Abstract
We assessed the pharmacokinetics and interactions of steady-state micafungin (Mycamine) or placebo with steady-state voriconazole in 35 volunteers. The 90% confidence intervals around the least-squares mean ratios for micafungin pharmacokinetic parameters and placebo-corrected voriconazole pharmacokinetic parameters were within the 80%-to-125% limits, indicating an absence of drug interaction.
Recently, drugs from two new antifungal classes became commercially available. Micafungin (Mycamine) is an echinocandin which inhibits the synthesis of (1,3)-β-d-glucan, a critical component of fungal cell walls (1, 3). It has potent activity in vitro and in vivo against Candida species, including azole-resistant strains, and Aspergillus species (4, 5, 6, 7, 9, 15). Micafungin is metabolized to M1 (catechol form) by arylsulfatase, with further metabolism to M2 (methoxy form) by catechol-O-methyltransferase. M5 is formed by hydroxylation at the side chain (omega-1 position) of micafungin by cytochrome P450 enzymes (1).
Voriconazole, an extended-spectrum triazole approved by the Food and Drug Administration, is fungicidal for Aspergillus species and a range of other molds. Voriconazole inhibits 14-alpha-lanosterol demethylation, which is an essential step in the synthesis of fungal ergosterol, a component of fungal cell walls. Voriconazole undergoes extensive metabolism by hepatic cytochrome P450 isoenzymes, primarily CYP2C19 and CYP2C9 but also CYP3A4 (10).
The availability of these antifungal agents has generated much interest in using combination therapy in an effort to improve treatment for life-threatening infections, particularly aspergillosis (8). The present study was conducted to identify potential pharmacokinetic drug interactions and safety concerns which might occur upon repeated, concomitant administration of micafungin and voriconazole at clinically relevant doses.
This randomized, double-blind, placebo-controlled, drug interaction study was conducted at Northwest Kinetics in accordance with FDA Regulations Relating to Good Clinical Practice and Clinical Trials (http://www.fda.gov/oc/gcp/regulations.html) and all subjects provided written informed consent. Adult male and female volunteers were eligible for the study if they were 18 to 50 years of age (inclusive), weighed at least 40 kg, were medically healthy with no concomitant illness or disease, were not taking medication, and had no clinically significant findings at baseline. Because 15% to 20% of Asian populations may be expected to be poor metabolizers of voriconazole due to the CYP2C19 genotype (10), Asians were excluded from participation as a safety precaution.
All subjects received oral voriconazole on days 1 to 4 and on days 21 to 24 and either micafungin (treatment sequence I) or placebo (treatment sequence II) on days 11 to 24. Voriconazole was administered at 400 mg twice daily on days 1 and 21 and at 200 mg twice daily on days 2 to 4 and days 22 to 24. Micafungin (150 mg/day in 100 ml of 0.9% sodium chloride) or placebo (100 ml of 0.9% sodium chloride) was infused over 1 h on days 11 to 24.
Physical examinations, vital signs, electrocardiograms, and clinical laboratory tests were used to monitor subject safety. Serial blood samples were collected at presumed steady state over days 4 to 7 for voriconazole, on day 20 for micafungin, and over days 24 to 27 for both drugs. Additionally for voriconazole predose trough samples were drawn on the day prior to the days of serial sampling; for micafungin predose and end-of-infusion samples were drawn 3 and 6 days prior to the day of serial sampling for micafungin alone.
Micafungin, M1, M2, and M5 levels were determined for plasma by using a validated high-performance liquid chromatography method with a lower limit of quantification of 0.0500 μg/ml. Voriconazole concentrations in plasma were determined by using a validated high-performance liquid chromatography-tandem mass spectrometry method with a lower limit of quantification of 5.00 ng/ml.
Noncompartmental methods (WinNonlin version 4.0; Pharsight Corporation, Mountain View, CA) were used to calculate pharmacokinetic parameters, including the maximum concentration of study drug in serum (Cmax) and the area under the concentration-time curve over 12 h after the morning dose (AUC0-12) for voriconazole and over 24 h postdose (AUC0-24) for micafungin.
The effect of each drug on the pharmacokinetics of the other was assessed for treatment sequence I by using a two one-sided test procedure. A least-squares (geometric) mean ratio from a one-way analysis of variance (ANOVA) model with the subject as the random effect and 90% confidence intervals was constructed for each parameter. If the 90% confidence intervals for both AUC and Cmax fell within the 80%-to-125% confidence limits, it was concluded that concomitant administration had “no effect.” The same analysis was performed for treatment sequence II to compare voriconazole pharmacokinetics with and without the coadministration of placebo.
Since a change in voriconazole pharmacokinetics was observed after the placebo was administered, the geometric mean ratios for sequence I were divided by the corresponding ratios for sequence II, and the 90% confidence intervals around the adjusted ratios were constructed by using an ANOVA model with treatment sequence as the main effect. This correction was previously described (12, 14).
A total of 35 subjects were enrolled in the study; 23 of the planned 24 subjects were randomized to treatment sequence I, and 12 subjects were randomized to treatment sequence II. Both drugs were well tolerated when administered alone or concomitantly. Headache, abnormal vision, and photophobia were the more common adverse events when voriconazole was administered alone. A procedural complication, described as phlebitis or pain at the intravenous cannula or venipuncture site, was the most common adverse event when micafungin was administered alone. The adverse events observed during this study were consistent with the safety profiles previously reported for each drug (Astellas Pharma US, Inc., unpublished data; 1, 10, 13).
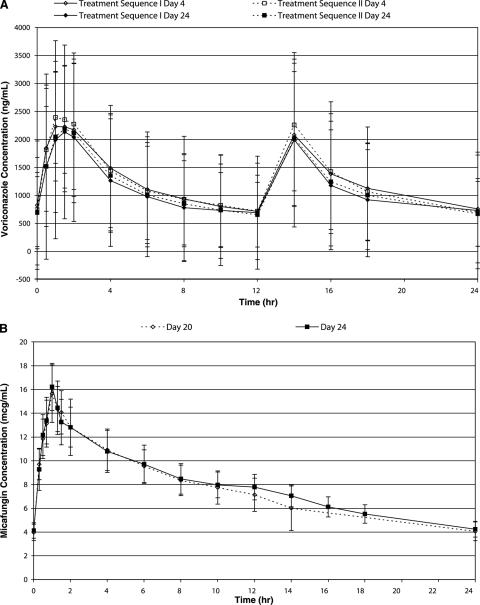
The mean concentrations for voriconazole are shown in Fig. 1. Mean Cmax and AUC0-12 for voriconazole in the presence of either micafungin or placebo (day 24) were slightly lower than those observed for voriconazole alone (day 4), and the 90% confidence intervals around the parameter ratios (day 24/day 4) were outside the 80%-to-125% confidence limits (Table 1). When the sequence I voriconazole data were corrected for the placebo finding, the 90% confidence intervals around the adjusted ratios fell within the 80%-to-125% confidence limits. A repeated-measures ANOVA (P = 0.059) of the trough voriconazole concentrations confirmed that steady state was attained by the regimen administered.
FIG. 1.
Plasma concentrations (arithmetic means ± standard deviations) of voriconazole over 24 h (2 doses) on days 4 and 24 (A) and of micafungin over 24 h on days 20 and 24 (B).
TABLE 1.
Steady-state pharmacokinetic parameters for voriconazole
| Pharmacokinetic parameter | Sequence | Resulta
|
Statistical comparisonb
|
|||
|---|---|---|---|---|---|---|
| Day 4 | Day 24 | Geometric mean ratio (%) | 90% CI | Adjusted ratio (90% CI) | ||
| AUC0-12 (μg · h/ml) | I | 15.2 ± 11.77 | 13.6 ± 13.55 | 80.7 | 73.4 to 88.8 | 92.4 (80.2 to 106.4) |
| II | 15.4 ± 10.24 | 14.0 ± 10.24 | 87.4 | 78.9 to 96.8 | ||
| Cmax (μg/ml) | I | 2.6 ± 1.0 | 2.5 ± 1.6 | 88.3 | 76.9 to 101.4 | 98.1 (80.1 to 120.2) |
| II | 2.7 ± 1.1 | 2.5 ± 1.2 | 90.0 | 77.9 to 104.1 | ||
Result (arithmetic mean ± standard deviation) after administration of voriconazole alone or in combination with micafungin (sequence I) or placebo (sequence II).
CI, confidence interval.
The coadministration of voriconazole had no effect on micafungin pharmacokinetics, as illustrated in Fig. 1. Mean micafungin AUC0-24 and Cmax values in the presence (day 24) and absence (day 20) of voriconazole were similar, and the 90% confidence intervals around the parameter ratios (day 24/day 20) were within the 80%-to-125% limits (Table 2). The attainment of steady state for micafungin was confirmed by a repeated-measures ANOVA of micafungin trough concentrations (P = 0.2990).
TABLE 2.
Steady-state pharmacokinetic parameters for micafungin
| Pharmacokinetic parameter | Resulta
|
Statistical comparison
|
||
|---|---|---|---|---|
| Day 20 | Day 24 | Geometric mean ratio (%) | 90% confidence interval | |
| AUC0-24 (μg · h/ml) | 180.9 ± 29.88 | 189.2 ± 23.95 | 105.6 | 99.3 to 112.2 |
| Cmax (μg/ml) | 15.9 ± 2.03 | 16.2 ± 1.98 | 102.9 | 99.0 to 107.1 |
Result (arithmetic mean ± standard deviation) after administration of micafungin alone or in combination with voriconazole (sequence I).
Consistent with previous studies (Astellas Pharma US, Inc., unpublished data; 1), micafungin metabolite concentrations were negligible relative to the parent compound (data not shown), with mean AUC0-24 values being 8% or less and mean Cmax values being 4% or less than those observed for micafungin, regardless of the presence or absence of voriconazole.
There were no unexpected safety or pharmacokinetic findings during this study. High intersubject variability (coefficient of variation, >60%) was observed with regard to voriconazole kinetics, a result which was not surprising given the drug's metabolism by polymorphically expressed CYP enzymes. Similar findings have also been reported in the literature and the voriconazole product labeling information (2, 10, 11). The mean systemic exposure (AUC0-12) of voriconazole was reduced by approximately 19% or 13%, respectively, upon coadministration of micafungin or placebo. A similar effect was observed for Cmax, which was decreased by approximately 12% or 10%, respectively. When the data were corrected for the placebo finding according to a previously reported method (12, 14), the 90% confidence intervals for both AUC0-12 and Cmax were within the 80%-to-125% boundaries. It is noteworthy that the degree of variability in voriconazole kinetics observed in the current study was similar to that reported in the presence and in the absence of anidulafungin, another echinocandin for which a drug interaction with voriconazole was investigated (2).
The coadministration of voriconazole did not significantly affect micafungin or metabolite kinetics. Steady state, as determined by trough concentrations, was attained after six doses, with 93% of steady-state levels being achieved after only three doses. This time course is consistent with that seen in previous studies in which micafungin was administered at a dose of 150 mg/day (Astellas Pharma US, Inc., unpublished data).
The data suggest that micafungin and voriconazole can be safely coadministered at clinically relevant doses.
Acknowledgments
Financial support for this clinical trial was provided by Astellas Pharma US, Inc., Deerfield, IL.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Astellas Pharma US, Inc. April 2005. Mycamine (micafungin sodium) for injection: full U.S. prescribing information. Astellas Pharma US, Inc., Deerfield, IL.
- 2.Dowell, J. A., J. Schranz, A. Baruch, and G. Foster. 2005. Safety and pharmacokinetics of coadministered voriconazole and anidulafungin. J. Clin. Pharmacol. 45:1373-1382. [DOI] [PubMed] [Google Scholar]
- 3.Hatano, K., Y. Morishita, T. Nakai, and F. Ikeda. 2002. Antifungal mechanism of FK463 against Candida albicans and Aspergillus fumigatus. J. Antibiot. 55:219-222. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maesaki, S., M. Hossain, Y. Miyazaki, K. Tomono, T. Tashiro, and S. Kohno. 2000. Efficacy of FK463, a (1,3)-β-d-glucan synthase inhibitor, in disseminated azole-resistant Candida albicans infection in mice. Antimicrob. Agents Chemother. 44:1728-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto, S., Y. Wakai, T. Nakai, K. Hatano, T. Ushitani, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob. Agents Chemother. 44:619-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikamo, H., Y. Sato, and T. Tamaya. 2000. In vitro antifungal activity of FK463, a new water-soluble echinocandin-like lipopeptide. J. Antimicrob. Chemother. 46:485-487. [DOI] [PubMed] [Google Scholar]
- 8.Petraitis, V., R. Petraitiene, A. A. Sarafandi, A. M. Kelaher, C. A. Lyman, H. E. Casler, T. Sein, A. H. Groll, J. Bacher, N. A. Avila, and T. J. Walsh. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J. Infect. Dis. 187:1834-1843. [DOI] [PubMed] [Google Scholar]
- 9.Petraitis, V., R. Petraitiene, A. H. Groll, K. Roussillon, M. Hemmings, C. A. Lyman, T. Sein, J. Bacher, I. Bekersky, and T. J. Walsh. 2002. Comparative antifungal activities and plasma pharmacokinetics of mica-fungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 46:1857-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfizer, Inc. March 2005. VFEND IV (voriconazole) for injection, VFEND (voriconazole) tablets, VFEND (voriconazole) for oral suspension: full U.S. prescribing information. Pfizer, Inc., New York, NY.
- 11.Purkins, L., N. Wood, D. Kleinermans, K. Greenhalgh, and D. Nichols. 2003. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br. J. Clin. Pharmacol. 56:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purkins, L., N. Wood, D. Kleinermans, and E. Love. 2003. No clinically significant pharmacokinetic interactions between voriconazole and indinavir in healthy volunteers. Br. J. Clin. Pharmacol. 56:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purkins, L., N. Wood, and K. Greenhelgh. 2003. Voriconazole, a novel wide-spectrum triazole: oral pharmacokinetics and safety. Br. J. Clin. Pharmacol. 56:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purkins, L., N. Wood, P. Ghahramani, D. Kleinermans, G. Layton, and D. Nichols. 2003. No clinically significant effect of erythromycin or azithromycin on the pharmacokinetics of voriconazole in healthy male volunteers. Br. J. Clin. Pharmacol. 56:30-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tamoshima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]