Abstract
The extracts and pure major constituents of Chios mastic gum (resin of Pistacia lentiscus var. chia) were tested for their activities against Helicobacter pylori. A total mastic extract without polymer (TMEWP) was prepared after removal of the contained insoluble polymer in order to ameliorate solubility and enhance in vivo activity. Administration of TMEWP to H. pylori SS1-infected mice over the period of 3 months with an average dose of 0.75 mg/day led to an approximately 30-fold reduction in the H. pylori colonization (1.5 log CFU/g of tissue). However, no attenuation in the H. pylori-associated chronic inflammatory infiltration and the activity of chronic gastritis was observed. To further characterize potential active mastic constituents, the TMEWP was separated into an acidic and a neutral fraction. Both were extensively characterized by nuclear magnetic resonance and mass spectroscopy to elucidate the structure of the components contained within each fraction. After chromatographic separation, the acid fraction gave the major triterpenic acids, while the neutral fraction gave several triterpenic alcohols and aldehydes. Mastic extracts and isolated pure triterpenic acids were tested for in vitro activity against a panel of 11 H. pylori clinical strains. The acid fraction was found to be the most active extract (minimum bactericidal concentration [MBC], 0.139 mg/ml), and the most active pure compound was isomasticadienolic acid (MBC, 0.202 mg/ml [0.443 mM]). Our results show that administration of TMEWP may be effective in reducing H. pylori colonization and that the major triterpenic acids in the acid extract may be responsible for such an activity.
Pistacia lentiscus L. is an evergreen shrub of the Anacardiaceae family, very common in the eastern Mediterranean area. The variety chia (Duham) is uniquely cultivated in southern Chios, a Greek island in the Aegean. The resin of that plant, mastic gum, is obtained as an exudate after “hurting” the trunk and branches.
Mastic gum has been used in traditional Greek medicine for various gastrointestinal disorders like gastralgia, dyspepsia, and peptic ulcer for more than 2,500 years. Ancient Greek physicians, such as Hippocrates, Dioscorides, Theophrastos, and Galenos, mentioned its properties and recommended its use. In modern times, it is used as a seasoning in Mediterranean cuisine, in the production of chewing gum, in perfumery, in dentistry, and by the local population of Chios for the relief of gastralgia and protection against peptic ulcer. Early studies involving mastic administration to rats with experimentally induced gastric and duodenal ulcers indicated a significant decrease of free acidity (2). Moreover, a double-blind clinical trial carried out on patients with symptomatic and endoscopically proven duodenal ulcer showed increased symptomatic relief in patients on mastic (1 g daily) compared to patients on placebo, while endoscopically proven healing occurred in 70% of the patients on mastic (1).
In 1983, Barry Marshall and Robin Warren suggested that gastric inflammation and peptic ulceration were the result of an infection caused by Helicobacter pylori (34). In the years to follow, the presence of H. pylori infection was shown to be the etiologic determinant of chronic active gastritis and a major risk factor for the development of peptic ulcer disease, gastric atrophy, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma (23). Antibiotic eradication schemes have proved very effective in clearing the infection; however, low patient compliance and the development of antibiotic resistance have created the need for new H. pylori eradication strategies. Mastic gum has been reported to possess considerable in vitro antibacterial and antifungal activities (32). A few years ago, it was specifically reported to be effective against Helicobacter pylori in vitro (7, 12, 21). However, in a more recent in vivo study of H. pylori infection, the activity of mastic gum was compared with antibiotic eradication schemes, and after a 7-day therapy no eradication of the bacterium from the stomachs of mice receiving mastic was observed (17). Finally, H. pylori-positive patients were treated with mastic capsules for 7 days, and they all remained H. pylori positive after the administration (6). The last two studies concluded that no “antibiotic-like” activity should be expected from crude mastic.
The crude resin that was used in all previous studies contained a high percentage (30%) of an insoluble and sticky polymer (poly-β-myrcene) (33) that obviously hinders its oral administration and reduces the bioavailability of the contained active compounds. To bypass such problems, we prepared a total mastic extract without polymer (TMEWP) and tested its activity against H. pylori in mice infected with the H. pylori SS1 strain. In addition, well-characterized mastic gum fractions as well as isolated pure compounds were tested in vitro against H. pylori in an effort to identify the most active constituents.
MATERIALS AND METHODS
General procedures.
Optical rotations were measured with a Perkin-Elmer 341 polarimeter. Nuclear magnetic resonance (NMR) spectra were recorded on Bruker DRX 400 and Bruker AC 200 spectrometers (1H [400 and 200 MHz] and 13C [50 MHz]); chemical shifts are expressed in ppm downfield from tetramethyl silane. The 1H-1H and the 1H-13C NMR experiments (distortionless enhancement by polarization transfer, correlated spectroscopy [COSY], COSY for long-range couplings, heteronuclear multiple quantum coherence, heteronuclear multiple-bond correlation, and nuclear Overhauser effect spectroscopy [NOESY]) were performed using standard Bruker microprograms. Gas chromatography-mass spectroscopy (MS) analysis was performed on a Finnigan GCQ Plus mass spectrometer. Electron impact and chemical ionization-MS spectra were determined on a Finnigan GCQ Plus mass spectrometer using CH4 as the chemical ionization reagent and high-resolution mass spectrometry on an AEI MS-90 spectrometer. Medium-pressure liquid chromatography (MPLC) was performed with a Büchi model 688 apparatus on columns containing Si gel (type 60; 20 to 40 μm; Merck).
Extraction process.
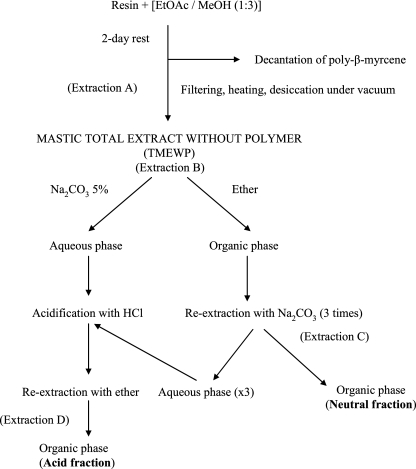
Commercial mastic gum was supplied by the Chios Mastic Growers Association, which is the exclusive worldwide producer of the resin. A quantity of mastic gum (500 g) was diluted in ethyl acetate (500 ml), and then methanol (1,500 ml) was added (Fig. 1). The mixture was let stand, and after a period of 2 days, a layer of poly-β-myrcene (150 g) was decanted. The clear supernatant solution was obtained by filtration, and the solvent mixture was evaporated in a rotary evaporator at 45°C with an 80-kPa vacuum (extraction A). The resulting semisolid residue was dried in a desiccator at 70°C and 1,000-mbar vacuum and gave a white powder (350 g). The TMEWP was freely soluble in ethanol, which is feasible for the crude resin only under protracted heating. TMEWP was partitioned between aqueous 5% Na2CO3 (1 liter) and ether (3.5 liter) as described by Barton (5) (extraction B). The organic phase was reextracted three times with 5% Na2CO3 (1 liter each time) (extraction C) and afforded the neutral fraction of mastic (135 g) as the organic phase. The aqueous phase was added to that of extraction B and acidified with 1 N HCl (3 liters). The acidic solution was reextracted with ether (6 liters) (extraction D), and the organic phase afforded the acid fraction of mastic (190 g).
FIG. 1.
Extraction of Chios mastic gum.
Comparison of crude mastic gum and TMEWP chemical profile.
Crude mastic gum and the TMEWP were submitted to thin-layer chromatography over silica gel using a mixture of CH2Cl2 and methanol (MeOH) (98/2) as the solvent system. The chromatographic zones were detected after spraying with a solution of vanillin and sulfuric acid in methanol. The chromatograms of both substances were identical except for poly-β-myrcene, which was present only in the crude resin (data not shown).
Isolation of pure triterpenic acids and neutral compounds.
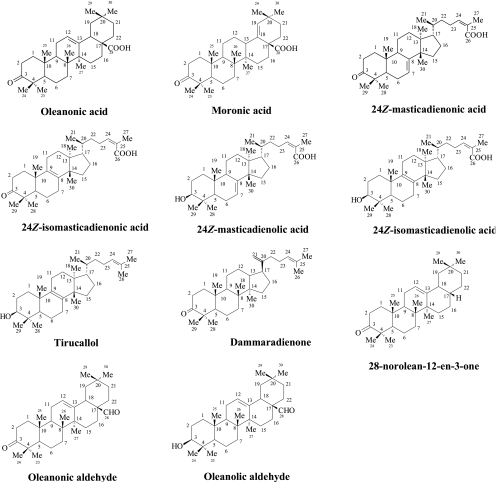
A part of the acidic fraction (20 g) was submitted to MPLC over normal-phase silica gel first with a cyclohexane-CH2Cl2 gradient (from cyclohexane-CH2Cl2 at 50/50 to CH2Cl2 at 100%) and then with a CH2Cl2-MeOH gradient (from CH2Cl2 at 100% to CH2Cl2-MeOH at 80/20). The total solvent volume used was 17.3 liters. A total of 24 fractions were obtained, the separation of which was decided on the basis of thin-layer chromatography (with several systems used as solvents), and the chromatographic zones were detected as mentioned above (the same separation procedure was followed in all chromatographic separations mentioned below). Fraction 11 (1.252 g) was separated by MPLC over normal-phase silica gel with a CH2Cl2-MeOH gradient (from CH2Cl2 at 100% to CH2Cl2-MeOH at 80/20; total solvent volume used, 2.1 liters) and afforded oleanonic acid (515 mg) (29, 30) and moronic acid (338 mg) (13). Fraction 12 (2.431 g) was separated by MPLC over normal-phase silica gel eluted with a CH2Cl2-MeOH gradient (from CH2Cl2 at 100% to CH2Cl2-MeOH at 80/20; total solvent volume used, 3.3 liters) and afforded 24Z-masticadienonic acid (1.1 g) (5, 8, 22) and 24Z-isomasticadienonic acid (1.0 g) (26). Fraction 17 (198 mg) was separated by column chromatography over silica gel eluted with a CH2Cl2-MeOH gradient (from CH2Cl2-MeOH from 99/1 to 80/20; total solvent volume used, 1.1 liter) and afforded 24Z-masticadienolic acid (85 mg) (9, 22) and 24Z-isomasticadienolic acid (92 mg) (24). The molecular structures are displayed in Fig. 2. All the above constituents were identified by one-dimensional (1D) and 2D NMR and MS and by comparison with data in the literature. Full NMR data for 24Z-isomasticadienonic acid, which have never been reported, are presented below.
FIG. 2.
Triterpenic compounds isolated from mastic acidic and neutral fractions.
24Z-Isomasticadienonic acid.
White “powder,” m.p. 166 to 167°C; [α]D: +34° (c 1.10, MeOH), EI-MS (m/z): 454 (34), 439 (100), 421 (62), 393 (13), 271 (11), 257 (19). 1H-NMR (CDCl3, 400 MHz): δ = 0.70 (3H, s, H-18), 0.82 (3H, s, H-30), 0.86 (3H, d, J = 5.8 Hz, H-21), 0.98 (3H, s, H-28), 1.02 (3H, s, H-19), 1.04 (3H, s, H-29), 1.08 (1H, H-22α), 1.19 (1H, H-15α), 1.38 (1H, H-20), 1.45 (1H, H-17), 1.46 (1H, H-22β), 1.48 (1H, H-15β), 1.57 (1H, H-1α), 1.64 (1H, H-5), 1.67 (2H, H-12), 1.80 (2H, H-6), 1.84 (3H, s, H-27), 1.88 (1H, H-16α), 1.90 (2H, H-11), 1.99 (1H, H-16β), 1.93 (1H, H-1β), 2.06 (2H, dd, J = 7 Hz, 14 Hz, H-7), 2.39 (1H, H-23α), 2.42 (1H, H-2α), 2.49 (1H, H-2β), 2.51 (1H, H-23β), 6.01 (1H, t, J = 7 Hz, H-24). 13C-NMR (CDCl3, 100 MHz): δ = 15.51 (C-18), 18.53 (C-21), 19.74 (C-27), 20.22 (C-6), 20.51 (C-19), 21.06 (C-28), 21.32 (C-16), 24.15 (C-30), 26.65 (C-29), 26.83 (C-23), 27.42 (C-7), 28.01 (C-11), 29.74 (C-15), 30.65 (C-12), 34.55 (C-2), 35.51 (C-1), 35.80 (C-22), 36.39 (C-20), 37.09 (C-10), 49.99 (C-13), 47.23 (C-4), 49.99 (C-14), 49.99 (C-17), 51.42 (C-5), 125.81 (C-25), 132.61 (C-9), 134.63 (C-8), 147.35 (C-24), 173.40 (C-26), 218.32 (C-3).
A part of the neutral fraction (17.2 g) was submitted to column liquid chromatography over normal-phase silica gel with a cyclohexane-CH2Cl2 gradient (from cyclohexane at 100% to CH2Cl2 at 100%) to afford 22 fractions. The total solvent volume used was 21 liters. Fraction 5 (839 mg) was separated by MPLC over normal-phase silica gel with a cyclohexane-CH2Cl2 gradient (from 90/10 to 100% CH2Cl2; total volume used, 2 liters) and afforded tirucallol (110 mg) (23) and dammaradienone (128 mg) (20). Fraction 8 (533 mg) was separated by MPLC over normal-phase silica gel eluted with a cyclohexane-CH2Cl2 gradient (from 80/20 to 100% CH2Cl2; total volume used, 1.5 liters) and afforded 28-norolean-12-en-3-one (206 mg) (20). Fraction 10 (396 mg) was separated by MPLC over normal-phase silica gel eluted with a cyclohexane-CH2Cl2 gradient (from 80/20 to 100% CH2Cl2; total volume used, 1.4 liters) and afforded oleanonic aldehyde (152 mg) (25) and oleanolic aldehyde (98 g) (3). The molecular structures are displayed in Fig. 2. All the above constituents were identified by 1D and 2D NMR and MS and by comparison with data in the published literature.
The neutral fraction was also submitted to analytical separation by gas chromatography-MS. Comparison of mass spectra with MS data library Wiley 275.l and data in the literature (4) resulted in the identification of the five compounds mentioned above as the major neutral ones, while several minor diterpenes or triterpenes with aldehyde, ketone, or hydroxyl groups were detected on the basis of molecular weight but not thoroughly identified.
In vitro test of mastic total extract, fractions, and pure compounds.
Minimum bactericidal concentrations (MBC) were evaluated utilizing H. pylori reference strain CCUG 38771 and another 10 clinical strains belonging to the Hellenic Pasteur Institute collection, isolated from gastric antrum biopsies from patients suffering from gastritis or duodenal or gastric ulcer (LAVHP-1 to LAVHP-10). H. pylori isolates were stored in brain heart infusion broth (BHIB) supplemented with 20% glycerol at −80°C. All strains prior to use were cultured twice under microaerophilic conditions (CampyPak Plus; Becton-Dickinson, Cockeysville, MD) for 24 h at 37°C, in Chalgren's-Wilkins (CHW) agar plates supplemented with 7% (vol/vol) horse blood and 1% (vol/vol) Vitox (Oxoid, Basingstoke, United Kingdom). Liquid cultures of H. pylori bacteria to a density of 109 CFU/ml were prepared by suspension in BHIB (Oxoid) supplemented with 10% fetal calf serum (Flow Laboratories, Irvine, Scotland) and 0.25% yeast extract (Oxoid). Successive twofold dilutions of each mastic extract or pure compound in BHIB medium were placed in sterilized 96-well flat-bottom microplates (Sarstedt, Numbrecht, Germany) within a total volume of 100 μl. All extracts were tested at a final concentration range of 0.049 to 1.560 mg/ml, with the exception of the acidic fraction, for which successive twofold dilutions ranged from 0.060 to 1.920 mg/ml. To each well containing the mastic dilutions, approximately 107 CFU of H. pylori bacteria were added within a 100-μl volume, and the microplates were incubated at 37°C for 24 h with continuous agitation under microaerophilic conditions. Thereafter, viability of H. pylori was evaluated by determination of viable CFU in CHW agar plates following incubation at 37°C for 48 h under microaerophilic conditions. The MBC was defined as the lowest concentration of mastic extract or pure compound that killed at least 99.9% of the CFU contained in the original inoculum. The mean MBC for each mastic preparation was determined as the average of three independent experiments.
Infection of mice with H. pylori strain SS1.
Specific-pathogen-free 6- to 8-week-old female C57BL/6 mice were obtained from the Central Animal Facility of the Hellenic Pasteur Institute. They were housed according to relevant Greek national legislation, fed a commercial diet, and given water ad libitum, except as otherwise stated. H. pylori infections by the SS1 strain were carried out as described before (27, 28). Briefly, freshly prepared aliquots (100 μl; 108 CFU) of H. pylori strain SS1 in BHIB (Oxoid) were administered to mice via orogastric inoculation, three times within a week. Accordingly, all noninfected control mice were inoculated with the same volume of plain BHIB.
Administration of TMEWP in vivo.
TMEWP was diluted into ethanol at a concentration of 180 mg/ml and then dissolved into a final aqueous solution of 180 μg/ml. The extract was administered through the animals' drinking water, starting 1 month following initial H. pylori infection and for 3 more months. The following groups of animals were included in the study: H. pylori-infected mice administered TMEWP (SMH; n = 10); uninfected mice administered TMEWP (SM; n = 10); and H. pylori-infected mice left untreated (SH; n = 10) as a control group. Animal weight was monitored throughout the whole observation period as a measure of health of the animals. Daily water consumption was measured, and a mean daily TMEWP consumption per animal was calculated to be 0.75 mg throughout the whole administration period. In addition to the above therapeutic in vivo protocol, we conducted a preliminary prophylactic study where C57BL/6 mice (n = 5) were administered TMEWP for a week prior to H. pylori infection, and we assessed H. pylori colonization 2 weeks after the initial infection.
Assessment of H. pylori colonization levels.
At end of the 3-month observation period, blood samples were collected via the tail vein and animals were sacrificed by cervical dislocation. Excised stomachs were dissected along the lesser curvature, and H. pylori detection in the gastric tissue was accomplished by H. pylori quantitative culture and PCR. For H. pylori SS1 quantitative culturing, preweighed half-stomach samples were homogenized in thioglycolate medium (Oxoid), serially diluted in phosphate-buffered saline, and plated on CHW agar plates with antibiotics (vancomycin, 10 μg/ml; trimethoprim, 10 μg/ml; polymyxin B, 104 IU/liter; amphotericin B, 2 μg/ml; nalidixic acid, 10 μg/ml; bacitracin, 30 μg/ml; fluorocytosine, 5 μg/ml; all from Sigma, St. Louis, Mo.). The cultures were incubated under microaerophilic conditions at 37°C for up to 6 days. H. pylori colonies were visualized on the basis of urease activity, and results were expressed as log CFU per gram of gastric tissue. The minimum bacterial density detected by this method was 100 CFU per gram. Qualitative H. pylori detection in the gastric samples was performed by H. pylori-specific PCR utilizing primers for the ureC (glmM) gene as described before (18). Genomic DNA for the detection of H. pylori by PCR in tissue samples or bacterial colonies was isolated by using a DNeasy tissue kit (QIAGEN).
Histopathologic analysis of gastric tissue samples.
Excised stomachs were opened along the lesser curvature, and the longitudinal half was fixed in 10% neutral buffered formalin solution, embedded in paraffin, and processed for histopathologic analysis. Antral, body, and cardioesophageal mucosa samples were examined in the same section. Eleven serial longitudinal 4-μm sections were cut from each specimen; 9 of them were stained with hematoxylin-eosin for evaluation of gastric inflammation, and 2 were stained by the May-Grünwald Giemsa stain method for the assessment of H. pylori colonization. The bacterial density and the pathological characteristics of the gastric mucosa were assessed according to the updated Sydney system (11). Histopathologic evaluation was performed with no prior knowledge of the identity of the samples by the histopathologist.
Determination of serum anti-H. pylori immunoglobulin G levels.
Immunoglobulin G (IgG) levels of anti-H. pylori antibodies were detected in the serum samples collected by an in-house enzyme-linked immunosorbent assay method. Briefly, 15 μg of H. pylori SS1 antigen produced by sonication and subsequent dialysis (SpectraPor; cutoff pore size, 8 kDa) was used to coat 96-well plates. Collected mouse serum samples (diluted 1/50) were primarily incubated in the plates for 24 h at 4°C, and then rabbit anti-mouse IgG (whole molecule)-peroxidase conjugate (Sigma) was used for the secondary incubation (2 h at 37°C). Color was developed by addition of o-phenylenediamine (Sigma), and optical density at 492 nm was measured in a Sunrise microtiter plate reader (Tekan).
Statistical analysis.
Analysis of the results from the in vivo experiments was performed with respect to H. pylori colonization by two-tailed unpaired t test with Welch correction and with respect to the associated gastritis by the Wilcoxon rank sum test due to the ordinal nature of the data. A P value of <0.05 was considered significant in both tests.
RESULTS
Isolation of TMEWP, acid and neutral fractions, and triterpenic compounds.
The TMEWP was obtained from crude mastic gum in a 70% proportion using a modification of the method described by Barton (5). Ether was replaced by ethyl acetate, a more convenient and nonexplosive solvent, in order to minimize the risk when manipulating large quantities of resin.
The TMEWP was further divided into two fractions, an acidic and a neutral one. The acidic fraction of TMEWP after several chromatographic separations afforded the major triterpenic acids (Fig. 2) oleanonic acid (515 mg), moronic acid (338 mg), 24Z-masticadienonic acid (1.1 g), 24Z-isomasticadienonic acid (1.0 g), 24Z-masticadienolic acid (95 mg), and 24Z-isomasticadienolic acid (102 mg). The neutral fraction, after similar treatment, afforded five neutral triterpenic compounds: tirucallol (110 mg), dammaradienone (128 mg), 28-norolean-12-en-3-one (206 mg), oleanonic aldehyde (152 mg), and oleanolic aldehyde (98 mg).
All the above constituents were identified by NMR (1H, 13C, distortionless enhancement by polarization transfer, COSY, heteronuclear multiple quantum coherence, heteronuclear multple-bond correlation, and NOESY) and MS and by comparison with data in the literature. The 1H and 13C NMR data for 24Z-isomasticadienonic acid have never been reported, and the stereochemistry of the double bond at position 24 of masticadienonic acid, isomasticadienonic acid, masticadienolic acid, and isomasticadienolic acid has never been studied. In all cases, the NOESY correlation of Me-27 with the double bond proton H-24 confirmed the stereochemistry as Z. The 1H and 13C NMR data of 24Z-isomasticadienonic acid are reported herein for the first time.
In vivo activity of mastic TMEWP against H. pylori infection in vivo.
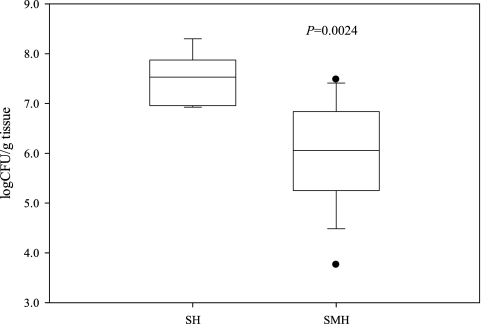
During the course of the experiment, none of the participating mice died and no statistical difference was observed between the three groups with regard to animal weight gain (data not shown). Mean water consumption was approximately the same for each animal group and ranged from 4.2 to 4.5 (± 0.1 [standard error of the mean]) ml, regardless of the presence of TMEWP. Average extract consumption was the same for both SM and SMH groups, calculated at 0.75 mg per mouse and taking into account mean water consumption volumes. In the control uninfected group, SM, absence of H. pylori infection was confirmed by PCR, serology, or H. pylori culture of the gastric samples. In the untreated H. pylori-infected SH group, the presence of H. pylori was confirmed by PCR and quantitative culturing in 9 out of 10 animals. In the H. pylori-negative animal, further analysis of serum anti-H. pylori IgG antibodies confirmed the absence of infection and, therefore, it was excluded from the study. The rest of the animals were positive for the presence of H. pylori by PCR, and the mean H. pylori colonization was calculated at 7.5 ± 0.18 log CFU/g of tissue. In the TMEWP-treated H. pylori-infected SMH group, the presence of H. pylori was confirmed by PCR in all the animals, and the mean H. pylori colonization was calculated at 6.0 ± 0.35 log CFU/g of gastric tissue. A statistically significant reduction in H. pylori viable counts was calculated between the two animal groups (P = 0.0024) (Fig. 3). These results were also consistent with the histopathologic observations, showing reduced colonization of the bacterium in the gastric antrum and corpus in the TMEWP-treated SMH group (P = 0.031 for antrum, P = 0.037 for corpus) (Table 1).
FIG. 3.
H. pylori colonization in H. pylori-infected mice following continuous administration of total mastic extract without the polymer (SMH; n = 10 animals) or left untreated (SH; n = 9 animals). Viable H. pylori counts are expressed in log CFU/g gastric tissue. A moderate 1.5-log reduction in H. pylori colonization was observed (P = 0.0024) in the SMH study group compared to the SH control group.
TABLE 1.
H. pylori colonization in H. pylori-infected mice treated with TMEWP
| Location | Group | No. of mice of gradea:
|
Pb | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Antrum | SH | 1 | 3 | 3 | 2 | |
| SMH | 3 | 6 | 1 | 0 | 0.031 | |
| Corpus | SH | 5 | 4 | 1 | 0 | |
| SMH | 10 | 0 | 0 | 0 | 0.038 | |
Colonization grades are according to the updated Sydney system (11), as follows: normal, 0; mild, 1; moderate, 2; marked, 3.
Statistical analysis with reference to the SH control group, done by Wilcoxon rank sum test. Both correlations shown were significant.
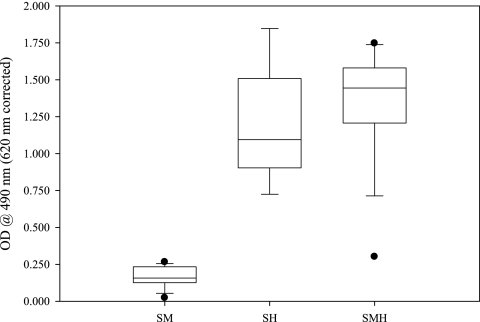
H. pylori-specific IgG antibodies are the dominant antibody class present in the sera of chronically H. pylori-infected mice and may serve as an indicator of successful H. pylori infection. A significant difference in anti-H. pylori IgG antibody titers was observed between H. pylori-infected (groups SH and SMH) and noninfected control mice (SM). However, no statistical difference was detected between the TMEWP-treated SMH group and the untreated H. pylori-infected SH group with regard to serum anti-H. pylori IgG levels (Fig. 4).
FIG. 4.
Serum anti-H. pylori IgG antibody response in H. pylori-infected animals treated with total mastic extract without the polymer (SMH) or left untreated (SH). Uninfected control mice that received total mastic extract without the polymer are also depicted (SM). Mouse sera were diluted to 1:50. No difference in anti-H. pylori titers was observed at 12 weeks postinfection between the SMH and SH groups.
Histopathologic evaluation of the gastric mucosa revealed a mild induction of chronic gastritis in the antrum and the corpus fundus in the animals of the untreated H. pylori-infected SH group (Tables 2 and 3). Gastric samples were characterized by mild infiltration of the lamina propria with scattered lymphocytes and neutrophils. Chronic inflammatory infiltration in the antrum was mild in eight animals and moderate in one animal. In the corpus, only six animals developed mild chronic inflammatory infiltration (Table 2). The activity of chronic gastritis that developed was mild to moderate (mild in six animals, moderate in two animals) (Table 3) in the antrum and milder in the corpus (normal in seven animals, mild in two animals) (Table 3). No development of glandular atrophy or intestinal metaplasia was observed, as the time interval from the onset of infection was too short. In the extract-treated H. pylori-infected SMH group, the development of chronic gastritis was similar to the SH group (Tables 2 and 3), with marginally reduced numbers of neutrophils infiltrating the lamina propria in some cases. However, statistical analysis with regards to chronic inflammatory infiltration or the development of chronic active gastritis revealed no significant differences between the two animal groups. The collected data from the animal studies suggested that continuous administration of 0.75 mg TMEWP to H. pylori-infected mice may moderately reduce H. pylori colonizing numbers without a profound effect on the associated gastritis. However, prophylactic administration of TMEWP did not prevent H. pylori colonization (data not shown).
TABLE 2.
Chronic inflammatory infiltrationa in H. pylori-infected mice treated with TMEWP
| Location | Group | No. of mice of gradeb:
|
Pc | ||
|---|---|---|---|---|---|
| 0 | 1 | 2a | |||
| Antrum | SH | 0 | 8 | 1 | |
| SMH | 0 | 8 | 2 | 0.713 | |
| Corpus | SH | 3 | 6 | 0 | |
| SMH | 7 | 3 | 0 | 0.569 | |
Lymphocyte infiltration.
Histopathology grades are according to the updated Sydney system (11) as follows: normal, 0; mild, 1; moderate, 2.
Statistical analysis with reference to the SH control group, done by Wilcoxon rank sum test.
TABLE 3.
Activity of chronic gastritisa in H. pylori-infected mice treated with TMEWP
| Location | Group | No. of mice of gradeb:
|
Pc | ||
|---|---|---|---|---|---|
| 0 | 1 | 2a | |||
| Antrum | SH | 1 | 6 | 2 | |
| SMH | 5 | 2 | 3 | 0.369 | |
| Corpus | SH | 7 | 2 | 0 | |
| SMH | 8 | 1 | 1 | 0.967 | |
Neutrophil infiltration.
Histopathology grades are according to the updated Sydney system (11) as follows: normal, 0; mild, 1; moderate, 2.
Statistical analysis with reference to the SH control group, done by Wilcoxon rank sum test.
In vitro activity of mastic total extract, fractions, and compounds against H. pylori strain SS1.
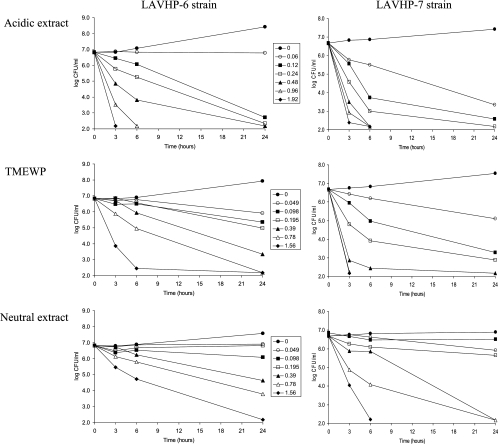
Having observed a moderate antimicrobial effect in vivo against H. pylori, we proceeded to investigate the potential in vitro anti-H. pylori activity of TMEWP and its acidic and neutral fractions against a panel of 10 clinical isolates of H. pylori and the CCUG 38771 reference strain (Table 4). Figure 5 depicts characteristic kill curves for strains LAVHP-6 (one of the least susceptible strains) and LAVHP-7 (the most susceptible strain). Mastic extracts exhibited concentration- and strain-dependent bactericidal activities. More specifically, in all strains tested, without exception the acidic fraction exhibited the highest activity, with a mean MBC of 0.136 mg/ml, followed by the TMEWP (MBC, 0.256 mg/ml). Reduced activity was observed for the neutral fraction of the TMEWP (0.638 mg/ml). Up to twofold differences were observed in the MBC between individual strains tested, and only in the case of LAVHP-7 strain was a higher susceptibility against the TMEWP and its acidic fraction observed.
TABLE 4.
Minimum bactericidal concentrations of mastic extractsa on H. pylori strains
| Strain | MBC (mg/ml)
|
||
|---|---|---|---|
| Total extract | Acidic fraction | Neutral fraction | |
| CCUG 38771 | 0.390 | 0.240 | 1.560 |
| LAVHP-1 | 0.195 | 0.120 | 0.390 |
| LAVHP-2 | 0.195 | 0.120 | 0.780 |
| LAVHP-3 | 0.195 | 0.120 | 0.780 |
| LAVHP-4 | 0.390 | 0.120 | 0.780 |
| LAVHP-5 | 0.195 | 0.120 | 0.390 |
| LAVHP-6 | 0.390 | 0.120 | 0.780 |
| LAVHP-7 | 0.090 | 0.060 | 0.390 |
| LAVHP-8 | 0.390 | 0.120 | 0.390 |
| LAVHP-9 | 0.195 | 0.240 | 0.390 |
| LAVHP-10 | 0.195 | 0.120 | 0.390 |
With the exception of the acidic fraction, for which successive twofold dilutions ranged from 0.060 to 1.920 mg/ml, all other extracts were tested at a final concentration range of 0.049 to 1.560 mg/ml.
FIG. 5.
Bactericidal activity of mastic gum extracts against H. pylori in a liquid medium. H. pylori strains LAVHP-6 (more resistant strain) and LAVHP-7 (most susceptible strain) were cultured under microaerophilic conditions in BHI as described in Materials and Methods and exposed to acidic, TMEWP, and neutral fractions at the concentrations depicted in the legend. After further incubation, viability was determined at each time point.
Having obtained the highest activity with the acidic fraction of the TMEWP, we proceeded to test the isolated pure acidic compounds for anti-Helicobacter activity. Highest overall activity was obtained consistently and for all 11 of the H. pylori strains tested with isomasticadienolic acid, with a mean MBC of 0.202 mg/ml (0.443 mM), followed by masticadienolic (0.220 mg/ml [0.482 mM]), oleanonic (0.292 mg/ml [0.643 mM]), and moronic acid (0.310 mg/ml [0.683 mM]) (Table 5). Interestingly, the 3-oxo derivatives, isomasticadienonic and masticadienonic acids, showed reduced activity compared to the corresponding 3-hydroxyl derivatives.
TABLE 5.
Mean MBC of mastic triterpenic acid compounds within the mastic acidic fraction against H. pyloria
| Substance | Mean MBC in mg/ml (mM) |
|---|---|
| 24Z-Isomasticadienonic acid | 0.333 (0.733) |
| 24Z-Masticadienonic acid | 0.350 (0.770) |
| 24Z-Isomasticadienolic acid | 0.202 (0.443) |
| 24Z-Masticadienolic acid | 0.220 (0.482) |
| Oleanonic acid | 0.292 (0.643) |
| Moronic acid | 0.310 (0.683) |
H. pylori strains are described in Table 4.
DISCUSSION
All previous in vivo studies evaluating activity of mastic gum against H. pylori have used a crude mastic preparation which contained a high percentage (30%) of an insoluble and sticky polymer. We speculated that the presence of the polymer hindered potential in vivo activity of mastic during oral administration, and for this reason we prepared TMEWP. We have verified that the chemical consistency of TMEWP was virtually identical to that of crude mastic gum, except for the absence of the polymer, and it also presented better solubility properties and increased concentration of active constituents. Previous animal studies for the determination of the potential anti-Helicobacter activity of mastic gum were organized on a short-term administration schedule. However, we have extended our administration and hence tested the TMEWP activity over a period of 3 months.
In the present study we utilized an established H. pylori infection model to evaluate the potential therapeutic effect of continuous TMEWP administration on H. pylori colonization and development of associated gastritis. This model involves the mouse-adapted H. pylori Sydney strain 1 (SS1), which colonizes the C57BL/6 mouse heavily and leads to the development of appreciable levels of gastritis closely mimicking human H. pylori infection (16, 19, 31). The particular infection model has been successfully utilized in the past to evaluate potential anti-H. pylori activity of a number of agents, such as antibiotics (14, 15) or lactic acid-producing bacteria (27, 28).
Our experiments showed that the mastic total extract could moderately reduce H. pylori colonization in the antrum and corpus of the stomach. The reduction in colonization levels calculated was approximately 30-fold, in the range of 1.5 log CFU/g of tissue. These results were in concurrence with the visible reduction in H. pylori colonization observed in the histopathology evaluations. However, such a moderate fall in H. pylori colonizing numbers could not support any attenuation of the H. pylori-associated chronic active gastritis. We have documented no such decline in either neutrophilic or lymphocytic infiltration within the lamina propria. Equally, no reduction was observed with reference to serum anti-H. pylori IgG antibodies between the mastic-treated and nontreated animal groups. Collectively, these results are in line with earlier observations involving the same infection model, in which 7-day monotherapy with crude mastic tear diluted in 100% ethanol failed to eradicate H. pylori (17). Nevertheless, we were able to document a moderate drop in colonization levels possibly by increasing the bioavailability of the active mastic constituents, following polymer removal. Another main advantage of our study was the application of a longer period of administration and observation. In this way we were able to administer 0.75 mg of TMEWP (the approximate equivalent of 1 mg total mastic gum) continuously through the animal water supply, for a period of 3 months, as opposed to only 1-week therapy regimens reported in other studies. Our mode of administration mimicked more closely real life conditions, where active mastic constituents can be released in a sustained release mode following daily consumption of mastic gum. Although 3 months proved too short a period for the mastic total extract to eradicate H. pylori, results suggest that in real life, habitual daily consumption of mastic gum over a much longer period may well create conditions favoring a decrease in H. pylori colonization levels. However, we observed no prophylactic activity of TMEWP on H. pylori infection, although our observations depend on a small number of animals. Equally, to date no epidemiological data exist to support such a prophylactic effect among the mastic gum-consuming population of Chios Island.
A detailed in vitro investigation was performed in order to identify the potentially most active fractions in mastic extracts. The TMEWP was further separated into an acidic and a neutral fraction, and the antimicrobial activities of these fractions, as well as that of mastic total extract, were determined. We observed a moderate activity for the total mastic extract against a panel of 11 H. pylori strains at a mean MBC of 0.256 mg/ml, which is higher than that previously reported against the H. pylori SS1 strain for mastic gum preparations diluted in ethanol (17). This could well be attributed to differences in methodology and the differential susceptibility of diverse H. pylori isolates, as our in vitro data clearly demonstrated. The acidic fraction of the TMEWP was found to be the most active, with an MBC as low as 0.136 mg/ml, consistently in all strains tested. In order to identify the most active constituent among the contained triterpenic acids, the acid fraction was further submitted to several chromatographic separations that afforded the six major constituents. They were all tested against the same H. pylori strains as mastic extracts, and the most active one was found to be isomasticadienolic acid. None of the pure compounds was more active than the whole acid fraction, suggesting that, although the anti-H. pylori activity of mastic could be located particularly in the acidic fraction, probably the final activity is a result of synergy between all the acid constituents. Although it is the first case in which specific mastic acid triterpenic compounds have been shown to exhibit anti-H. pylori activity, other tetracyclic acids similar to those contained in mastic have been reported to be active against Escherichia coli (35). In our study, however, due to limitations in the isolation and purification steps, we were not able to test the potential antimicrobial in vivo activity of individual triterpenic compounds.
Emerging antibiotic resistance and reduced patient compliance are the main reasons for failed H. pylori eradication therapy. In addition, the considerable expense of the antibiotic regimen coadministered with a proton pump inhibitor creates the need for alternative antibiotics for combination therapy. The present study demonstrated that a mastic gum extract without the polymer constituent poly-β-myrcene was effective in reducing H. pylori colonization levels by 30-fold in infected mice over an administration period of 3 months and that the activity could be attributed to triterpenic acids within the acid fraction of mastic extracts. These results suggest that removal of the constituent poly-β-myrcene polymer can produce an enhanced therapeutic moiety which may exhibit anti-H. pylori activity, detectable over a shorter time period, bearing all the advantages of a nutritional product. We plan to evaluate further the true therapeutic potential of the acidic and neutral fractions of mastic gum in extensive animal studies following administration of the respective triterpenic constituents, alone and in combination, to assess synergic effects. The role of such mastic constituents will also be assessed with reference to potential synergy with currently used antibiotic eradication schemes.
Finally, our results also suggest that habitual long-term mastic consumption may be effective in moderating H. pylori colonization, although to date no epidemiological data exist to support the hypothesis of a reduced prevalence of H. pylori infection among the mastic gum-consuming population of Chios Island.
Acknowledgments
We acknowledge the help and support of the staff in the Central Animal House of the Hellenic Pasteur Institute and in particular A. Marandidou.
The project was supported in part by the General Secretariat for Research and Technology of Greece PAVET, program 00BE308, entitled “Study of biologically active substances with potential antimicrobial and healing activity isolated from traditional plant origin” and LAVIPHARM S.A., a company manufacturing pharmaceutical, chemical, and cosmetic products.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Al Habbal, M. J., Z. Al Habbal, and F. U. Huwez. 1984. A double-blind controlled clinical trial of mastic and placebo in the treatment of duodenal ulcer. Clin. Exp. Pharmacol. Physiol. 11:541-544. [DOI] [PubMed] [Google Scholar]
- 2.Al Said, M., A. M. Ageel, N. S. Parmar, and M. Tariq. 1986. Evaluation of mastic, a crude drug obtained from Pistacia lentiscus for gastric and duodenal anti-ulcer activity. J. Ethnopharmacol. 15:271-278. [DOI] [PubMed] [Google Scholar]
- 3.Ampofo, S. A., and P. G. Waterman. 1986. Xanthones from three Garcinia species. Phytochemistry 25:2351-2355. [Google Scholar]
- 4.Assimopoulou, A. N., and V. P. Papageorgiou. 2004. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part I. Pistacia lentiscus var. Chia. Biomed. Chromatogr. 19:1077-1079. [DOI] [PubMed] [Google Scholar]
- 5.Barton, D. H. R., and E. Seoane. 1956. Triterpenoids. Part XXII. The constitution and stereochemistry of masticadienonic acid. J. Chem. Soc. 189:4150-4157. [Google Scholar]
- 6.Bebb, J. R., N. Bailey-Flitter, D. Ala Aldeen, and J. C. Atherton. 2003. Mastic gum has no effect on Helicobacter pylori load in vivo. J. Antimicrob. Chemother. 52:522-523. [DOI] [PubMed] [Google Scholar]
- 7.Bona, S., L. Bono, L. Daghetta, and P. Marone. 2001. Bactericidal activity of Pistacia lentiscus gum mastic against Helicobacter pylori. Am. J. Gastroenterol. 96:S49. [DOI] [PubMed] [Google Scholar]
- 8.da Silva, M. F. G. F., R. H. P. Fransisco, A. I. Gray, J. R. Lechatt, and P. G. Waterman. 1990. Lanost-7-en triterpenes from stem bark of Santiria trimera. Phytochemistry 29:1629-1632. [Google Scholar]
- 9.Deng, J.-D., S. R. Starck, D.-A. Sun, M. Sabat, and S. M. Hecht. 2000. A new 7,8-euphadien-type triterpenoid from Brackenridgea nitida and Bleasdalea bleasdalei that inhibits DNA polymerase β. J. Nat. Prod. 63:1356-1360. [DOI] [PubMed] [Google Scholar]
- 10.de Pascual Teresa, J., J. G. Urones, P. Basabe, M. J. Sexmero Cuadrado, and R. Fernandez Moro. 1986. Triterpens from Euphorbia broteri. Phytochemistry 26:1767-1776. [Google Scholar]
- 11.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney system. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 12.Huwez, F. U., D. Thirlwell, A. Cockayne, and D. A. Ala'Aldeen. 1998. Mastic gum kills Helicobacter pylori. N. Engl. J. Med. 339:1946. [DOI] [PubMed] [Google Scholar]
- 13.Ito, J., F.-R. Chang, H.-K. Wang, Y. K. Park, M. Ikegaki, N. Kilgore, and K.-H. Lee. 2001. Anti-AIDS agents. 48. Anti-HIV activity of moronic acid derivatives and the new melliferone-related trierpenoid isolated from Brazilian propolis. J. Nat. Prod. 64:1278-1281. [DOI] [PubMed] [Google Scholar]
- 14.Jenks, P. J., A. Labigne, and R. L. Ferrero. 1999. Exposure to metronidazole in vivo readily induces resistance in Helicobacter pylori and reduces the efficacy of eradication therapy in mice. Antimicrob. Agents Chemother. 43:777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenks, P. J., R. L. Ferrero, J. Tankovic, J. M. Thiberge, and A. Labigne. 2000. Evaluation of nitrofurantoin combination therapy of metronidazole-sensitive and -resistant Helicobacter pylori infections in mice. Antimicrob. Agents Chemother. 44:2623-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, A., J. O'Rourke, M. C. de Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 17.Loughlin, M. F., D. A. Ala'Aldeen, and P. J. Jenks. 2003. Monotherapy with mastic does not eradicate Helicobacter pylori infection from mice. J. Antimicrob. Chemother. 51:367-371. [DOI] [PubMed] [Google Scholar]
- 18.Lu, J. J., C. L. Perng, R. Y. Shyu, C. H. Chen, Q. Lou, S. K. Chong, and C. H. Lee. 1999. Comparison of five PCR methods for detection of Helicobacterpylori DNA in gastric tissues. J. Clin. Microbiol. 37:772-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mähler, M., S. Janke, S. Wagner, and H. J. Hedrich. 2002. Differential susceptibility of inbred mouse strains to Helicobacter pylori infection. Scand. J. Gastroenterol. 37:267-278. [DOI] [PubMed] [Google Scholar]
- 20.Marner, F.-J., A. Freyer, and J. Lex. 1991. Triterpenoids from gum mastic, the resin of Pistacia lentiscus. Phytochemistry 30:3709-3712. [Google Scholar]
- 21.Marone, P., L. Bono, E. Leone, S. Bona, E. Carretto, and L. Perversi. 2001. Bactericidal activity of Pistacia lentiscus mastic gum against Helicobacter pylori. J. Chemother. 13:611-614. [DOI] [PubMed] [Google Scholar]
- 22.Mulholland, D. A., and J. J. Nair. 1994. Triterpenoids from Disoxylum pettigrewianum. Phytochemistry 37:1409-1411. [Google Scholar]
- 23.Peek, R. M., and J. E. Crabtree. 2006. Helicobacter infection and gastric neoplasia. J. Pathol. 208:233-248. [DOI] [PubMed] [Google Scholar]
- 24.Pozzo-Balbi, T., L. Nobile, G. Scapini, and M. Cini. 1978. The triterpenoid acids of Schinus molle. Phytochemistry 17:2107-2110. [Google Scholar]
- 25.Reddy, G. C., S. Rangaswami, and R. Sunder. 1977. Triterpenoids of the stem bark of Gardenia gummifera. Planta Med. 32:206-211. [DOI] [PubMed] [Google Scholar]
- 26.Seoane, E. 1956. Further crystalline constituents of gum mastic. J. Am. Chem. Soc. 189:4158-4160. [Google Scholar]
- 27.Sgouras, D. N., E. G. Panayotopoulou, B. Martinez-Gonzalez, K. Petraki, S. Michopoulos, and Α. Mentis. 2005. Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces the levels of proinflammatory chemokines in C57BL/6 mice. Clin. Diagn. Lab. Immunol. 12:1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sgouras, D., P. Maragkoudakis, K. Petraki, B. Martinez-Gonzalez, E. Eriotou, S. Michopoulos, G. Kalantzopoulos, E. Tsakalidou, and A. Mentis. 2004. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl. Environ. Microbiol. 70:518-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirane, N., Y. Hashimoto, K. Ueda, H. Takenaka, and K. Katoh. 1996. Ring-A cleavage of 3-oxo-olean-12-3n-28-oic acid by the fungus Chaetomium longirostre. Phytochemistry 43:99-104. [Google Scholar]
- 30.Sung, T. V., J. Peter-Katalinic, and G. Adam. 1991. A bidesmosidic triterpenoid saponin from Schefflera octophylla. Phytochemistry 30:3717-3720. [DOI] [PubMed] [Google Scholar]
- 31.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 18:2677-2685. [DOI] [PubMed] [Google Scholar]
- 32.Tassou, C. C., and G. J. E. Nychas. 1995. Antimicrobial activity of the essential oil of mastic gum (Pistacia lentiscus var. chia) on gram positive and gram negative bacteria in broth and model food system. Int. Biodeter. Biodegradation 36:411-420. [Google Scholar]
- 33.van den Berg, K. J., J. van der Horst, J. J. Boon, and O. O. Sudmeijer. 1998. cis-1,4-Poly-β-myrcene: the structure of the polymeric fraction of mastic resin (Pistacia lentiscus L.) elucidated. Tetrahedron Lett. 39:2645-2648. [Google Scholar]
- 34.Warren, J. R., and B. Marshall. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 321:1273-1275. [PubMed] [Google Scholar]
- 35.Yang, S.-P., and J.-M. Yue. 2001. Two novel cytotoxic and antimicrobial triterpenoids from Pseudolarix kaempferi. Bioorg. Med. Chem. Lett. 11:3119-3122. [DOI] [PubMed] [Google Scholar]