Abstract
Choline kinase is the first enzyme in the Kennedy pathway (CDP-choline pathway) for the biosynthesis of the most essential phospholipid, phosphatidylcholine, in Plasmodium falciparum. In addition, choline kinase also plays a pivotal role in trapping essential polar head group choline inside the malaria parasite. Recently, Plasmodium falciparum choline kinase (PfCK) has been cloned, overexpressed, and purified. However, the function of this enzyme in parasite growth and survival has not been evaluated owing to the lack of a suitable inhibitor. Purified recombinant PfCK enabled us to identify an inhibitor of PfCK, hexadecyltrimethylammonium bromide (HDTAB), which has a very close structural resemblance to hexadecylphosphocholine (miltefosin), the well-known antiproliferative and antileishmanial drug. HDTAB inhibited PfCK in a dose-dependent manner and offered very potent antimalarial activity in vitro against Plasmodium falciparum. Moreover, HDTAB exhibited profound antimalarial activity in vivo against the rodent malaria parasite Plasmodium yoelii (N-67 strain). Interestingly, parasites at the trophozoite and schizont stages were found to be particularly sensitive to HDTAB. The stage-specific antimalarial effect of HDTAB correlated well with the expression pattern of PfCK in P. falciparum, which was observed by reverse transcription-PCR and immunofluorescence microscopy. Furthermore, the antimalarial activity of HDTAB paralleled the decrease in phosphatidylcholine content, which was found to correlate with the decreased phosphocholine generation. These results suggest that inhibition of choline kinase by HDTAB leads to decreased phosphocholine, which in turn causes a decrease in phosphatidylcholine biosynthesis, resulting in death of the parasite.
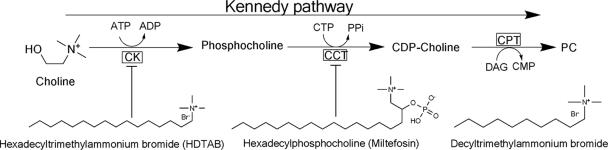
Phosphatidylcholine (PC) is the most abundant phospholipid in Plasmodium falciparum and is synthesized mainly by (i) the Kennedy or CDP-choline pathway (40) and (ii) the serine decarboxylation-phosphoethanolamine methylation pathway, which eventually shunts to the Kennedy pathway (31). So far, the Bremer-Greenberg pathway (phosphatidylethanolamine methylation pathway) for the synthesis of PC has not been characterized in P. falciparum probably due to the lack of phosphatidylethanolamine methyltransferase, the key enzyme in the Bremer-Greenberg pathway in the P. falciparum genome (www.plasmodb.org). The relative contributions of the first two pathways for the synthesis of PC have not been addressed; however, the Kennedy pathway has been suggested to be the main pathway (31, 40). Choline kinase (ATP:choline phosphotransferase [EC 2.7.1.32]) is the first enzyme in the Kennedy pathway (CDP-choline pathway) (see Fig. 1), and inhibition of this pathway prevents parasite growth (1, 3, 36). Moreover, PC biosynthesis has been suggested as a realistic target for development of new pharmacophores even against pharmacoresistant strains (1, 43). Choline kinase (CK) catalyzes the initial step in the CDP-choline pathway and can be regulatory for PC biosynthesis in various biological systems (42, 45), but so far, a regulatory role for CK in malaria parasites has not been reported, although a positive correlation between the intracellular phosphocholine pool and PC content had been found under certain quaternary ammonium compound treatment (4), indicating CK may have the potential to regulate PC biosynthesis in the malaria parasite also. Recently, we have cloned, overexpressed, and characterized Plasmodium falciparum choline kinase (PfCK) (16). The availability of catalytically active recombinant PfCK opened an opportunity to identify potent PfCK inhibitors with a view to explore the function of PfCK. Here, evidence is presented to establish that hexadecyltrimethylammonium bromide (HDTAB) inhibits purified PfCK as well as CK of P. falciparum in culture. The structure of HDTAB closely resembles the structure of hexadecylphosphocholine (miltefosin) (Fig. 1), the well-known drug used in chemotherapy for cancer (10) and leishmaniasis (11, 30). HDTAB exhibits potent antimalarial activity in vitro and in vivo. Furthermore, evidence has been presented to show that inhibition of CK in P. falciparum by HDTAB perturbs the Kennedy pathway which results in parasite death.
FIG. 1.
Kennedy pathway for phosphatidylcholine biosynthesis and the structures of choline, decyltrimethylammonium bromide, HDTAB, and hexadecylphosphocholine (miltefosin). The arrow indicates the direction of the pathway. Abbreviations, CK, choline kinase; CCT, choline phosphate cytidylyltransferase; CPT, choline phosphotransferase; DAG, diacylglycerol; PC, phosphatidylcholine. The probable target enzymes of the HDTAB and miltefosin are indicated by ⊤ bars.
MATERIALS AND METHODS
Materials.
RPMI 1640 medium, gentamicin, hypoxanthine, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), hemicholinium-3 (HC-3), HDTAB, trichloroacetic acid, ATP, d-sorbitol, Dowex-50W (H+-form), and anti-rabbit immunoglobulin G (IgG) coupled to horseradish peroxidase were procured from Sigma (St. Louis, MO). [3H]hypoxanthine, [methyl-14C]choline chloride, RNase-free DNase, Ready.To.Go reverse transcription-PCR (RT-PCR) beads, and Cy2-tagged anti-rabbit IgG antibodies were purchased from Amersham Biosciences, NJ. RNeasy protect kit was purchased from QIAGEN, Germany. AlbuMax II and PTC-200 were procured from Gibco and MJ Research, respectively. Diamidino phenylindole (DAPI) and MitoTracker Red CMXRos were from MBI Fermentas, Hanover, MD, and Molecular Probes, OR, respectively. The instruments used for cell harvesting and radioactivity measurements were the Harvester 96 MACH III M (Tomtec, CT) and LS6500 multipurpose scintillation counter (Beckman Coulter Counter), respectively. Agarose was from Genei, Banglore, India. Dimethyl sulfoxide (DMSO) was from Thomas Baker, India. All other chemicals were of analytical grade.
Parasite culture.
P. falciparum (clone NF-54) was grown in human B+ erythrocytes at a hematocrit level of 5% in complete RPMI 1640 medium (CRPMI) (RPMI 1640 medium supplemented with 25 mM HEPES, 50 μg ml−1 gentamicin, 370 μM hypoxanthine, and 0.5% [wt/vol] AlbuMax II) in tissue culture flasks (25 cm2 and 75 cm2) with loose screw caps by the method of Trager and Jensen (39). Used medium was changed with fresh medium once in 24 h, and the culture was routinely monitored through Giemsa staining of thin smears.
Isolation of parasites from infected erythrocytes.
Parasites were isolated as described previously (41). Briefly, erythrocytes with ∼10% parasitemia were centrifuged at 800 × g for 5 min, washed, and resuspended in cold phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 5.3 mM Na2HPO4, and 1.8 mM KH2PO4). An equal volume of 0.5% saponin in PBS was added to the erythrocyte suspension (final concentration, 0.25%) and kept on ice for 15 min. It was centrifuged at 1,300 × g for 5 min to obtain a parasite pellet, and then pellet was washed with PBS thrice. Isolated parasites were either used immediately or kept at −80°C for later use.
Extraction of RNA, PCR, cloning, overexpression, and purification.
Freshly isolated parasites were immediately suspended in RNAlater solution (QIAGEN), and RNA was extracted from the parasites using QIAGEN RNeasy protect kit according to the manufacturer's instructions. Nucleic acid bound to a RNeasy column was incubated with 5 Kunitz units of RNase-free DNase in 50 mM Tris-HCl (pH-7.5) and 10 mM MgCl2 for 20 min at 37°C to remove DNA contamination in RNA preparations. The gene for putative choline kinase (PfCK) from total RNA was reverse transcribed and PCR amplified with forward primer 5′-GCGGGATCCGGATGGAAAGCAAAATCTGTGACCCC-3′ (BamHI restriction site is underlined) and reverse primer 5′CCGGAATTCATGATGATGATGATGATGATCGTCATAATCCTTGATAATATTTTTGG-3′ (EcoRI restriction site is underlined and contains codons for the six-His tag downstream of the EcoRI site). The RT-PCR-amplified PfCK gene was cloned in pRSET-C, an Escherichia coli expression vector. The host strain was optimized, and overexpressed PfCK protein was purified to homogeneity using Ni- nitrilotriacetic acid agarose affinity and gel filtration chromatography as described previously (16).
Inhibition of PfCK.
Choline kinase activity was measured by the method described earlier (17) with the following modifications. The activity of PfCK was evaluated in 96-well plates by incubating PfCK (5 μg) in 100 mM Tris-Cl (pH 8.8), 5 mM ATP, 6 mM MgCl2, and 250 μM [methyl-14C]choline chloride (specific activity, 5.6 mCi/mmol) in the absence or presence of different concentrations of H-89, HC-3, and HDTAB at 37°C for 45 min in a total volume of 100 μl. H-89 and HC-3 are known choline kinase inhibitors (8, 44), and HDTAB has quaternary ammonium groups, which can function as a structural analogue of choline and may bind to PfCK. For a few experiments with H-89, PfCK activity was also evaluated at 100 μM ATP and 120 μM MgCl2, keeping remaining conditions as such. Similarly, PfCK activity was also evaluated at 100 μM choline in the cases of HC-3 and HDTAB without modifying other variables. Since compounds were dissolved in DMSO, PfCK activity was also evaluated in the presence of DMSO and considered 100% PfCK activity. Reactions were terminated by the addition of a 50% slurry of Dowex-50W [H+] resin prepared in absolute alcohol. Following vigorous mixing, resin was allowed to settle under gravity. Equal volumes of supernatants containing 14C-labeled phosphocholine formed by PfCK were mixed with 10 ml of scintillation fluid and counted in a liquid scintillation β-counter (Beckman).
Antimalarial activity of HDTAB.
The antimalarial activity of HDTAB was determined in vitro by the [3H]hypoxanthine uptake assay described earlier (20). Two hundred microliters of P. falciparum asynchronous culture at 1% hematocrit and 0.5 to 0.7% parasitemia was exposed to various concentrations of HDTAB in the wells of a 96-well plate for one full parasite cycle (48 h). HDTAB concentrations were prepared in DMSO and diluted in RPMI 1640 medium. DMSO-treated parasites were used as a control. After completion of incubation, cells were washed thrice with CRPMI medium, and [3H]hypoxanthine (0.7 μCi/well) was added followed by a further incubation for 48 h under optimum growth conditions. Parasite viability was evaluated by the ability of the parasite to incorporate [3H]hypoxanthine in its nucleic acid. On completion of incubation, the cells were harvested on Whatman GF/C glass filters using a cell harvester. Subsequently, cells were lysed by triple distilled water, and unincorporated radioactivity on glass filters was removed by three successive washes with triple distilled water. Radioactivity was retained on filters and represented the incorporated [3H]hypoxanthine in parasite nucleic acid. After complete drying of filters at 30°C, scintillation fluid was added and kept for 24 h before counting in a liquid scintillation β-counter (Beckman).
Antimalarial effect of HDTAB on different stages of Plasmodium falciparum.
The stage-specific activity of HDTAB was evaluated by using the method described previously (3). The P. falciparum culture was synchronized as described earlier (27) using 5% d-sorbitol. The parasitemia in the synchronized culture was determined from Giemsa-stained thin smears and diluted to desired parasitemia of 0.5% (ring stage) with uninfected human B+ red blood cells (RBCs) to a final hematocrit level of 1%. The diluted culture was kept under optimum growth conditions, and aliquots were removed at time intervals coinciding with the ring, trophozoite, and schizont stages. At the appropriate times, rings (0 h), trophozoites (24 h), and schizonts (36 h) were incubated with various concentrations of HDTAB in 200 μl CRPMI for 8 h followed by three successive washings with CRPMI without hypoxanthine to remove the compound completely. Subsequently, parasites were further incubated in CRPMI without hypoxanthine until [3H]hypoxanthine (0.7 μCi/well) was added at 24 h, 48 h, and 60 h for rings, trophozoites, and schizonts, respectively. Cultures were finally harvested at 72 h from Whatman GF/C glass filters with a 96-well cell harvester, and samples were processed as described above. The DMSO control for each stage was run in parallel and had received the same treatment as the corresponding stage had.
Stage-specific expression of PfCK.
Different erythrocytic stages of parasites were obtained after preparing ring-synchronized cultures of P. falciparum. Synchronization was done as described earlier (27). Briefly, ring-stage-rich parasite culture (60 ml, 5% hematocrit) of approximately 10% parasitemia was pelleted at 800 × g, and the pellet (3 ml) was resuspended in 60 ml of 5% d-sorbitol and incubated for 10 min at 30°C with occasional shaking. Intact cells were separated from lysed ones by centrifugation at 800 × g for 5 min. Cells were aseptically washed thrice in CRPMI medium and finally diluted to the desired parasitemia with fresh uninfected human B+ RBCs, keeping the final hematocrit level 5%. Synchronization of the culture was confirmed by microscopic examination of Giemsa-stained thin smears. Parasites harvested from synchronized cultures at 0 h, 24 h, and 36 h when the parasites were in the ring, trophozoite, and schizont stages, respectively (3). Total RNA was isolated as described above from each parasite stage. An equal amount of RNA (1 μg) from each stage was used as a template to amplify PfCK, keeping other conditions the same as mentioned above for RT-PCR amplification. Simultaneously, positive-control primers for seryl tRNA synthetase were added in each RT-PCR. Seryl tRNA synthetase is expressed equally in each stage of P. falciparum (9). Primer sequences used for seryl tRNA synthetase were 5′-GAGGAATTTTACGTGTTCATCAA-3′ (forward) and 5′-GATTACTTGTAGGAAAGAATCCTTC-3′ (reverse). RT-PCR products were analyzed through electrophoresis on 1% agarose gel in Tris-acetate-EDTA buffer at 10 V/cm. The gel was photographed with a Gel Documentation system (Alpha Infotech, India). The intensity of bands was measured with densitometric software (Lab Image beta version; Kapelan GmbH, Germany).
To investigate the pattern of expression of PfCK in different stages of P. falciparum at the protein level, immunofluorescence microscopy was performed. In brief, asynchronous P. falciparum culture (50 μl in CRPMI medium at 4 to 5% parasitemia) was incubated with 250 nM MitoTracker Red CMXRos for 30 min under optimum growth conditions in the dark. Three successive washings with CRPMI medium removed unincorporated MitoTracker Red CMXRos. In order to obtain a concentrated suspension, the cells were finally resuspended in 10 μl of CRPMI medium and smeared on poly-l-lysine-coated glass coverslips. Smears were allowed to air dry and fixed in methanol:acetone (7:3 [vol/vol]) for 20 min at −20°C. Subsequently, cells were washed and hydrated in PBS and then permeabilized in 0.1% Triton X-100 for 20 min at 30°C. Permeabilized cells on the coverslips were washed four or five times with PBS for complete removal of Triton X-100. Blocking was performed with 5% (wt/vol) bovine serum albumin (BSA) in PBS for 20 min at 30°C, followed by four or five washes in PBS. Subsequently, smears were incubated for 16 h at 4°C with anti-PfCK antibodies (at a dilution of 1:50 in 2% [wt/vol] BSA in PBS) and washed with ice-cold PBS four or five times. Smears were further incubated with anti-rabbit IgG coupled to Cy2 at a dilution of 3.6 μg/ml (protein) in 2% BSA in PBS for 5 to 6 h at 4°C and then were washed with PBS four or five times. Subsequently, nuclear DNA of the parasite was stained with 20 μg/ml DAPI in PBS for 45 min at 30°C with gentle agitation and then washed with PBS four or five times. Finally, coverslips were mounted on slides in mounting medium containing paraphenyl diamine (Sigma) dissolved in 90% glycerol. Fluorophores were excited with excitation wavelengths of 350 nm (for DAPI), 579 nm (for MitoTracker Red CMXRos), and 489 nm (for Cy2-tagged anti-rabbit IgG), and their emissions at 470-nm (for DAPI), 599-nm (for MitoTracker Red CMXRos), and 506-nm (for Cy2-tagged anti-rabbit IgG) wavelengths were observed with a fluorescence microscope.
Effects of HDTAB on phosphocholine and phosphatidylcholine formation in P. falciparum.
To study the effect of HDTAB on the Kennedy pathway of malaria parasite, an experiment was performed to investigate the incorporation of radiolabeled choline into different metabolites of the pathway by the method described earlier (36). P. falciparum culture (5 ml) at 15% parasitemia and 5% hematocrit level was incubated with DMSO (control) or various concentrations of HDTAB for 1 hour under the optimum growth conditions (at 37°C under 90% N2 and 5% CO2). Subsequently, 40 μM [methyl-14C]choline chloride (specific activity, 5.6 mCi/mmol) was added to the culture and further grown under the identical conditions for 4 h. On completion of incubation, cells were pelleted at 800 × g, the culture medium was discarded, and the cells were washed three times with ice-cold PBS containing 40 μM nonradiolabeled choline chloride. Choline, phosphocholine, and phosphatidylcholine were extracted from the cells by a combination of the methods described earlier (18, 35). Cells were lysed in 5% trichloroacetic acid and then subjected to three cycles of freeze-thawing for complete lysis of the cells (35). Cell lysates were centrifuged at 12,000 × g and 4°C for 30 min to precipitate proteins and membranes (containing phosphatidylcholine) in the pellet. Pellets were dissolved in 500 μl of 1 N NaOH and added to 10 ml scintillation fluid. Supernatant containing phosphocholine and choline was extracted five times with diethyl ether to remove trichloroacetic acid, and then the pH of the aqueous phase was adjusted with 0.1 N NaOH to pH 7. For separation of choline and phosphocholine, the aqueous phase was vigorously mixed with 300 mg of Dowex-50W [H+] resin which binds only choline (18). Resin was allowed to settle, and supernatant containing phosphocholine was mixed in 10 ml of scintillation fluid. Choline from the Dowex-50W [H+] resin was recovered by vigorously mixing the resin in 500 μl of 1 N HCl for 5 min. Resin was allowed to settle under gravity, and supernatant was added to 10 ml of scintillation fluid. Counts were taken in a liquid scintillation β-counter (Beckman).
Toxicity assay.
Hemolytic activity of HDTAB was evaluated by the method described earlier (7) with slight modifications. Human B+ RBCs were incubated in 96-well plates either in the presence of DMSO (control) or in the presence of various concentrations of HDTAB for 24 h in 200 μl complete RPMI medium at 5% hematocrit level. RBCs were washed thrice in CRPMI following HDTAB removal and inoculated with 20 μl of P. falciparum parasitized RBCs (human B+) growing at 5% hematocrit level and 4 to 5% parasitemia (mostly trophozoites). Parasites were allowed to complete one intraerythrocytic cycle, 0.7 μCi [3H]hypoxanthine was added to each well, and the culture was further incubated for 24 h under optimum conditions. On completion of incubation, cells were harvested on Whatman GF/C glass filters with a cell harvester, and samples were processed as described above.
Cytotoxicity of HDTAB was also evaluated against growing nucleated mammalian cells (MCF-7) using a colorimetric assay for lactate dehydrogenase (LDH) release. Exponentially growing MCF-7 cells were seeded in 96-well tissue culture plates at a density of 2 × 104 cells per well in triplicate and cultured for 24 h in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum at 37°C in 5% humidified CO2. Following incubation, culture medium was replaced with 200 μl of fresh medium containing either different concentrations of HDTAB (dissolved in DMSO) or DMSO (as a negative control). As a positive control, cells were treated with 0.9% Triton X-100. The culture plate was then incubated for 7 h, and 50 μl of the supernatant from each well of the assay plate was taken from the corresponding well of a flat-bottom 96-well plate. Color reaction for LDH assay was performed using the CytoTox kit (Promega) by following the instructions of the manufacturer. Optical densities at 490 nm were measured in a microplate reader. Comparative analysis of LDH release was performed by setting LDH release in Triton X-100-treated cells at 100%.
In vivo antimalarial activity.
The in vivo efficacy of HDTAB was evaluated against P. yoelii (N-67 strain) in Swiss mice at three dose levels. In the first experiment, a group of five mice (22 ± 2 g) were inoculated intraperitoneally (i.p.) with 1 × 105 parasitized RBCs on day 0 and HDTAB was administered after 6 h of parasite inoculation via the i.p. route. In another experiment, a group of five mice (22 ± 2 g) were inoculated intravenously (i.v.) with 1 × 104 parasitized RBC on day 0, and HDTAB was administered after 6 h of parasite inoculation via the i.p. route. In both experiments, the treatment was continued at each dose level from day 0 to 3 via the intraperitoneal route. The aqueous suspension was prepared so as to obtain the required drug dose per animal in 0.20ml volume. Parasitemia levels from individual mice were recorded in Giemsa-stained thin blood smears on day 4. The mean value determined for a group of five mice was used to calculate the percentage of suppression in parasitemia with respect to the vehicle control group.
Statistical analysis.
The data were analyzed by Student's t test and one-way analysis of variance, followed by multiple-comparison t test.
RESULTS
HDTAB inhibits purified PfCK.
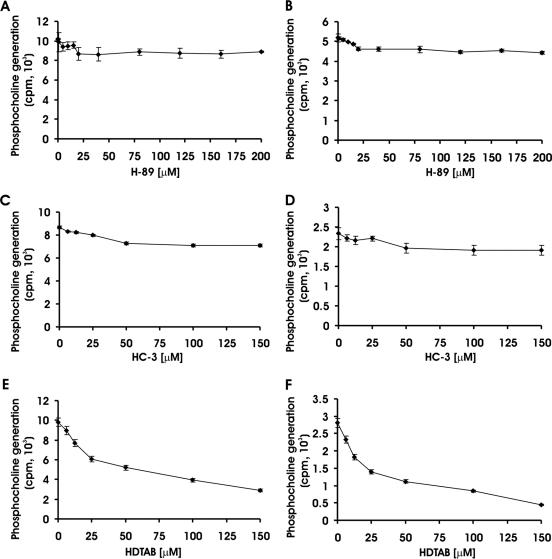
H-89 and HC-3 are known to inhibit choline kinase in various cells (8, 44). The effects of these inhibitors and choline analogue HDTAB on PfCK were investigated with the view of identifying a selective and potent inhibitor to explore the function of CK in P. falciparum growth and survival during intraerythrocytic stages. H-89, the known protein kinase A inhibitor (14), has also been reported to inhibit choline kinase of HeLa cells (44) but failed to inhibit purified PfCK (Fig. 2A and B). PfCK activity was measured at two different concentrations of ATP (5 mM and 100 μM) in the presence of H-89, and DMSO-treated PfCK was used as a control (as H-89 was dissolved in DMSO). H-89 had no effect on PfCK at an ATP concentration of 5 mM, since PfCK treated with H-89 catalyzed the formation of phosphocholine as efficiently as control cells (DMSO-treated PfCK) did (Fig. 2A). Since inhibition of protein kinase A by H-89 was found to be competitive in nature with respect to ATP (25), the concentration of ATP was decreased to 100 μM to assay PfCK in the presence or absence of H-89. Decreasing the ATP concentration to 100 μM had no effect on PfCK, as H-89-treated PfCK displayed activity comparable to control PfCK activity (Fig. 2B). A possible explanation of this inability of H-89 to inhibit purified PfCK is that H-89 offered its choline kinase inhibitory effect in intact HeLa cells where it could affect choline kinase activity via inhibition of protein kinase A and C, as growing evidence has established that phosphorylation of choline kinase by protein kinase A and C increases its activity (15, 45). The known choline transport inhibitor HC-3 (22, 37) has been reported to have a choline kinase-inhibitory effect (8). Therefore, the effect of HC-3 on PfCK was evaluated. Since HC-3 contains a quaternary ammonium group that mimics choline structure, PfCK activity was studied at two concentrations of choline, namely, 250 μM and 100 μM. At both choline concentrations, PfCK was not significantly inhibited by HC-3 up to 25 μM (Fig. 2C and D). However, poor inhibition of only 16% and 18% was observed at 50 μM and 150 μM, respectively, at both 100 and 250 μM choline. A similar level of inhibition by HC-3 has also been reported earlier in the malaria parasite lysate for choline kinase activity (6). These results indicated that HC-3 is not a strong inhibitor of PfCK. Development of specific choline kinase inhibitors based on the structure of HC-3 (23, 24) and a mild inhibition of PfCK by HC-3 induced interest in studying the effects of other quaternary ammonium compounds on PfCK activity. The quaternary ammonium compound selected for the study was HDTAB because it has a quaternary ammonium group as well as its structure is very similar to the structure of hexadecylphosphocholine (miltefosin), a molecule widely used as an antiproliferative (10) and antileishmanial drug (11, 30). HDTAB inhibited PfCK in a dose-dependent manner and showed 60% (P < 0.001) inhibition at 100 μM (Fig. 2E). To investigate the type of inhibition exerted by HDTAB, choline concentration was decreased from 250 μM to 100 μM in the assay system. Comparison of the percentages of inhibition offered by different concentrations of HDTAB at both choline concentrations suggested that a decrease in the choline concentration shifted the concentration required for 60% inhibition of PfCK from 100 μM to 50 μM (Fig. 2F). The observed shift indicated that HDTAB may compete with choline for the choline binding site of PfCK and may offer competitive inhibition with respect to choline.
FIG. 2.
Effects of H-89, HC-3, and HDTAB on PfCK activity. PfCK activity was evaluated in the presence or absence of the indicated concentrations of H-89, HC-3, and HDTAB. The effect of H-89 on PfCK was evaluated at 5 mM (A) and 100 μM (B) ATP. The effect of HC-3 on PfCK was studied at 250 μM (C) and 100 μM (D) choline concentrations. Similarly, the effect of HDTAB on PfCK was also studied at 250 μM (E) and 100 μM (F) choline concentrations as described in Materials and Methods. PfCK activity was expressed as the amount of phosphocholine generated (counts per minute). Data are presented as means ± standard errors of the means (error bars) for three different experiments performed in triplicate.
Antimalarial effect of HDTAB.
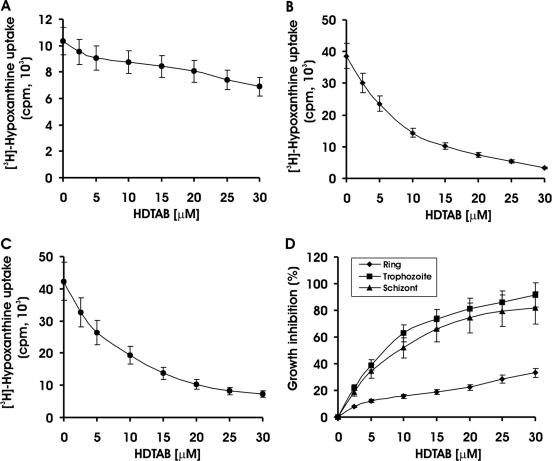
We showed above that HDTAB had an inhibitory effect on PfCK. Therefore, to evaluate the importance of choline kinase in P. falciparum growth and survival, P. falciparum was cultured in the presence or absence of HDTAB. Interestingly, HDTAB inhibited the growth of malaria parasite in a dose-dependent manner with 62% (P < 0.001) and 81% (P < 0.001) growth inhibition at 10 μM and 20 μM, respectively (Fig. 3).
FIG. 3.
Antimalarial effect of HDTAB. Asynchronous P. falciparum culture (200 μl, 0.5 to 0.7% parasitemia) was incubated either with the indicated concentrations of HDTAB or with DMSO (control) for one complete cycle (48 h). Incorporation of [3H]hypoxanthine in P. falciparum was determined as described in Materials and Methods. Incorporation of [3H]hypoxanthine is presented in counts per minute. (Inset) Percent growth inhibition offered by HDTAB calculated from the incorporation of [3H]hypoxanthine in HDTAB-treated cells compared to the DMSO control, which was taken as 100% growth or 0% growth inhibition. Data are presented as means ± standard errors of the means (error bars) for three different experiments performed in triplicate.
Sensitivity of different erythrocytic stages of Plasmodium falciparum to HDTAB.
It is evident from the existing literature that antimalarial compounds have stage specificity in their antimalarial effect. For instance, artemisinin exerts its antimalarial effect by killing malaria parasites at early stages of intraerythrocytic growth (34), whereas quinoline derivatives like chloroquine kill malaria parasites in its mature stage (33). Therefore, the sensitivity of different erythrocytic stages of P. falciparum toward HDTAB was evaluated. HDTAB at a concentration of 10 μM inhibited 62% (P < 0.001) and 51% (P < 0.001) growth of P. falciparum at trophozoite and schizont stages, respectively (Fig. 4B and C). Whereas even at threefold-higher concentration (30 μM), it inhibited only 33.3% of growth of parasites at the ring stage (Fig. 4A). Comparison of growth inhibition among these stages clearly suggested that HDTAB had the highest inhibitory activity toward trophozoites (Fig. 4D). Thus, results clearly indicated the stage specificity of antimalarial action of HDTAB. The results also raised the question of why this sensitivity is to trophozoites and schizonts only and not to rings. Is there any stage specificity in the expression pattern of PfCK? Is PfCK expressed strongly at the trophozoite and schizont stages? To test the hypothesis that HDTAB inhibits PfCK and thus offers an antimalarial effect, RT-PCR and immunofluorescence microscopic analysis were performed to investigate any possible stage specificity in the expression pattern of PfCK.
FIG. 4.
Stage-specific antimalarial effect of HDTAB. P. falciparum culture was synchronized using 5% d-sorbitol, and parasitemia was adjusted to 0.5 to 0.7% at a hematocrit level of 1% with fresh and uninfected B+ human RBCs. Incorporated [3H]hypoxanthine was determined as described in Materials and Methods. [3H]hypoxanthine incorporation in ring (A), trophozoite (B), and schizont (C) stages of P. falciparum is presented as counts per minute. (D) Growth inhibitory effect of HDTAB toward rings, trophozoites, and schizonts. Incorporation of [3H]hypoxanthine in HDTAB-treated cells was measured and compared to the value in the DMSO control, which was taken as 100% growth or 0% growth inhibition. Data are presented as means ± standard errors of the means (error bars) for three different experiments performed in triplicate.
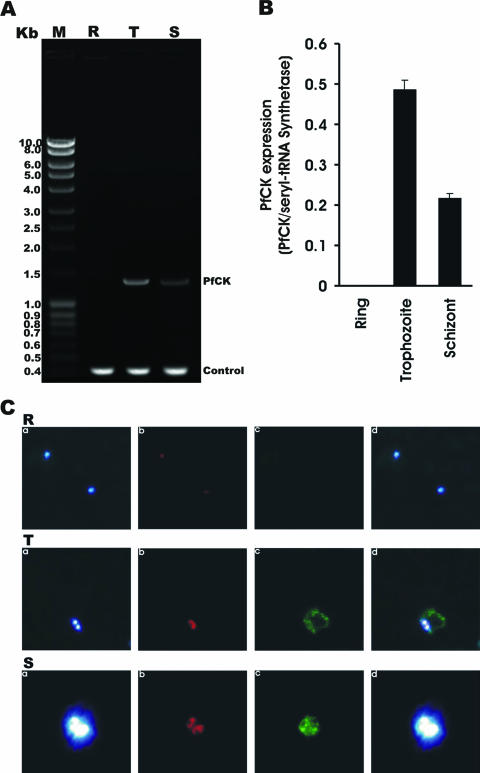
Evidence for stage-specific expression of PfCK.
P. falciparum expresses proteins differentially depending on the need for a particular stage. To check whether PfCK is expressed at a specific stage, RT-PCR was performed using total RNA (as a template) extracted separately from the ring, trophozoite, and schizont stages of P. falciparum and using a set of primers specific for PfCK (Fig. 5A). Amplification of a fragment of 1.3 kb, the predicted size of PfCK, was found by RT-PCR with the RNA from trophozoites (Fig. 5A, lane T) and schizonts (Fig. 5A, lane S) but not from the ring stage (Fig. 5A, lane R), indicating stage-specific expression of the PfCK gene. In contrast, the expression pattern of the seryl tRNA synthetase (control) was not affected by the different stages of the malaria parasite (Fig. 5A, lanes R, T, and S). Densitometric analysis suggested that the highest expression of PfCK takes place at the trophozoite stage of the malaria parasite (Fig. 5B). To further confirm the stage specificity of PfCK expression in P. falciparum at the protein level, immunofluorescence microscopic analysis was performed using anti-PfCK antibodies (Fig. 5C). Immunofluorescence microscopic results clearly suggested that PfCK expression predominates in the mature stages of the malaria parasite, i.e., trophozoites and schizonts (Fig. 5C, T and S panels), whereas expression of PfCK was completely absent in the ring stage of malaria parasites (Fig. 5C, R panels). These results corroborated the RT-PCR results and confirmed that higher expression of PfCK in P. falciparum takes place in the mature stages of the parasite, which are the most sensitive stages for HDTAB as described above. These results including in vitro PfCK inhibition by HDTAB together indicated that HDTAB might utilize PfCK as its target to exert antimalarial activity. Examining the effect of HDTAB on the CDP-choline pathway in the biosynthesis of PC would certainly provide further details on the mode of action of HDTAB.
FIG. 5.
Stage-specific expression of PfCK. (A) Agarose gel of RT-PCR products. Total RNA from a synchronized P. falciparum culture was isolated at 0 h, 24 h, and 36 h when the parasites were in the ring, trophozoite, and schizont stages, respectively. Total RNA (1 μg) was subjected to RT-PCR using specific primers for PfCK and seryl tRNA synthetase (control) as described in Materials and Methods. Lane M, DNA ladder; lane R, ring stage; lane T, trophozoite stage; lane S, schizont stage. (B) Densitometry of PfCK expression. Densitometric analysis of PfCK expression in different stages of Plasmodium falciparum. The intensity of PfCK was normalized to that of seryl tRNA synthetase. (C) Immunofluorescence microscopy. Slides were prepared as described in Materials and Methods. Different fields were observed with excitation wavelengths of 350 nm (DAPI), 579 nm (MitoTracker Red CMXRos), and 489 nm (Cy2). Blue, red, and green fluorescence indicate the locations of nucleus (a), mitochondria (b), and PfCK (c) in Plasmodium falciparum, respectively. (d) Merged picture of panel a, b, and c pictures. Mononuclear (R panels), binuclear (T panels), and multinuclear (S panels) P. falciparum cells represent rings, trophozoites, and schizonts, respectively.
HDTAB inhibits choline kinase of Plasmodium falciparum in culture.
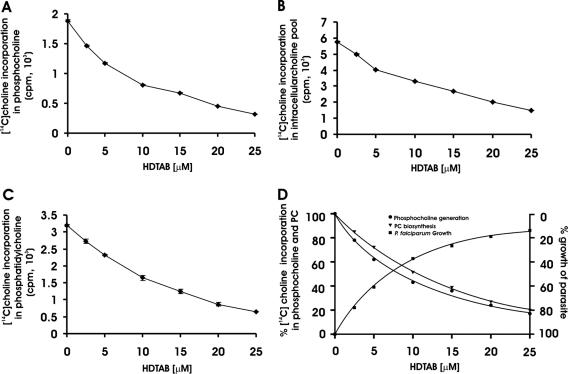
So far, results have indicated that purified PfCK is inhibited by HDTAB, which has antimalarial activity. The antimalarial activity of HDTAB is specific for the trophozoite and schizont stages, where the highest PfCK expression occurs, suggesting that HDTAB may exhibit antimalarial activity through PfCK inhibition. Direct measurement of phosphocholine (as a measure of PfCK activity) in HDTAB-treated and nontreated P. falciparum cells would be useful in determining the mode of action of HDTAB. The effect of HDTAB on phosphatidylcholine biosynthesis in P. falciparum was studied. For this, P. falciparum was cultured in CRPMI containing [methyl-14C]choline chloride after HDTAB or DMSO (control) treatment, and the incorporation of radiolabeled choline into different metabolites of the Kennedy pathway was monitored. HDTAB significantly inhibited the incorporation of [methyl-14C]choline into phosphocholine in a dose-dependent manner with a 57% (P < 0.001) decrease at 10 μM (Fig. 6A). This reduction in phosphocholine could be a result of either choline kinase inhibition or inhibition of choline transport, which in turn resulted in decreased phosphocholine generation. Therefore, to determine intracellular free choline, control parasites and HDTAB-treated parasites were compared. Parasites treated with 10 μM HDTAB showed 42% (P < 0.001) less intracellular free choline than control parasites did (Fig. 6B). The reduction in intracellular free choline and phosphocholine generation clearly suggested that not only choline kinase activity of malaria parasite but also choline transport was inhibited by the HDTAB. These results supported the earlier reported role of choline kinase in trapping choline inside the P. falciparum cells by its phosphorylation (29). In addition, the decrease in phosphocholine generation resulted in a decrease in phosphatidylcholine biosynthesis (Fig. 6C). HDTAB treatment caused a dose-dependent decrease in phosphatidylcholine biosynthesis (Fig. 6C), which correlated well with the decrease in phosphocholine generation, indicating that choline kinase may regulate PC biosynthesis in P. falciparum (Fig. 6D). Parallel decreases in phosphocholine and phosphatidylcholine were found and also correlated well with the decrease in the parasitic growth (Fig. 6D), indicating that the perturbation of PC biosynthesis led to the death of the parasite. A similar correlation in phosphocholine generation and PC biosynthesis after treatment with other quaternary ammonium compounds was earlier reported (4). These results clearly indicated that HDTAB offered antimalarial activity via inhibition of choline kinase and also advocated the critical role of choline kinase in P. falciparum growth and stage progression.
FIG. 6.
Effect of HDTAB on the CDP-choline pathway. P. falciparum culture (5 ml) at 15% parasitemia and 5% hematocrit was exposed to the indicated concentrations of HDTAB or DMSO (control) for 1 hour. Subsequently, HDTAB was removed and cells were washed thrice with CRPMI medium, and then 40 μM [methyl-14C]choline chloride (specific activity, 5.6 mCi/mmol) was added. Cells were further grown for 4 h under optimum growth conditions. Following incubation, choline, phosphocholine, and phosphatidylcholine were extracted as described in Materials and Methods. (A) Incorporation of [methyl-14C]choline in phosphocholine; (B) intracellular free [methyl-14C]choline in P. falciparum cells; (C) incorporation of [methyl-14C]choline in phosphatidylcholine; (D) percent [methyl-14C]choline incorporation in phosphocholine and phosphatidylcholine and its correlation with percent growth of P. falciparum at different concentrations of HDTAB. Data are presented as means ± standard errors of the means (error bars) of three different experiments performed in triplicate.
HDTAB has no toxic effect on the viability of RBC.
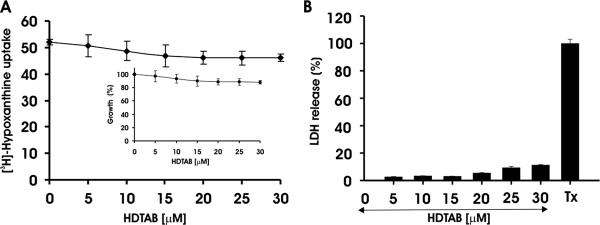
HDTAB has a very long aliphatic chain, which causes its detergent-like properties. Thus, to investigate whether HDTAB has any specificity for the malaria parasite or whether it lyses any type of biological membrane including RBC membrane, normal uninfected RBCs were incubated with HDTAB at different concentrations. Following incubation, RBCs were infected with P. falciparum-infected erythrocytes and [H3]hypoxanthine uptake was monitored. Incorporation of [H3]hypoxanthine in P. falciparum cultured in RBCs treated with 5, 10, 15, 20, 25, and 30 μM HDTAB was comparable to that in the control as evident from Fig. 7A. These results suggest that HDTAB up to 30 μM did not affect the ability of RBCs to support parasite invasion and growth. Therefore, it can be concluded that HDTAB does not affect the viability of the RBCs and that its antimalarial action is specific to the parasite only. To study the toxicity of HDTAB on nucleated mammalian cells, MCF-7 cells were incubated with 5 to 30 μM HDTAB and then the LDH release assay was conducted. It was found that HDTAB had no significant toxicity for mammalian nucleated cells (Fig. 7B).
FIG. 7.
Toxicity of HDTAB toward RBCs and MCF-7 cells. (A) Fresh and uninfected human B+ RBCs were incubated with the indicated concentrations of HDTAB for 24 h. After completion of incubation, cells were washed thrice with CRPMI medium and infected with P. falciparum culture. [3H]hypoxanthine uptake was studied as described in Materials and Methods. Incorporation of [3H]hypoxanthine is presented in counts per minute. (Inset) Percent growth inhibition offered by HDTAB calculated from incorporation of [3H]hypoxanthine in HDTAB-treated cells compared to the DMSO control, which was taken as 100% growth or 0% growth inhibition. (B) Exponentially growing MCF-7 cells were seeded in 96-well plates at a concentration of 2 × 104 cells per well and cultured for 24 h in Dulbecco modified Eagle medium. Subsequently, culture medium was changed, and in fresh medium, cells were incubated with the indicated concentrations of HDTAB, DMSO (negative control), and 0.9% Triton X-100 (Tx) (positive control) for 7 h. Then, LDH release was estimated as described in Materials and Methods. Data are presented as means ± standard errors of the means (error bars) for three different experiments performed in triplicate.
In vivo antimalarial activity against rodent malaria parasite P. yoelii.
The in vitro antimalarial activity of HDTAB encouraged us to evaluate the effect of HDTAB against the rodent malarial parasite Plasmodium yoelii (N-67 strain) in vivo. Two approaches were used. In the first approach, HDTAB treatment was administered 6 h after the inoculation of infection from the same route, i.e., intraperitoneally. Following this regimen, HDTAB suppressed the day 4 mean parasitemia by 36.77, 52.44, and 77.17% at dose levels of 5 mg/kg of body weight, 10 mg/kg, and 20 mg/kg, respectively (Table 1). These results suggested that HDTAB also has a potent antimalarial activity in vivo, but this antimalarial activity could have resulted from a local effect of HDTAB, since infection or treatment was given by the same route, which might not have allowed parasites to circulate and grow in the animal body. To rule out this possibility, another approach in which parasites were inoculated via the i.v. route and treatment was administered intraperitoneally was used. The antimalarial activity of HDTAB was also unaffected by this regimen, and mean parasitemia suppression values of 48.70, 64.25, and 83.68% were observed at day 4 at dose levels of 5 mg/kg, 10 mg/kg, and 20 mg/kg, respectively (Table 1). The results suggest that the time lag between parasite inoculation and administration of HDTAB and the different routes for inoculation and treatment did not influence the antimalarial activity of HDTAB, indicating a systemic mode of antimalarial effect of HDTAB.
TABLE 1.
In vivo antimalarial activity of HDTAB against the chloroquine-resistant rodent malaria parasite Plasmodium yoelii (strain N-67)
| HDTAB dose (mg/kg)a | Mean % parasitemia suppression on day 4b
|
|
|---|---|---|
| i.v. | i.p. | |
| 5 | 48.70 | 36.77 |
| 10 | 64.25 | 52.44 |
| 20 | 83.68 | 77.17 |
| 0 (vehicle) (control) | 0 | 0 |
HDTAB treatment was given by the intraperitoneal route in each case.
Parasites were inoculated intravenously or intraperitoneally. Percent suppression was calculated as [(C − T)/C] × 100, where C is parasitemia in the control group and T is parasitemia in the treated group.
DISCUSSION
The ability of quaternary ammonium compounds to interfere with different enzymatic steps of the CDP-choline pathway led us to study the effect of HDTAB on PfCK activity. A smaller derivative of the same compound, decyltrimethylammonium bromide shown in Fig. 1, has already been reported to have antimalarial activity without having any adverse effect on RBCs (7). Miltefosin inhibits the CDP-choline pathway (26) (Fig. 1) and offers antiproliferative, antileishmanial, and antimalarial drug activity (10, 11, 30, 32). The mode of action in the latter two cases has not been well defined. For antiproliferative activity, miltefosin acts by inhibiting choline phosphate cytidylyltransferase (CCT), the regulatory enzyme of the CDP-choline pathway, since miltefosin mimics the structure of phosphocholine, the substrate for CCT (Fig. 1).
The importance of choline kinase in P. falciparum was evaluated by incubating the P. falciparum culture in the presence of HDTAB. HDTAB inhibited the growth of P. falciparum very efficiently. The concentration of HDTAB required to inhibit P. falciparum growth by 62% was 10 times lower than the concentration required to inhibit purified PfCK by 60% in vitro. The apparent difference in action could be attributed to the choline transporter in the malaria parasite membrane, which has been found to accumulate quaternary ammonium compounds inside the malarial parasite to the millimolar range from the nanomolar external concentration (12, 13, 38, 43). There are several other mono and bis quaternary ammonium compounds with long aliphatic chains that have been found to inhibit P. falciparum growth in vitro (1, 3, 4, 7) and in vivo (2, 43). This class of antimalarials is not only active against chloroquine-sensitive strains of P. falciparum but is also equally effective against multiple drug-resistant strains of P. falciparum (1, 3). Similarly, HDTAB has also exhibited potent antimalarial activity in vivo against the chloroquine-resistant rodent malarial parasite Plasmodium yoelii (N-67 strain). The exact mode of action of these quaternary ammonium compounds is not fully understood, but ample evidence suggested that these compounds target enzymatic steps of the Kennedy pathway, including choline kinase (3, 36).
The results of our stage-specific RT-PCR and immunofluorescence microscopic analysis suggested that PfCK is expressed during the mature intraerythrocytic stages (trophozoites and schizonts) of the parasite, which coincides perfectly with the timing of higher phosphatidylcholine biosynthesis in the parasite (5). These results were also in agreement with the DNA microarray data available at www.PlasmoDB.org for the expression profile of the PfCK gene. The stage-specific expression of PfCK and antiplasmodial activity of HDTAB raised interest in investigating whether HDTAB has any stage specificity in its action. Interestingly, we found that the mature stages, i.e., trophozoites and schizonts, of the intraerythrocytic cycle of P. falciparum were much more susceptible to HDTAB than malaria parasites in the ring stage were. At these stages, malaria parasites multiply, and inhibition of choline kinase results in the inhibition of phosphocholine production, which in turn affects the biosynthesis of phosphatidylcholine, the major component of the malaria parasite membrane. Moreover, phosphocholine has also been recognized as an important secondary signal molecule in the process of mitogenesis (19, 21, 28). Reduced phosphocholine generation via inhibition of choline kinase may also have an adverse effect on the ongoing mitogenic process during the mature stages of malaria parasites, thereby explaining the higher susceptibility of the mature stages of the malaria parasite to HDTAB compared to that of malaria parasites in the ring stage.
To investigate whether HDTAB actually inhibits the choline kinase of P. falciparum inside the cell, radiolabeled choline incorporation into different metabolites of the CDP-choline pathway was monitored. The incorporation of radiolabeled choline into phosphocholine was reduced by 57% in the 10 μM HDTAB-treated P. falciparum culture compared to the control culture, and a concomitant reduction in the incorporation of radiolabeled choline into phosphatidylcholine was also observed. This indicates that choline kinase may have a regulatory role in phosphatidylcholine biosynthesis, as its inhibition by HDTAB reduced the production of phosphocholine, which in turn decreased phosphatidylcholine biosynthesis. These results are consistent with the earlier report where a good correlation between the intracellular phosphocholine pool and phosphatidylcholine content had been found by treatment with certain quaternary ammonium compounds (4). Choline kinase has been reported to assist choline transport by its phosphorylation in P. falciparum (29). Therefore, intracellular free choline levels in control parasites and HDTAB-treated parasites were compared. Parasites treated with HDTAB at a concentration of 10 μM had 42% less intracellular free choline than control parasites did, reconfirming the previously reported role of choline kinase in choline transport by phosphorylation (29).
HDTAB is well-known for its detergent properties and may act by solubilizing biological membranes nonspecifically. Therefore, to investigate whether HDTAB has any specificity for the malaria parasite or whether it lyses any type of biological membrane, including the RBC membrane, normal uninfected RBCs were incubated with HDTAB. Subsequently, treated RBCs were infected with P. falciparum, and [3H]hypoxanthine uptake was monitored. Incorporation of [3H]hypoxanthine into P. falciparum cultured in HDTAB-treated RBCs was comparable with that of the control, indicating that incubation of RBCs with HDTAB did not affect the ability of RBCs to support parasite invasion and growth up to 30 μM concentration. Therefore, HDTAB does not affect the viability of RBCs or the integrity of the RBC membrane. HDTAB did not show any cytolytic effect on proliferating nucleated mammalian cells (MCF-7) as measured by LDH release up to 30 μM. Thus, the exhibited antimalarial action of HDTAB is specific to the parasite only.
Acknowledgments
We gratefully acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing grants through the CSIR Network Project [SMM0003 (P22)] and providing a Senior Research fellowship to Vinay Choubey to carry out this work.
We thank Sudhir Sinha for helpful and valuable suggestions during the course of this work.
This report is CDRI communication no. 7068.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Ancelin, M. L., M. Calas, J. Bompart, G. Cordina, D. Martin, M. Ben Bari, T. Jei, P. Druilhe, and H. J. Vial. 1998. Antimalarial activity of 77 phospholipid polar head analogs: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood 91:1426-1437. [PubMed] [Google Scholar]
- 2.Ancelin, M. L., M. Calas, A. Bonhoure, S. Herbute, and H. J. Vial. 2003. In vivo antimalarial activities of mono- and bis quaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 47:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancelin, M. L., M. Calas, V. Vidal-Sailhan, S. Herbute, P. Ringwald, and H. J. Vial. 2003. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob. Agents Chemother. 47:2590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ancelin, M. L., and H. J. Vial. 1986. Quaternary ammonium compounds efficiently inhibit Plasmodium falciparum growth in vitro by impairment of choline transport. Antimicrob. Agents Chemother. 29:814-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancelin, M. L., and H. J. Vial. 1989. Regulation of phosphatidylcholine biosynthesis in Plasmodium-infected erythrocytes. Biochim. Biophys. Acta 1001:82-89. [DOI] [PubMed] [Google Scholar]
- 6.Ancelin, M. L., and H. J. Vial. 1986. Several lines of evidence demonstrating that Plasmodium falciparum, a parasitic organism, has distinct enzymes for the phosphorylation of choline and ethanolamine. FEBS Lett. 202:217-223. [DOI] [PubMed] [Google Scholar]
- 7.Ancelin, M. L., H. J. Vial, and J. R. Philippot. 1985. Inhibitors of choline transport into Plasmodium-infected erythrocytes are effective antiplasmodial compounds in vitro. Biochem. Pharmacol. 34:4068-4071. [DOI] [PubMed] [Google Scholar]
- 8.Ansell, G. B., and S. G. Spanner. 1974. The inhibition of brain choline kinase by hemicholinium-3. J. Neurochem. 22:1153-1155. [DOI] [PubMed] [Google Scholar]
- 9.Ben Mamoun, C., I. Y. Gluzman, C. Hott, S. K. MacMillan, A. S. Amarakone, D. L. Anderson, J. M. Carlton, J. B. Dame, D. Chakrabarti, R. K. Martin, B. H. Brownstein, and D. E. Goldberg. 2001. Co-ordinated programme of gene expression during asexual intraerythrocytic development of the human malaria parasite Plasmodium falciparum revealed by microarray analysis. Mol. Microbiol. 39:26-36. [DOI] [PubMed] [Google Scholar]
- 10.Berkovic, D. 1998. Cytotoxic etherphospholipid analogues. Gen. Pharmacol. 31:511-517. [DOI] [PubMed] [Google Scholar]
- 11.Berman, J. 2005. Clinical status of agents being developed for leishmaniasis. Expert Opin. Investig. Drugs 14:1337-1346. [DOI] [PubMed] [Google Scholar]
- 12.Biagini, G. A., E. M. Pasini, R. Hughes, H. P. De Koning, H. J. Vial, P. M. O'Neill, S. A. Ward, and P. G. Bray. 2004. Characterization of the choline carrier of Plasmodium falciparum: a route for the selective delivery of novel antimalarial drugs. Blood 104:3372-3377. [DOI] [PubMed] [Google Scholar]
- 13.Biagini, G. A., E. Richier, P. G. Bray, M. Calas, H. Vial, and S. A. Ward. 2003. Heme binding contributes to antimalarial activity of bis-quaternary ammoniums. Antimicrob. Agents Chemother. 47:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chijiwa, T., A. Mishima, M. Hagiwara, M. Sano, K. Hayashi, T. Inoue, K. Naito, T. Toshioka, and H. Hidaka. 1990. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 265:5267-5272. [PubMed] [Google Scholar]
- 15.Choi, M. G., V. Kurnov, M. C. Kersting, A. Sreenivas, and G. M. Carman. 2005. Phosphorylation of the yeast choline kinase by protein kinase C. Identification of Ser25 and Ser30 as major sites of phosphorylation. J. Biol. Chem. 280:26105-26112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choubey, V., M. Guha, P. Maity, S. Kumar, R. Raghunandan, P. R. Maulik, K. Mitra, U. C. Halder, and U. Bandyopadhyay. 2006. Molecular characterization and localization of Plasmodium falciparum choline kinase. Biochim. Biophys. Acta 1760:1027-1038. [DOI] [PubMed] [Google Scholar]
- 17.Cook, S. J., and M. J. Wakelam. 1989. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem. J. 263:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crilly, K. S., M. Tomono, and Z. Kiss. 1998. The choline kinase inhibitor hemicholinium-3 can inhibit mitogen-induced DNA synthesis independent of its effect on phosphocholine formation. Arch. Biochem. Biophys. 352:137-143. [DOI] [PubMed] [Google Scholar]
- 19.Cuadrado, A., A. Carnero, F. Dolfi, B. Jimenez, and J. C. Lacal. 1993. Phosphorylcholine: a novel second messenger essential for mitogenic activity of growth factors. Oncogene 8:2959-2968. [PubMed] [Google Scholar]
- 20.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong, Z., C. Huang, W. Y. Ma, B. Malewicz, W. J. Baumann, and Z. Kiss. 1998. Increased synthesis of phosphocholine is required for UV-induced AP-1 activation. Oncogene 17:1845-1853. [DOI] [PubMed] [Google Scholar]
- 22.Guyenet, P., P. Lefresne, J. Rossier, J. C. Beaujouan, and J. Glowinski. 1973. Inhibition by hemicholinium-3 of (14C)acetylcholine synthesis and (3H)choline high-affinity uptake in rat striatal synaptosomes. Mol. Pharmacol. 9:630-639. [PubMed] [Google Scholar]
- 23.Hernandez-Alcoceba, R., F. Fernandez, and J. C. Lacal. 1999. In vivo antitumor activity of choline kinase inhibitors: a novel target for anticancer drug discovery. Cancer Res. 59:3112-3118. [PubMed] [Google Scholar]
- 24.Hernandez-Alcoceba, R., L. Saniger, J. Campos, M. C. Nunez, F. Khaless, M. A. Gallo, A. Espinosa, and J. C. Lacal. 1997. Choline kinase inhibitors as a novel approach for antiproliferative drug design. Oncogene 15:2289-2301. [DOI] [PubMed] [Google Scholar]
- 25.Hidaka, H., M. Inagaki, S. Kawamoto, and Y. Sasaki. 1984. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23:5036-5041. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez-Lopez, J. M., M. P. Carrasco, J. L. Segovia, and C. Marco. 2002. Hexadecylphosphocholine inhibits phosphatidylcholine biosynthesis and the proliferation of HepG2 cells. Eur. J. Biochem. 269:4649-4655. [DOI] [PubMed] [Google Scholar]
- 27.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 28.Lee, M., and S. S. Han. 2002. Choline phosphate potentiates sphingosine-1-phosphate-induced Raf-1 kinase activation dependent of Ras-phosphatidylinositol-3-kinase pathway. Cell Signal. 14:373-379. [DOI] [PubMed] [Google Scholar]
- 29.Lehane, A. M., K. J. Saliba, R. J. Allen, and K. Kirk. 2004. Choline uptake into the malaria parasite is energized by the membrane potential. Biochem. Biophys. Res. Commun. 320:311-317. [DOI] [PubMed] [Google Scholar]
- 30.Murray, H. W., J. D. Berman, C. R. Davies, and N. G. Saravia. 2005. Advances in leishmaniasis. Lancet 366:1561-1577. [DOI] [PubMed] [Google Scholar]
- 31.Pessi, G., J. Y. Choi, J. M. Reynolds, D. R. Voelker, and C. B. Mamoun. 2005. In vivo evidence for the specificity of Plasmodium falciparum phosphoethanolamine methyltransferase and its coupling to the Kennedy pathway. J. Biol. Chem. 280:12461-12466. [DOI] [PubMed] [Google Scholar]
- 32.Pessi, G., G. Kociubinski, and C. B. Mamoun. 2004. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc. Natl. Acad. Sci. USA 101:6206-6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters, W. 1987. Introduction, p. 1-18. In Chemotherapy and drug resistance in malaria, 2nd ed. Academic Press, London, United Kingdom.3493885
- 34.Price, R. N. 2000. Artemisinin drugs: novel antimalarial agents. Expert Opin. Investig. Drugs 9:1815-1827. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez de Molina, A., V. Penalva, L. Lucas, and J. C. Lacal. 2002. Regulation of choline kinase activity by Ras proteins involves Ral-GDS and PI3K. Oncogene 21:937-946. [DOI] [PubMed] [Google Scholar]
- 36.Roggero, R., R. Zufferey, M. Minca, E. Richier, M. Calas, H. Vial, and C. Ben Mamoun. 2004. Unraveling the mode of action of the antimalarial choline analog G25 in Plasmodium falciparum and Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 48:2816-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandberg, K., and J. T. Coyle. 1985. Characterization of [3H]hemicholinium-3 binding associated with neuronal choline uptake sites in rat brain membranes. Brain Res. 348:321-330. [DOI] [PubMed] [Google Scholar]
- 38.Stead, A. M., P. G. Bray, I. G. Edwards, H. P. DeKoning, B. C. Elford, P. A. Stocks, and S. A. Ward. 2001. Diamidine compounds: selective uptake and targeting in Plasmodium falciparum. Mol. Pharmacol. 59:1298-1306. [DOI] [PubMed] [Google Scholar]
- 39.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 40.Vial, H. J., and M. L. Ancelin. 1998. Malarial lipids, p. 159-175. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. ASM Press, Washington, DC.
- 41.Wallach, M. 1982. Efficient extraction and translation of Plasmodium falciparum messenger RNA. Mol. Biochem. Parasitol. 6:335-342. [DOI] [PubMed] [Google Scholar]
- 42.Warden, C. H., and M. Friedkin. 1985. Regulation of choline kinase activity and phosphatidylcholine biosynthesis by mitogenic growth factors in 3T3 fibroblasts. J. Biol. Chem. 260:6006-6011. [PubMed] [Google Scholar]
- 43.Wengelnik, K., V. Vidal, M. L. Ancelin, A. M. Cathiard, J. L. Morgat, C. H. Kocken, M. Calas, S. Herrera, A. W. Thomas, and H. J. Vial. 2002. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 295:1311-1314. [DOI] [PubMed] [Google Scholar]
- 44.Wieprecht, M., T. Wieder, and C. C. Geilen. 1994. N-[2-bromocinnamyl(amino)ethyl]-5-isoquinolinesulphonamide (H-89) inhibits incorporation of choline into phosphatidylcholine via inhibition of choline kinase and has no effect on the phosphorylation of CTP:phosphocholine cytidylyltransferase. Biochem. J. 297:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu, Y., A. Sreenivas, D. B. Ostrander, and G. M. Carman. 2002. Phosphorylation of Saccharomyces cerevisiae choline kinase on Ser30 and Ser85 by protein kinase A regulates phosphatidylcholine synthesis by the CDP-choline pathway. J. Biol. Chem. 277:34978-34986. [DOI] [PubMed] [Google Scholar]