Abstract
A structure-guided drug design approach was used to optimize a novel series of aminobenzimidazoles that inhibit the essential ATPase activities of bacterial DNA gyrase and topoisomerase IV and that show potent activities against a variety of bacterial pathogens. Two such compounds, VRT-125853 and VRT-752586, were characterized for their target specificities and preferences in bacteria. In metabolite incorporation assays, VRT-125853 inhibited both DNA and RNA synthesis but had little effect on protein synthesis. Both compounds inhibited the maintenance of negative supercoils in plasmid DNA in Escherichia coli at the MIC. Sequencing of DNA corresponding to the GyrB and ParE ATP-binding regions in VRT-125853- and VRT-752586-resistant mutants revealed that their primary target in Staphylococcus aureus and Haemophilus influenzae was GyrB, whereas in Streptococcus pneumoniae it was ParE. In Enterococcus faecalis, the primary target of VRT-125853 was ParE, whereas for VRT-752586 it was GyrB. DNA transformation experiments with H. influenzae and S. aureus proved that the mutations observed in gyrB resulted in decreased susceptibilities to both compounds. Novobiocin resistance-conferring mutations in S. aureus, H. influenzae, and S. pneumoniae were found in gyrB, and these mutants showed little or no cross-resistance to VRT-125853 or VRT-752586 and vice versa. Furthermore, gyrB and parE double mutations increased the MICs of VRT-125853 and VRT-752586 significantly, providing evidence of dual targeting. Spontaneous frequencies of resistance to VRT-752586 were below detectable levels (<5.2 × 10−10) for wild-type E. faecalis but were significantly elevated for strains containing single and double target-based mutations, demonstrating that dual targeting confers low levels of resistance emergence and the maintenance of susceptibility in vitro.
The emergence of antibiotic-resistant bacteria is a growing clinical problem that can cause treatment failures, which lead to increased rates of morbidity and mortality. During the past decade there have been numerous reports on the emergence of bacteria resistant to frontline antibacterial therapies, such as vancomycin-resistant enterococci, methicillin-resistant and vancomycin-intermediate Staphylococcus aureus, and penicillin-, macrolide-, and fluoroquinolone-resistant Streptococcus pneumoniae (1, 2, 21, 22, 27, 34, 40). While the rate of drug resistance among bacteria is on the rise, there have been very few examples of the development of structurally new classes of antibiotics with truly novel mechanisms of action that can circumvent the prevailing resistance problems (2, 46). Linezolid and daptomycin are the only examples of such novel antibiotics that were developed in the last few decades. With recent reports of emerging resistance to linezolid (32), there is a continued need for the discovery and development of novel antibiotics.
Bacterial DNA gyrase and topoisomerase IV (topo IV) are highly conserved type II topoisomerases that play essential roles in promoting DNA replication and transcription and are attractive targets for antibacterial drug discovery (10, 15, 30). DNA gyrase is a heterotetramer comprising two GyrA and two GyrB subunits, whereas topo IV is a heterotetramer comprising two ParC and two ParE subunits (GrlA and GrlB, respectively, in S. aureus). DNA gyrase provides the essential function of introducing negative supercoils into DNA and regulates the superhelical state of the bacterial chromosome, while topo IV provides the decatenation function required for the segregation of daughter chromosomes after DNA replication (8, 10, 23, 48). Both enzymes introduce double-strand breaks into DNA, and ATP hydrolysis provides the energy needed for the strand passage and resealing reactions (5). The fluoroquinolones target the GyrA subunit of gyrase and/or the ParC subunit of topo IV and act by trapping the enzyme-DNA complex in the double-strand break stage, resulting in DNA synthesis arrest (10). While the bactericidal activities of the fluoroquinolones are thought to be a consequence of the release of double-strand breaks from these enzyme-DNA complexes, the situation is more complex and not completely understood (6, 11, 38, 49). The essentiality and evolutionary conservation of gyrase and topo IV in bacteria impart broad-spectrum antibacterial activity to the fluoroquinolones (15, 30).
The essential ATPase activity of gyrase (a function of the GyrB subunit) and topo IV (a function of the ParE subunit) that is required for the strand-passage reaction is an attractive but relatively less exploited target for antibacterial drug discovery. The coumarin class of antibiotics, represented by novobiocin, exhibit antibacterial activity via inhibition of gyrase and, to a lesser extent, topo IV ATPase activities (3, 13, 30, 31, 44). However, their use has been limited due to poor antibacterial spectrum, rapid resistance development, and mammalian toxicities (29). By using a structure-guided drug design approach, a novel series of compounds belonging to the aminobenzimidazole class were optimized to inhibit bacterial gyrase and topo IV ATPase activities. VRT-125853 and VRT-752586 (Fig. 1) are representatives of this class of antibacterials that exhibit broad-spectrum antibacterial activities against both susceptible and multidrug-resistant clinical isolates; in addition, the frequencies of spontaneous resistance to these antibacterials in vitro are low, consistent with their novel dual-targeting mechanisms of action (28). Here we present genetic, biochemical, and physiological data that demonstrate their mechanisms of action against Escherichia coli, S. aureus, S. pneumoniae, Enterococcus faecalis, and Haemophilus influenzae and confirm their in vitro dual-targeting activities, as defined by effective inhibition of both gyrase and topo IV at biologically relevant concentrations. We also show that in the case of E. faecalis, balanced dual targeting of gyrase and topo IV appears to be responsible for low rates of the spontaneous emergence of resistance in vitro at concentrations near the MIC.
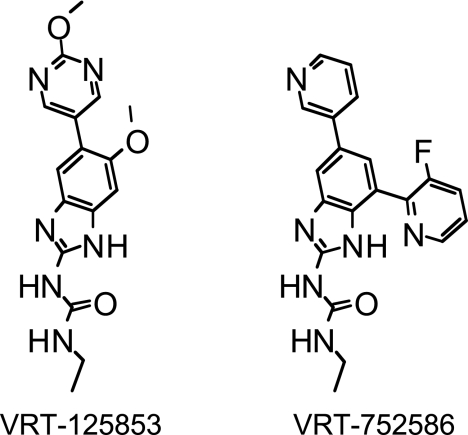
FIG. 1.
Chemical structures of dual-targeting aminobenzimidazoles.
MATERIALS AND METHODS
Bacterial strains and growth media.
S. aureus ATCC 29213, S. pneumoniae R6 (ATCC/BAA-255), E. faecalis ATCC 29212, and H. influenzae ATCC 51907 were obtained from the American Type Culture Collection (Manassas, VA). E. coli tolC strain CAG 12184, used for plasmid supercoiling assays, was from the Coli Genetic Stock Center (New Haven, CT). Routinely, liquid cultures of E. coli and S. aureus were grown in cation-adjusted Mueller Hinton broth (caMHB; Fisher Scientific, Hampton, NH), S. pneumoniae was grown in caMHB containing laked horse blood (Quad Five, Ryegate, MT) at a final concentration of 3%, and H. influenzae was grown in haemophilus test medium (HTM) (33). Solid medium for E. coli, S. aureus, and H. influenzae was prepared by adding Bacto agar to a final concentration of 1.5%. Brain heart infusion (BHI; Difco; Fisher Scientific, Hampton, NH) medium containing Bacto agar at a final concentration of 1.5% and either fresh defibrinated sheep blood (Bioreclamation, Inc., Hicksville, NY) or laked horse blood at a final concentration of 5% was added for the growth of S. pneumoniae and E. faecalis, respectively, on solid medium. VRT-125853 and VRT-752586 were synthesized at Vertex Pharmaceuticals Incorporated (Cambridge, MA). Ciprofloxacin was obtained from US Biological (Swampscott, MA). Other antibiotics were obtained from Sigma Chemical Co. (St. Louis, MO). Antibiotics or compounds were initially dissolved in 100% dimethyl sulfoxide (DMSO) at 25.6 mg/ml and were added to the growth medium as indicated. The final concentration of DMSO in all cultures was ≤0.5%.
Determination of MICs.
MICs were determined by the methods described by the Clinical and Laboratory Standards Institute (formerly the NCCLS) guidelines (33). The S. aureus, S. pneumoniae, E. faecalis, and H. influenzae strains were grown on appropriate solid growth medium as described above with or without appropriate antibiotics or compounds and were incubated overnight at 35°C. The test compounds were serially diluted in 100% DMSO at a 200× concentration and then diluted 1:200 into medium, resulting in a final concentration of 0.5% DMSO. Inocula were prepared by using the BBL Prompt system (Becton Dickinson, Cockeysville, MD), according to the manufacturer's directions. MIC assay plates were incubated at 35°C for 18 to 20 h and were visually scored by using a test-reading mirror. A twofold variation in the MIC was considered within the error of the assay.
In vivo plasmid DNA supercoiling assay.
E. coli tolC strain CAG 12184 carrying plasmid pUC18 (47) was grown at 37°C in a 250-ml shake flask in caMHB medium containing 100 μg/ml ampicillin to an optical density at 600 nm of ∼0.5. Two milliliter of cells was then distributed into multiple tubes, and drug or compound was added to create a twofold dilution series that spanned the MICs of the respective compounds. The tubes were further incubated at 37°C with shaking for 1 h. Plasmid DNA was isolated from compound-treated cells by using a plasmid miniprep kit (QIAGEN, Valencia, CA), according to the manufacturer's instructions. The DNA was recovered with 100 μl of double-distilled H2O, concentrated fivefold in a Speedvac (Savant Instruments Inc., Holbrook, NY), and electrophoresed along with supercoiled DNA size markers (Sigma Chemical Co.) on a 1% Tris-borate-EDTA (TBE)-agarose gel at 45 V/cm for ∼16 h. After electrophoresis, the gel was stained with ethidium bromide and photographed by standard procedures (41).
Macromolecular synthesis assays.
The synthesis of DNA (as measured by [3H]thymidine incorporation), RNA (as measured by [3H]uridine incorporation), and protein (as measured by 3H-amino acid mixture incorporation) in S. aureus in the presence of compounds was essentially performed by the method described previously (16), with the following specifics. Log-phase cells of S. aureus ATCC 29213 were exposed to 5× the MICs of the respective compounds. All treatments were performed in duplicate. DNA and RNA synthesis was carried out in the presence of compounds for 15 min, while protein synthesis was carried out for 25 min before the reactions were stopped by addition of 10% trichloroacetic acid. The level of incorporation of the radioactive precursors in the absence of any compound was considered 100%, and the relative level of incorporation in the presence of compounds was calculated as a percentage of that for the untreated control.
Isolation of resistant mutants, DNA isolation, PCR amplification, and DNA sequencing.
Mutants of S. aureus, S. pneumoniae, and H. influenzae resistant to VRT-125853, VRT-752586, and novobiocin were isolated by plating ∼109 to 1010 cells on the appropriate solid medium containing compounds at either 2×, 4×, or 8× their respective MICs. In the case of E. faecalis, no mutant arose at the lowest concentration tested of 2× the MIC for VRT-125853, VRT-752586, or novobiocin; and therefore, a serial passaging approach was used to isolate resistant mutants of these organisms. Mutants selected at the highest concentration of the compound were plated onto the same selection medium to reconfirm the resistant phenotype. Double mutants of S. aureus, S. pneumoniae, and E. faecalis with sequence-verified mutations in both gyrB and parE, selected in other studies in our laboratory, were used as tools to probe the secondary target interaction of VRT-125853 and VRT-752586.
In order to isolate genomic DNA, the resistant mutants of S. aureus, H. influenzae, and E. faecalis were grown overnight in the appropriate liquid growth medium containing compounds at 2× to 4× the wild-type MICs to ensure the presence of the mutation in the isolated DNA. Cells were collected by centrifugation, and genomic DNA was isolated by standard procedures (41). For S. pneumoniae, the mutant strains were grown on BHI agar medium containing 5% defibrinated sheep blood and selective concentrations of the compound. The plates were incubated overnight at 37°C in a humidified 5% CO2 incubator, and on the following day the cells were scraped from the surface of the agar plates and washed with 0.2 ml BHI medium. Cells were recovered by centrifugation, and genomic DNA was isolated by using a QIAamp DNA isolation kit (QIAGEN), according to the manufacturer's instructions.
Genomic DNA preparations were used as templates to amplify the gyrB and parE ATP-binding regions from the wild type and mutants by PCR with the primer pairs listed in Table 1. The PCR amplification was carried for 35 cycles with denaturation at 95°C for 1 min, annealing at 40 to 50°C for 1 min, and extension with Taq DNA polymerase (Stratagene, La Jolla, CA) at 72°C for 1 min. The PCR products were electrophoresed on 1% TBE-agarose gels containing ethidium bromide, excised and purified with a gel extraction kit (QIAGEN), and sequenced by standard automated sequencing methods to determine the mutations.
TABLE 1.
Sequences of primers used for PCR amplification and DNA sequencing of gyrB and parE DNA encoding the ATP binding region of mutants
| Primer name | DNA sequence |
|---|---|
| S. aureus gyrB forward | CGATTCAGCATAAAGTACAAACATTTGTCACTACGACATCTG |
| S. aureus gyrB reverse | CCGCCCTCATAGTGATAGGAGTCTTCTCTAACG |
| S. aureus grlB forward | GCATTTTACGCTGATTTATATAAGAATAACTATTGTATAGTTTTAAAAACGAAC |
| S. aureus grlB reverse | GCTCTTGACGCTCTTTACCACTGCGTAAATCATTAAGCG |
| E. faecalis gyrB forward | CGTCATTGTATTGATAAGCGACCTCTACTTCG |
| E. faecalis gyrB reverse | CGGGGATCCCTTCAATTGAAATGTGTAAGCCTCG |
| E. faecalis parE forward | CGCGGATCCGGCTCACTCTTCTTCTAATTC |
| E. faecalis parE reverse | CGGGGATCCGGGTTCTTCCCCACGTTCATC |
| S. pneumoniae gyrB forward | CGCTCTAGAGCTGCAGGACTTGATACCCACTTG |
| S. pneumoniae gyrB reverse | GCCTCTAGACAGTGATAGAAATTTGAAGACCGCG |
| S. pneumoniae parE forward | CGGTCTAGACTCTGCTGAAATTGTCACATCGGAG |
| S. pneumoniae parE reverse | CGGTCTAGACGCTTGTCCGTTAAAGACAAGGTCAC |
| H. influenzae gyrB forward | CGTTTTCCTTTCCCGAAAAGCATCGCCAAAACGGCG |
| H. influenzae gyrB reverse | CTACGAATGCTTGAATACCGCCTTCATAATGGAAATGATC |
| H. influenzae parE forward | GTGCGGTCGAATATCACCGCACTTTTTGATAG |
| H. influenzae parE reverse | CGGAAGGGTTTCAAAGCCATTTACTGCTTCG |
DNA transformations.
E. coli was used as a cloning host for the construction of S. aureus shuttle plasmids. The S. aureus gyrB open reading frame, including the native promoter, was amplified from the genomic DNA of the mutant strains by PCR with the following oligonucleotides: 5′-ggatccGAAGTTGGGGAATATCCCATCTTATT-3′ containing an engineered BamHI site (lowercase and underlined) and 5′-ggtaccTCCTTCAAAAGTTCAGTTCACAGCGC-3′ with an engineered KpnI site (lowercase and underlined). The PCR products were cloned into plasmid pCR 2.1 (Invitrogen, Carlsbad, CA), and the DNA sequence was confirmed by standard automated sequencing methods. The gyrB BamHI and KpnI fragment was subcloned into the corresponding sites in plasmid pUC18 (47). The resulting plasmid was digested with HindIII and ligated to HindIII-digested plasmid pC194 (4) to form the E. coli/S. aureus shuttle plasmids (see Table 3). The shuttle plasmids were transformed into S. aureus RN4220, a restriction-deficient strain (24), by electroporation (43). Plasmids isolated from RN4220 were used to transform S. aureus ATCC 29213 by electroporation, as described above. S. aureus and Escherichia coli were grown at 37°C in Luria-Bertani (LB) broth or were plated on LB agar with the appropriate antibiotics for plasmid selection or maintenance (chloramphenicol at 10 μg/ml for S. aureus; ampicillin at 100 μg/ml for E. coli).
TABLE 3.
Genetic proof that mutations in GyrB/ParE confer resistance to VRT-125853 and VRT-752586 in S. aureus and H. influenzaea
| Strain | Chromosome | Plasmid | MIC (μg/ml)
|
No. of transformants/ selection plate
|
||||
|---|---|---|---|---|---|---|---|---|
| VRT-125853 | VRT-752586 | Novo | Cipro | VRT-125853 (32 μg/ml) | Novo (0.5 μg/ml) | |||
| S. aureus ATCC 29213 GyrB | WT | No plasmid | 2 | 0.06 | 0.13 | 0.25 | ||
| WT | WT | 2 | 0.06 | 0.13 | 0.25 | |||
| T173N | No plasmid | 8 | 0.25 | 0.25 | 0.25 | |||
| WT | T173N | 8 | 0.25 | 0.13 | 0.25 | |||
| T173I | No plasmid | 8 | 0.25 | 2 | 0.25 | |||
| WT | T173I | 8 | 0.13 | 0.5 | 0.25 | |||
| R144I | No plasmid | 2 | 0.06 | 8 | 0.25 | |||
| WT | R144I | 2 | 0.06 | 8 | 0.25 | |||
| H. influenzae ATCC 51907 GyrB | WT | 0 | 0 | |||||
| T169I | 71 | ND | ||||||
| R140C | ND | 141 | ||||||
| R140H | ND | 53 | ||||||
| R140L | ND | 245 | ||||||
Abbreviations: WT, wild type; Novo, novobiocin; Cipro, ciprofloxacin; ND, not determined.
H. influenzae ATCC 51907 competent cells were prepared by the method described previously (39). For the H. influenzae transformation experiments, frozen competent cells were thawed to room temperature, centrifuged to remove the storage medium, and suspended in the same volume of MIV (m-four) medium (39). Approximately ∼300 ng of DNA (gel-purified PCR product) was added to 0.25 ml of competent cell preparation, and the cells were mixed and incubated in a 37°C incubator for 30 min. At the end of incubation period the cells were plated on HTM agar plates containing the selective concentration of the compound. The plates were incubated in a 37°C incubator for 48 to 72 h and compound-resistant colonies were counted. Competent cells with no DNA added or PCR product from wild-type cells added served as the background controls.
Determination of spontaneous resistance frequencies.
Wild-type and sequence-verified E. faecalis mutant strains were grown and plated as described previously (28). Resistance frequencies were minimally determined in duplicate with two independently inoculated cultures and (see Table 4 for representative results). E. faecalis wild-type and mutant cultures were grown to late log phase (approximately 108 to 109 CFU/ml) at 37°C, concentrated 10-fold by low-speed centrifugation, and plated on selection plates. Plates were prepared by adding an appropriate dilution of VRT-752586 in 100% DMSO to MHB agar with 5% laked horse blood, and agar was poured into sterile polystyrene petri plates (150 by 15 mm). The final DMSO concentration was <0.1%. A sample of the inoculum was serially diluted to confirm the starting number of CFU. The plates were incubated at 35°C for a minimum of 3 days before the colonies were counted. The resistance frequency was calculated as the number of compound-resistant colonies divided by the total number of CFU plated.
TABLE 4.
Dual targeting leads to lower frequencies of spontaneous resistance to VRT-752586 in E. faecalis
| Mutation
|
VRT-752586 resistance frequency at:
|
VRT-752586 MIC (μg/ml) | |||
|---|---|---|---|---|---|
| GyrB | ParE | 2× MIC | 4× MIC | 8× MIC | |
| WTa | WT | <5.2 × 10−10 | <5.2 × 10−10 | <5.2 × 10−10 | 0.032 |
| WT | T169A | 8.7 × 10−9 | 5.2 × 10−9 | 1.2 × 10−9 | 0.064 |
| T167A | WT | 9.1 × 10−7 | 2.4 × 10−8 | 6.0 × 10−9 | 0.032 |
| T167A | T169A | 1.2 × 10−8 | 3.7 × 10−8 | 1.2 × 10−8 | 0.500 |
WT, wild type.
RESULTS
VRT-125853 inhibits RNA and DNA synthesis but not protein synthesis in S. aureus.
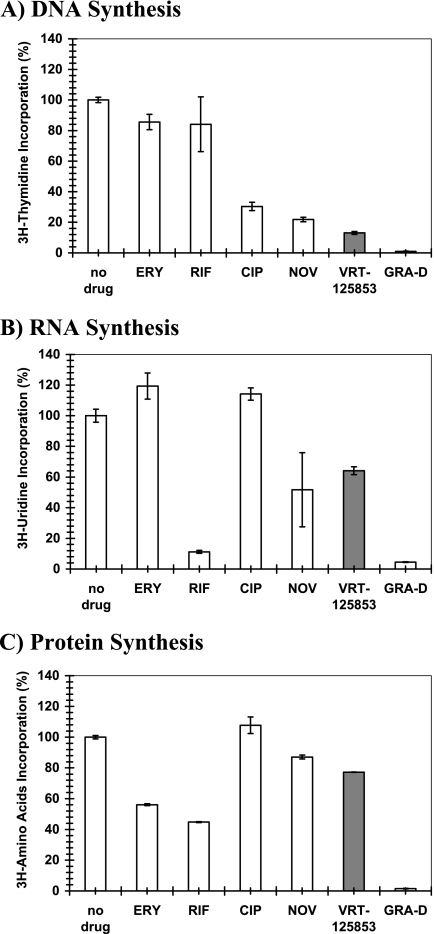
The effect of VRT-125853 on the synthesis of DNA ([3H]thymidine), RNA ([3H]uridine), and protein (3H-amino acid) was evaluated with S. aureus ATCC 29213 and compared with the effects of antibiotics that preferentially target specific pathways (ciprofloxacin, novobiocin, erythromycin, rifampin, and gramicidin D) (Fig. 2). VRT-125853 had a pronounced effect on DNA synthesis (∼80% inhibition), as expected for DNA gyrase inhibitors (Fig. 2A), while the effect on RNA (Fig. 2B) and protein synthesis (Fig. 2C) was modest (∼20 to 40% inhibition). The inhibition profile for VRT-125853 was most similar to that for novobiocin, which also inhibits GyrB ATPase activity in S. aureus. As expected, ciprofloxacin, an inhibitor of the other subunits of gyrase (GyrA) and topo IV (ParC), showed specific inhibition of DNA synthesis and no inhibition of RNA or protein synthesis. Erythromycin showed a specific effect on protein synthesis, consistent with its mechanism of action. Both rifampin and gramicidin D also showed the expected inhibition profiles: rifampin inhibited RNA and protein synthesis, whereas gramicidin D, a membrane-active agent, showed nonspecific inhibition of DNA, RNA, and protein synthesis.
FIG. 2.
Effect of VRT-125853 on DNA, RNA, and protein synthesis in S. aureus. Metabolite incorporation assays were performed as described in Materials and Methods. The synthesis of DNA (A), RNA (B), and protein (C) were measured in S. aureus cells exposed to five times the MIC of VRT-125853 or control compounds; and the levels of incorporation of [3H]thymidine (DNA synthesis), [3H]uridine (RNA synthesis) or 3H-amino acid mixture (protein synthesis) during the course of the experiment were measured and are expressed as a percentage of that for the untreated (no drug) control. Ciprofloxacin (CIP), novobiocin (NOV), erythromycin (ERY), rifampin (RIF), and gramicidin D (GRA-D) were the control compounds used for comparison. The results for VRT-125853 are shown as shaded bars.
VRT-125853 and VRT-752586 cause a reduction in steady-state levels of plasmid supercoiling in E. coli.
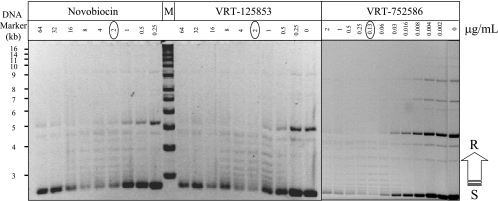
VRT-125853 and VRT-752586 are potent inhibitors of both E. coli and S. aureus gyrase and topo IV in enzyme assays (28). In order to demonstrate that this potent inhibition in vitro translated into inhibition of gyrase and topo IV inside bacterial cells, we tested the effects of VRT-125853 and VRT-752586 on the steady-state levels of plasmid supercoiling in E. coli. Since these compounds were found to be effluxed from wild-type E. coli (28), it was necessary to use an efflux-deficient strain (tolC mutant) to detect the effects of compound in E. coli cells. Both VRT-125853 and VRT-752586 reduced the steady-state levels of supercoiled plasmid pUC18, as evidenced by a decrease in the negatively supercoiled DNA band and a concomitant increase in plasmid topoisomers with reduced negative supercoils (laddering effect), in a manner dependent on the concentration of the compound present in the growth medium (Fig. 3). As expected, a similar effect on plasmid supercoiling was also observed in the case of novobiocin, a known inhibitor of GyrB ATPase activity (Fig. 3).
FIG. 3.
VRT-125853 and VRT-752586 cause a reduction in steady-state levels of plasmid supercoiling in E. coli. The effects of VRT-125853, VRT-752586, and novobiocin on the steady-state levels of plasmid DNA supercoiling were determined with E. coli cells, as described in Materials and Methods. The MIC of each compound is circled. The positions of migration of the supercoiled (S) and relaxed (R) DNA forms of plasmid DNA are indicated. M, DNA size marker (sizes are indicated on the left).
Mutations conferring resistance to VRT-125853 and VRT-752586 are found in DNA sequences encoding the N-terminal region of GyrB or ParE.
DNA sequencing of gyrB and parE regions encoding the ATP-binding site of first-step mutants resistant to VRT-125853 and VRT-752586 of S. aureus, S. pneumoniae, H. influenzae, and E. faecalis revealed single point mutations that caused specific amino acid substitutions in the resistant variants. VRT-125853 and VRT-752586 resistance-conferring mutations were all located in gyrB in S. aureus and H. influenzae, whereas they were all located in parE in S. pneumoniae. Interestingly, in the case of E. faecalis, the primary target of VRT-125853 was found to be ParE, whereas for VRT-752586 it was found to be GyrB. On the other hand, S. aureus, H. influenzae, and S. pneumoniae selected on novobiocin showed mutations in their gyrB genes.
One of the VRT-125853 and VRT-752586 resistance-conferring mutations common to all four bacteria studied corresponded to T165 of GyrB or T163 of ParE (E. coli numbering), a highly conserved residue among bacterial GyrB and ParE proteins and implicated in novobiocin resistance (44). The novobiocin-selected mutation in S. pneumoniae encoding a S127L substitution in GyrB corresponds to GyrB-V120/ParE-I116 residues in E. coli and has been reported previously (31). The novobiocin-selected mutations in gyrB of S. aureus (encoding R144) and H. influenzae (encoding R140), corresponding to R136 encoded by E. coli gyrB, has also been implicated in conferring high-level resistance to novobiocin in other bacteria (9, 17, 42, 44).
VRT-125853- and VRT-752586-resistant mutants show differential patterns of cross-resistance to novobiocin.
The MICs of the wild types and the resistant mutants of S. aureus, S. pneumoniae, E. faecalis, and H. influenzae isolated with VRT-125853, VRT-752586, and novobiocin were determined as described in Materials and Methods and are shown in Table 2. S. aureus gyrB mutants (encoding the T173N and T173I substitutions) isolated under selection with VRT-125853 and VRT-752586 showed resistance to both compounds, as expected; however, they showed differential sensitivities to novobiocin (Table 2). While the gyrB mutation encoding a T173N substitution caused only a twofold increase in the MIC of novobiocin compared to that for the wild-type parent, the gyrB mutation encoding the T173I substitution increased the MIC of novobiocin by eightfold. In contrast, the novobiocin resistance-conferring gyrB mutation encoding a R144I substitution increased the MIC of novobiocin 64-fold over that for the wild-type strain but caused only 2-fold increases in the MICs of VRT-125853 and VRT-752586.
TABLE 2.
Dual targeting of GyrB and ParE by VRT-125853 and VRT-752586a
| Strain | GyrB | ParE (GrlB) | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|---|
| VRT-125853 | VRT-752586 | Novo | Cipro | |||
| S. aureus ATCC 29213b | WT | WT | 2 | 0.03 | 0.13 | 0.25 |
| T173N | WT | 16 | 0.5 | 0.25 | 0.25 | |
| T173I | WT | 16 | 0.5 | 1 | 0.25 | |
| R144Ic | WT | 4 | 0.06 | 8 | 0.25 | |
| T173N | T166A | >64 | >8 | 0.25 | 0.25 | |
| T173I | T166A | >64 | 4 | 2 | 0.25 | |
| S. pneumoniae ATCC BAA-255 | WT | WT | 0.5 | 0.004 | 0.5 | 0.5 |
| S127Lc | WT | 0.5 | 0.002 | 8 | 0.25 | |
| WT | T172A | 8 | 0.016 | 0.5 | 0.25 | |
| WT | T172I | 4 | 0.016 | 0.5 | 0.5 | |
| T172A | T172A | 64 | 0.25 | 4 | 0.25 | |
| E. faecalis ATCC 29212 | WT | WT | 1 | 0.032 | 8 | 1 |
| WT | T169A | 8 | 0.064 | 8 | 1 | |
| T167A | WT | 0.5 | 0.032 | 16 | 0.5 | |
| T167I | WT | 1 | 0.064 | 32 | 1 | |
| T167I | T169A | >64 | 1 | 16 | 0.5 | |
| T167A | T169A | >64 | 0.5 | 16 | 0.5 | |
| H. influenzae ATCC 51907 | WT | WT | 4 | 1 | 0.064 | 0.004 |
| R140Cc | WT | 2 | 1 | 8 | 0.004 | |
| R140Hc | WT | 2 | 1 | 4 | 0.004 | |
| R140Lc | WT | 4 | 1 | 4 | 0.004 | |
| T169I | WT | >64 | >64 | 0.25 | 0.004 | |
Abbreviations: WT, wild type; Novo, novobiocin; Cipro, ciprofloxacin.
In S. aureus, ParE is GrlB.
Mutants selected with novobiocin.
As shown in Table 2, the S. pneumoniae novobiocin resistance-conferring gyrB mutation encoding the S127L substitution increased the MIC of novobiocin 16-fold over that for the wild-type strain but did not increase the MICs of VRT-125853 and VRT-752586. In contrast, strains with parE mutations isolated with VRT-125853 and VRT-752586, which encoded a T172A or T172I substitution, showed resistance to both compounds, as expected, but the mutations did not increase the MICs of novobiocin.
As shown in Table 2, the E. faecalis VRT-125853 resistance-conferring mutation (parE encoding a T169A substitution) increased the MIC of VRT-125853 by eightfold but caused only a twofold increase in the MIC of VRT-752586. However, gyrB mutations encoding T167I or T167A substitutions, isolated with VRT-752586, had only a modest effect on the MICs of both VRT-125853 and VRT-752586. The effects of these gyrB single mutations on novobiocin MICs were also small (in the two- to fourfold range).
As shown in Table 2, the novobiocin-resistant strains of H. influenzae carrying gyrB mutations encoding the R140C, R140H, or R140L substitution had novobiocin MICs that were increased 64- to 128-fold, but the MICs of VRT-125853 and VRT-752586 were unaffected by these mutations. On the other hand, strains containing gyrB mutations encoding the T169I substitution, selected with VRT-125853 and VRT-752586, were resistant to both compounds but showed only a fourfold increase in the MIC of novobiocin.
In all of the cases described above, the MICs of ciprofloxacin, which targets the GyrA and ParC subunits of gyrase or topo IV, respectively, were unaffected by gyrB or parE mutations selected by VRT-125853, VRT-752586, and novobiocin (Table 2). The MICs of ethidium bromide for these mutants were comparable to those for their wild-type parents, ruling out the presence of general efflux mechanisms (data not shown). Similarly, no significant effects on the MICs of other, mechanistically unrelated antibiotics, such as ampicillin (S. pneumoniae) or vancomycin and linezolid (S. aureus and E. faecalis), were observed for strains carrying the gyrB and/or parE mutation mentioned above (data not shown).
Genetic evidence that GyrB ATP-binding site mutations confer resistance to VRT-125853 and VRT-752586.
In order to confirm that the mutations observed in the DNA sequences encoding the ATP-binding site of GyrB or ParE were the only mutations present in the full-length genes, we determined the DNA sequences of the entire gyrB and parE genes from the S. aureus, S. pneumoniae, E. faecalis, and H. influenzae compound-resistant strains. No other mutations outside of the ATP-binding regions were detected. In order to demonstrate unambiguously that the mutations observed in the gyrB DNA sequence encoding the ATP-binding site were responsible for the corresponding resistance phenotypes, DNA transformation experiments were performed with S. aureus and H. influenzae.
The full-length gyrB genes of the S. aureus wild-type and the gyrB mutant strains were subcloned with the native promoter into an E. coli-S. aureus shuttle vector and reintroduced into an S. aureus wild-type background. The compound MICs for the S. aureus wild-type and gyrB mutant strains selected with VRT-125853, VRT-752586, and novobiocin were compared with those for the S. aureus wild-type strains carrying plasmids expressing either the wild-type or mutant gyrB alleles (Table 3). Introduction of a plasmid carrying the wild-type gyrB allele into the wild-type parent strain did not alter the MICs of VRT-125853, VRT-752586, novobiocin, or ciprofloxacin. However, when a plasmid expressing a mutant gyrB allele encoding either a T173N or T173I substitution was introduced into a wild-type S. aureus background, the MICs of VRT-125853 and VRT-752586 increased by the same magnitude as that for S. aureus carrying the corresponding mutations in the chromosome (Table 3). These results confirmed that the T173N and T173I mutations in gyrB are responsible for decreased susceptibility to VRT-125853 and VRT-752586 in S. aureus. Similar results were obtained for the novobiocin resistance-conferring mutation in gyrB encoding the R144I substitution, thereby linking this mutation to the novobiocin resistance phenotype in S. aureus.
We used the natural transformation ability of H. influenzae to carry out allele replacement experiments to unambiguously demonstrate the role of chromosomal mutations in gyrB in conferring resistance to the compound used for its selection. DNA fragments of gyrB carrying different mutations were amplified from the chromosome of the VRT-125853-resistant mutant (encoding the T169I substitution) and the novobiocin-resistant mutants (encoding R140C, R140H, or R140L) by PCR, and the purified DNA fragments were transformed into wild-type H. influenzae. Transformants were plated on HTM agar plates containing 8× the MIC for VRT-125853 or novobiocin for the wild type. As shown in Table 3, transformants receiving DNA fragments carrying the VRT-125853 resistance-conferring mutation, gyrB encoding the T169I substitution, were resistant to VRT-125853. Similarly, transformants receiving DNA fragments carrying novobiocin resistance-conferring mutations, gyrB encoding the R140C, R140H, or R140L substitution, were resistant to novobiocin. In contrast, a DNA fragment containing the wild-type gyrB sequence resulted in no transformants that could grow in the presence of VRT-125853 or novobiocin.
Dual targeting of GyrB and ParE by VRT-125853 and VRT-752586.
The abilities of VRT-125853 and VRT-752586 to inhibit both GyrB and ParE (i.e., dual targeting) in cells were demonstrated by using sequence-verified mutants of S. aureus, S. pneumoniae, and E. faecalis with double mutations which were isolated during other studies in our laboratory. Mutants with double mutations of S. aureus (encoding the GyrB-T173N and ParE-T166A or GyrB-T173I and ParE-T166A substitutions), S. pneumoniae (encoding the GyrBT172A and ParE-T172A substitutions), and E. faecalis (encoding the GyrB-T167I and ParE-T169A and the GyrB-T167A and ParE-T169A substitutions) showed significant increases in their MICs of VRT-125853 and VRT-752586 compared to the MICs for their wild-type strains and the mutants with single gyrB or parE mutations, thereby providing strong evidence that these compounds interact with both targets in living cells (Table 2). On the other hand, the novobiocin MICs for these mutants with double mutations either were comparable to those for gyrB mutants with single mutations or in some cases cross-resistance was observed, consistent with its differential target interactions and preferences in these three bacteria.
Dual targeting of gyrase and topo IV leads to lower spontaneous resistance frequencies.
The spontaneous frequencies of resistance of E. faecalis mutants with single gyrB and parE mutations and mutants with sequence-verified double mutations were determined for VRT-752586 at 2×, 4×, and 8× the MIC to demonstrate that effective dual targeting at biologically relevant compound concentrations leads to low frequencies of resistance in the wild-type parent in vitro. Representative values from at least two independent experiments with each strain are presented in Table 4. At all concentrations of VRT-752586 tested, the mutants with single and double gyrB and parE mutations showed elevated frequencies of resistance compared to that of the wild-type parent (Table 4).
DISCUSSION
VRT-125853 and VRT-752586 (Fig. 1) are two new aminobenzimidazoles that display potent antibacterial activities, to which resistance emerges at low frequencies in vitro, and that are bactericidal against gram-positive and some gram-negative bacteria (28). Structurally, VRT-125853 carries a 5-(-2-methoxypyrimidin-5-yl) substituent and a 6-methoxy substituent on the aminobenzimidazole urea core scaffold, whereas VRT-752586 carries a 5-(-3-pyridine) substitution and a 7-(-3-fluoropyridin-2-yl) substitution on the aminobenzimidazole urea core scaffold. While both VRT-125853 and VRT-752586 showed potent inhibition of E. coli and S. aureus gyrase and topo IV enzymes, the latter compound was especially more potent (at least 30-fold better) against topo IV (28). We believe that the superior enzyme inhibition activity of VRT-752586 contributes to its improved antibacterial activity (28).
Gyrase uses ATP hydrolysis to introduce negative supercoils into DNA and in doing so facilitates the movement of replication and transcription complexes on DNA (10). Similarly, topo IV uses the energy from ATP hydrolysis to decatenate DNA catenanes and also contributes to DNA relaxation (48). Consistent with these critical roles, addition of VRT-125853, an inhibitor of the ATPase activities of gyrase and topo IV, to S. aureus cells reduced the level of incorporation of [3H]thymidine into DNA by ∼80%, indicating an arrest of DNA synthesis (Fig. 2). The overall effect of VRT-125853 on the metabolism of S. aureus was comparable to that of novobiocin, consistent with the similarities in their mechanisms of action.
In plasmid supercoiling assays with E. coli, both VRT-125853 and VRT-752586 reduced the steady-state levels of negatively supercoiled plasmid DNA in a dose-dependent manner. The maximal reduction in the negatively supercoiled plasmid DNA band coincided with the MICs of VRT-125853 and VRT-752586 for the E. coli tolC strain (Fig. 3), consistent with a gyrase-mediated inhibition of growth. Novobiocin showed a similar effect, although in this case the maximal reduction of supercoiled plasmid DNA band occurred at four times the MIC of novobiocin. Moreover, in these experiments, VRT-125853, VRT-752586, and novobiocin each produced slightly different DNA banding patterns at concentrations greater than the MIC. At higher concentrations of both the aminobenzimidazoles and novobiocin, a reappearance of the rapidly migrating band was noted. This may have been due to compound insolubility or the secondary effects of the compounds. Further characterization of the differences observed between the aminobenzimidazole compounds and novobiocin, as well as the significance of the rapidly migrating band, will be addressed in future studies.
VRT-125853 and VRT-752586 showed interesting patterns of target preference in S. aureus, S. pneumoniae, E. faecalis, and H. influenzae. In S. aureus and H. influenzae, both compounds selected for first-step mutations in gyrB, whereas in S. pneumoniae both compounds selected for first-step mutations in parE. In E. faecalis, however, the primary target specificities for VRT-125853 (ParE) and VRT-752586 (GyrB) differed, suggesting that both differences in the inhibitor structure and differences in the ATP-binding site structure influence the target preference. Such differences in target preference have been well documented among the fluoroquinolones that target the GyrA and ParC subunits of gyrase and topo IV, respectively. For example, ciprofloxacin primarily targets GyrA in E. coli (7), whereas its primary target in S. aureus has been shown to be ParC (35). Some of the newer fluoroquinolones, such as sparfloxacin and moxifloxacin, target GyrA in S. pneumoniae (26, 36) and S. aureus (12). In the case of moxifloxacin, some reports show that the primary target in S. aureus is ParC (20). Gemifloxacin, on the other hand, has been reported to target ParC in S. aureus, while its primary target in S. pneumoniae is GyrA (14, 19).
Analysis of VRT-125853 and VRT-752586 with S. aureus, S. pneumoniae, and E. faecalis mutants carrying single or double mutations in the DNA regions encoding the GyrB or/and ParE ATP-binding sites revealed the abilities of the two compounds to inhibit both targets in vitro at biologically relevant concentrations. Single target-based mutations only modestly elevated the MICs of VRT-125853 and VRT-752586 in each of these organisms. Significant increases in the MICs of VRT-125853 and VRT-752586 over the MICs of their wild-type strains and single-target mutants were observed when both gyrB and parE mutations were simultaneously present in the same strain. In the case of E. faecalis, single mutations in either gyrB or parE showed little effect, whereas double mutations caused a synergistic increase in MICs. The observation that individual mutations in either gyrB or parE had only modest effects on the MIC of VRT-752586 suggests that the bias for GyrB as its primary target identified by serial passage selection (see Materials and Methods) is very slight (Table 2). These results indicated that the wild-type MICs of VRT-125853 and VRT-752586 are the results of dual targeting of both GyrB and ParE in E. faecalis (Table 2). VRT-752586 is ∼64-fold more potent than VRT-125853 against S. aureus (Table 2). This is consistent with the superior activity of VRT-752586 against both the gyrase and the topo IV enzymes (28), supporting the idea that improved potency against the two enzymes could also be contributing to improved potency against S. aureus. A comparison of the MICs of VRT-125853 and VRT-752586 for S. pneumoniae, E. faecalis, and H. influenzae also suggests that a similar correlation exists. However, currently we do not have the supporting enzyme activity data for these three bacterial enzymes.
The coumarin antibiotic novobiocin has been well characterized with respect to its mode of interaction with the E. coli GyrB and ParE ATP-binding sites (3, 25). Biochemical, genetic, and crystallographic studies implicate R136 of GyrB (E. coli numbering) in novobiocin binding; and mutations in this residue have been shown to decrease the affinity of novobiocin to gyrase and confer novobiocin resistance to bacteria carrying these mutations (9, 13, 44). A comparison of novobiocin-resistant mutants carrying a mutation in the corresponding amino acid residue of S. aureus GyrB (R144I substitution) and H. influenzae GyrB (R140C, R140H, or R140L substitutions) showed that while these mutations increased the MIC of novobiocin significantly above that for the wild-type strain, little or no effect on the MICs of VRT-125853 or VRT-752586 was observed (Table 2). Similar results were observed with a novobiocin-resistant S. pneumoniae mutant carrying an S127L mutation in GyrB. This mutation is known to confer high-level novobiocin resistance in this bacterium, but the MICs of VRT-125853 and VRT-752586 were comparable to that for the wild-type parent. These results suggest that while novobiocin derives much of its binding energy from an interaction with these residues, VRT-125853 and VRT-752586 interact quite differently with the ATP-binding region of GyrB, a feature engineered by structure-guided drug design.
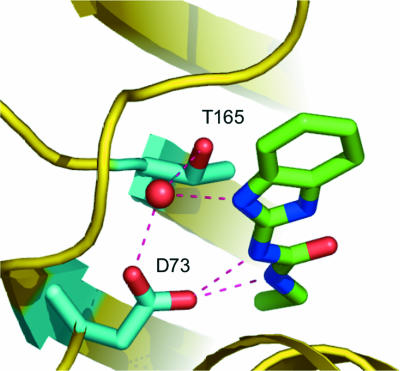
The T165 residue (E. coli GyrB numbering) in the ATP-binding site is a highly conserved amino acid in both GyrB and ParE from diverse bacteria. It plays a key role in ATP binding by coordinating a conserved water molecule via its side chain hydroxyl group and the N-7 nitrogen in the adenine ring, creating a tight hydrogen bond network (13, 25). Site-directed mutagenesis of the E. coli GyrB showed that a T165A amino acid substitution causes an approximately fivefold increase in the Km for ATP, while it still retains sufficient catalytic activity to complement a gyrB temperature-sensitive mutant of E. coli (13). S. aureus, S. pneumoniae, E. faecalis, and H. influenzae strains carrying gyrB or parE mutations encoding amino acid replacements in the threonine corresponding to T165 of E. coli GyrB all had significantly elevated MICs over those for the wild-type parent for both VRT-125853 and VRT-752586 (Table 2). This result is consistent with structural data indicating that the T165 residue (E. coli GyrB numbering) is involved in an extensive hydrogen bond network involving D73 (E. coli GyrB numbering), the aminobenzimidazole urea core of VRT-125853 or VRT-752586, and a highly conserved water (Fig. 4; unpublished crystallographic data).
FIG. 4.
Key interactions of the aminobenzimidazole core and GyrB. T165 is involved in an extensive hydrogen bond network involving D73, the aminobenzimidazole urea core, and a highly conserved water (unpublished crystallographic data).
Dual targeting of two essential bacterial proteins is expected to reduce the emergence of resistance at compound concentrations where both targets are similarly inhibited. New, more potent quinolones that equipotently target gyrase and topo IV show improved MICs and to which organisms show reduced frequencies of resistance in vitro (18, 37, 45). Consistent with this idea, the frequency of resistance emergence in E. faecalis was found to be low at concentrations near the MIC in a wild-type strain but significantly elevated in strains that carried either single or double mutations in gyrB and parE (Table 4). Presumably, the individual target-based mutations create an imbalance in the dual targeting of gyrase and topo IV, allowing resistance to be more readily selected for. The significant increase in MICs, accompanied by mutations in both gyrB and parE (Table 2) and low frequencies of resistance development, underscores the benefits of the dual targeting activity of an antibiotic.
In summary, our data demonstrate that VRT-125853 and VRT-752586 possess dual targeting activities in S. aureus, E. faecalis, and S. pneumoniae. Dual inhibition of GyrB and ParE translated into potent antibacterial activities and low frequencies of resistance development at biologically relevant concentrations in vitro, two properties that are expected to prolong the life of an antibiotic in the clinic. Both compounds were shown to be active against broad panels of susceptible and multidrug-resistant gram-positive bacteria and select gram-negative bacteria, especially those involved in causing respiratory infections (28). Since antibiotics targeting GyrB/ParE ATPase activity are not currently used in the clinic, these compounds are not expected to face the problem of cross-resistance that newer, other antibiotic classes encounter. VRT-125853 and VRT-752586 represent a novel class of dual-targeting compounds with the potential to evolve as a therapeutically useful class of antibacterial agents.
Acknowledgments
We thank John Thomson, Ann D. Kwong, William Markland, and Michael Briggs for support and helpful suggestions; Yeelan Wang for help with DNA sequencing; and Julia Sun and Jingbo Cai for exceptional media preparation services.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Aspa, J., O. Rajas, F. Rodriguez de Castro, J. Blanquer, R. Zalacain, A. Fenoll, R. de Celes, A. Vargas, F. R. Salvanes, P. P. Espana, J. Rello, and A. Torres. 2004. Drug-resistant pneumococcal pneumonia: clinical relevance and related factors. Clin. Infect. Dis. 38:787-798. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, C. T., and J. F. Barrett. 2003. Antibacterials: are the new entries enough to deal with the emerging resistance problems? Curr. Opin. Biotech. 14:621-626. [DOI] [PubMed] [Google Scholar]
- 3.Bellon, S., J. D. Parsons, Y. Wei, K. Haykawa, L. L. Swenson, P. S. Charifson, J. A. Lippke, R. Aldape, and C. H. Gross. 2004. Crystal structures of Escherichia coli topoisomerase IV ParE subunit (24 and 43 kilodaltons): a single residue dictates differences in novobiocin potency against topoisomerase IV and DNA gyrase. Antimicrob. Agents Chemother. 48:1856-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bron, S. 1990. Plasmids, p. 75-138. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 5.Champoux, J. J. 2001. DNA topoisomerases: structure, function and mechanism. Annu. Rev. Biochem. 70:369-413. [DOI] [PubMed] [Google Scholar]
- 6.Chen, C.-R., M. Malik, M. Snyder, and K. Drlica. 1996. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J. Mol. Biol. 258:627-637. [DOI] [PubMed] [Google Scholar]
- 7.Cullen, M. E., A. W. Wyke, R. Kuroda, and L. M. Fisher. 1989. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob. Agents Chemother. 33:886-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deibler, R. W., S. Rahmati, and E. L. Zechiedrich. 2001. Topoisomerase IV, alone, unknots DNA in E. coli. Genes Dev. 15:748-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Castillo, I., J. L. Vizan, M. C. Rodriguez-Sainz, and F. Moreno. 1991. An unusual mechanism for resistance to the antibiotic coumermycin A1. Proc. Natl. Acad. Sci. USA 88:8860-8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drlica, K., and X. Zhao. 1997. DNA gyrase, topoisomerse IV and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61:377-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gootz, T. D., and K. E. Brighty. 1998. Chemistry and mechanism of action of the quinolone antibacterials, p. 29-80. In V. T. Andriole (ed.), The quinolones, 2nd ed. Academic Press, Inc., New York, NY.
- 12.Griggs, D. J., H. Marona, and L. J. V. Piddock. 2003. Selection of moxifloxacin-resistant Staphylococcus aureus compared to five other fluoroquinolones. J. Antimicrob. Chemother. 51:1403-1407. [DOI] [PubMed] [Google Scholar]
- 13.Gross, C. H., J. D. Parsons, T. H. Grossman, P. S. Charifson, S. Bellon, J. Jernee, M. Dwyer, S. P. Chambers, W. Markland, M. Botfield, and S. Raybuck. 2003. Active-site residues of Escherichia coli DNA gyrase required in coupling ATP hydrolysis to DNA supercoiling and amino acid substitutions leading to novobiocin resistance. Antimicrob. Agents Chemother. 47:1037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heaton, V. J., J. E. Ambler, and L. M. Fisher. 2000. Potent antipneumococcal activity of gemifloxacin is associated with dual targeting of gyrase and topoisomerase IV, an in vivo target preference for gyrase, and enhanced stabilization of cleavable complexes in vitro. Antimicrob. Agents Chemother. 44:3112-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heisig, P. 2001. Inhibitors of bacterial topoisomerases: mechanisms of action and resistance and clinical aspects. Planta Med. 67:3-12. [DOI] [PubMed] [Google Scholar]
- 16.Hilliard, J. J., R. M. Goldschmidt, L. Licata, E. Z. Baum, and K. Bush. 1999. Multiple mechanisms of action for inhibitor of histidine protein kinase for bacterial two component systems. Antimicrob. Agents Chemother. 43:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes, M. L., and M. L. Dyall-Smith. 1991. Mutations in DNA gyrase result in novobiocin resistance in halophilic archaebacteria. J. Bacteriol. 173:642-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ince, D., X. Zhang, L. C. Silver, and D. C. Hooper. 2002. Dual targeting of DNA gyrase and topoisomerase IV: target interactions of garenoxacin (BMS-284756, T-3811ME), a new desfluoroquinolone. Antimicrob. Agents Chemother. 46:3370-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ince, D., X. Zhang, L. C. Silver, and D. C. Hooper. 2003. Topoisomerase targeting with and resistance to gemifloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:274-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ince, D., X. Zhang, and D. C. Hooper. 2003. Activity of and resistance to moxifloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1410-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacoby, G. A. 1996. Antimicrobial-resistant pathogens in the 1990s. Annu. Rev. Med. 47:169-179. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs, M. R. 2003. Worldwide trends in antimicrobial resistance among common respiratory tract pathogens. Pediatr. Infect. Dis. J. 22:S109-S119. [DOI] [PubMed] [Google Scholar]
- 23.Kato, J.-I., Y. Nishimura, R. Imamura, H. Niki, S. Hiraga, and H. Suzuki. 1990. New topoisomerases essential for chromosome segregation in E. coli. Cell 63:393-404. [DOI] [PubMed] [Google Scholar]
- 24.Kreiswirth, B. N., S. Lofdalh, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature (London) 305:709-712. [DOI] [PubMed] [Google Scholar]
- 25.Lewis, R. J., O. M. P. Singh, C. V. Smith, T. Skarzynski, A. Maxwell, A. J. Wonacott, and D. B. Wigley. 1996. The nature of inhibition of DNA gyrase by the coumarins and cyclothialidine revealed by X-ray crystallography. EMBO J. 15:1412-1420. [PMC free article] [PubMed] [Google Scholar]
- 26.Li, X., X. Zhao, and K. Drlica. 2002. Selection of Streptococcus pneumoniae mutants having reduced susceptibility to moxifloxacin and levofloxacin. Antimicrob. Agents Chemother. 46:522-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livermore, D. M. 2004. The need for new antibiotics. Clin. Microbiol. Infect. 10:(Suppl. 4)1-9. [DOI] [PubMed] [Google Scholar]
- 28.Mani, N., C. H. Gross, J. D. Parsons, B. Hanzelka, U. Müh, S. Mullin, Y. Liao, A.-L. Grillot, D. Stamos, P. S. Charifson, and T. H. Grossman. 2006. In vitro characterization of the antibacterial spectrum of novel bacterial type II topoisomerase inhibitors of the aminobenzimidazole class. Antimicrob. Agents Chemother. 50:1228-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maxwell, A. 1997. DNA gyrase as a drug target. Trends Microbiol. 5:102-109. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell, A., and D. M. Lawson. 2003. The ATP-binding site of type II topoisomerases as a target for antibacterial drugs. Curr. Top. Med. Chem. 3:283-303. [DOI] [PubMed] [Google Scholar]
- 31.Muñoz, R., M. Bustamante, and A. G. de la Campa. 1995. Ser-127-to-Leu substitution in the DNA gryase B subunit of Streptococcus pneumoniae is implicated in novobiocin resistance. J. Bacteriol. 177:4166-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutnick, A. H., V. Enne, and R. N. Jones. 2003. Linezolid resistance since 2001. SENTRY Antimicrobial Surveillance Program. Ann. Pharmacother. 37:769-774. [DOI] [PubMed] [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed., vol. 23, no. 2. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 34.National Nosocomial Infections Surveillance System. October. 2004, posting date. National Nosocomial Infections Surveillance (NNIS) System report. Data summary from January 1992 through June 2004. www.cdc.gov/ncidod/hip/NNIS/2004NNISreport.pdf. [DOI] [PubMed]
- 35.Ng, E. Y., M. Trucksis, and D. C. Hooper. 1996. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan, X. S., and L. M. Fisher. 1997. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob. Agents Chemother. 41:471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan, X. S., and L. M. Fisher. 1998. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pohlhaus, J. R., and K. N. Kreuzer. 2005. Norfloxacin-induced DNA gyrase cleavage complexes block Escherichia coli replication forks, causing double-stranded breaks in vivo. Mol. Microbiol. 56:1416-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poje, G., and R. J. Redfield. 2003. Transformation of Haemophilus influenzae. Methods Mol. Med. 71:57-70. [DOI] [PubMed] [Google Scholar]
- 40.Pottumarthy, S., T. R. Fritsche, and R. N. Jones. 2005. Comparative activity of oral and parenteral cephalosporins tested against multidrug-resistant Streptococcus pneumoniae: report from the SENTRY Antimicrobial Surveillance Program (1997-2003). Diagn. Microbiol. Infect. Dis. 51:147-150. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 42.Samuels, D. S., R. T. Marconi, W. M. Huang, and C. F. Garon. 1994. gyrB mutations in coumermycin A1-resistant Borrelia burgdorferi. J. Bacteriol. 176:3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenk, S., and R. A. Laddaga. 1992. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol. Lett. 73:133-138. [DOI] [PubMed] [Google Scholar]
- 44.Stieger, M., P. Angehrn, B. Wohlgensinger, and H. Gmunder. 1996. GyrB mutations in Staphylococcus aureus strains resistant to cyclothialidine, coumermycin, and novobiocin. Antimicrob. Agents Chemother. 40:1060-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strahilevitz, J., and D. C. Hooper. 2005. Dual targeting of topoisomerase IV and gyrase to reduce mutant selection: direct testing of the paradigm by using WCK-1734, a new fluroquinolone, and ciprofloxacin. Antimicrob. Agents Chemother. 49:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomson, C. J., E. Power, H. Ruebsamen-Waigmann, and H. Labischinski. 2004. Antibacterial research and development in the 21st century—an industry perspective of the challenges. Curr. Opin. Microbiol. 7:445-450. [DOI] [PubMed] [Google Scholar]
- 47.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 48.Zechiedrich, E. L., A. B. Khodrusky, S. Bachellier, R. Schneider, D. Chen, D. M. J. Lilley, and N. R. Cozzarelli. 2000. Roles of topoisomerases in maintaining steady-state DNA supercoiling in Escherichia coli. J. Biol. Chem. 11:8103-8113. [DOI] [PubMed] [Google Scholar]
- 49.Zhao, X., M. Malik, N. Chan, A. Drlica-Wagner, J.-Y. Wang, X. Li, and K. Drlica. 2006. Lethal action of quinolones against a temperature-sensitive dnaB replication mutant of Escherichia coli. Antimicrob. Agents Chemother. 50:362-364. [DOI] [PMC free article] [PubMed] [Google Scholar]