubMed] [Google Scholar]
- 30.Puren, A. J., C. Feldman, N. Savage, P. J. Becker, and C. Smith. 1995. Patterns of cytokine expression in community-acquired pneumonia. Chest 107:1342-1349. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld, M., B. W. Ramsey, and R. L. Gibson. 2003. Pseudomonas acquisition in young patients with cystic fibrosis: pathophysiology, diagnosis, and management. Curr. Opin. Pulm. Med. 9:492-497. [DOI] [PubMed] [Google Scholar]
- 31a.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, vol. 1, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Slopek, S., B. Weber-Dabrowska, M. Dabrowski, and A. Kucharewicz-Krukowska. 1987. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch. Immunol. Ther. Exp. (Warsaw) 35:569-583. [PubMed] [Google Scholar]
- 33.Smith, H. W., and M. B. Huggins. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhea in calves, piglets and lambs. J. Gen. Microbiol. 129:2659-2675. [DOI] [PubMed] [Google Scholar]
- 34.Smith, H. W., and M. B. Huggins. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J. Gen. Microbiol. 128:307-318. [DOI] [PubMed] [Google Scholar]
- 35.Smith, H. W., M. B. Huggins, and K. M. Shaw. 1987. The control of experimental Escherichia coli diarrhea in calves by means of bacteriophages. J. Gen. Microbiol. 133:1111-1126. [DOI] [PubMed] [Google Scholar]
- 36.Soothill, J. S. 1992. Treatment of experimental infections of mice with bacteriophages. J. Med. Microbiol. 37:258-261. [DOI] [PubMed] [Google Scholar]
- 37.Soothill, J. S. 1994. Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa. Burns 20:209-211. [DOI] [PubMed] [Google Scholar]
- 38.Speert, D. P., M. E. Campbell, D. A. Henry, R. Milner, F. Taha, A. Gravelle, A. G. Davidson, L. T. Wong, and E. Mahenthiralingam. 2002. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis in British Columbia, Canada. Am. J. Respir. Crit. Care Med. 166:988-993. [DOI] [PubMed] [Google Scholar]
- 39.Steinstraesser, L., Y. Oezdogan, S. C. Wang, and H. U. Steinau. 2004. Host defense peptides in burns. Burns 30:619-627. [DOI] [PubMed] [Google Scholar]
- 40.Sulakvelidze, A., Z. Alavidze, and J. G. Morris, Jr. 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tancrede, C. H., and A. O. Andremont. 1985. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J. Infect. Dis. 152:99-103. [DOI] [PubMed] [Google Scholar]
- 42.Weber-Dabrowska, B., M. Mulczyk, and A. Gorski. 2000. Bacteriophage therapy of bacterial infections: an update of our institute's experience. Arch. Immunol. Ther. Exp. (Warsaw) 48:547-551. [PubMed] [Google Scholar]