Abstract
Paracoccidioidomycosis (PCM) is an important systemic fungal disease, particularly among individuals living and working in rural areas of endemicity in Latin America, who, without antifungal therapy, may develop fatal acute or chronic infection. For such patients, the detection of antibody responses by immunodiffusion is of limited value due to false-negative results. In contrast, the detection of Paracoccidioides brasiliensis gp43 circulating antigen may represent a more practical approach to the rapid diagnosis of the disease. Accordingly, an inhibition enzyme-linked immunosorbent assay (inh-ELISA) was developed for the detection of a 43-kDa P. brasiliensis-specific epitope incorporating a species-specific murine monoclonal antibody. With sera from patients with acute and chronic forms of the disease (n = 81), the overall sensitivity of the test was found to be 95.1%, while specificity was found to be 97.5% compared to that with normal human sera from blood donors (n = 93) and sera from patients with other chronic fungal infections (histoplasmosis [n = 33] and cryptococcosis [n = 20]). The inh-ELISA detected circulating antigen in 100% of patients with the acute form of PCM and in 95.31 and 100% of patients with the chronic multifocal and unifocal forms of PCM according to the patient's clinical presentation. Cerebrospinal fluid from 14 patients with neuroparacoccidioidomycosis and 13 samples of bronchoalveolar lavage fluid from patients with pulmonary unifocal PCM were also tested for gp43 detection, with the test showing 100% sensitivity and specificity. This novel, highly specific inh-ELISA represents a significant addition to the existing tests for the diagnosis of PCM.
Paracoccidioidomycosis (PCM) is a systemic granulomatous disease caused by Paracoccidioides brasiliensis, a thermal dimorphic fungus. PCM is widespread in Latin America, mainly in Brazil, Argentina, Colombia, and Venezuela, affecting mainly rural workers. According to McEwen et al. (22), approximately 10 million people may be infected with this fungus, and up to 2% of them may develop the disseminated forms of the disease. The incidence may increase due to forest destruction and a rise in iatrogenic immunosuppression procedures (39). The acute or subacute form of PCM affects both sexes and chiefly involves the reticuloendothelial system. The chronic form affects adult males with predominantly pulmonary and/or mucocutaneous involvement (12). A definitive diagnosis is usually made by visualization or isolation of the fungus from the lesions, which is time-consuming and lacks sensitivity. Detection of specific antibodies in serum has also been one of the main tools for the diagnosis of PCM and may be useful to monitor the evolution of the disease and its response to treatment (24). The most common serological tests used for diagnosis are immunodiffusion (4, 31), immunoenzymatic assays (3, 5, 25), and counterimmunoelectrophoresis (7). Unfortunately, there is extensive antigenic cross-reactivity between P. brasiliensis and other fungi, limiting the value of the tests that are currently employed. In view of cross-reactivity and variations in the isolates used as sources for the production of antigens, it is advisable to employ more than one test for diagnosis of PCM
The gp43-glycoprotein from P. brasiliensis, secreted exocellularly during the infective yeast phase (4, 28, 34), is the main PCM diagnostic antigen (4, 28), being recognized by virtually all sera from PCM patients in various test formats (1, 4, 25, 35, 36). With respect to cellular immunity, gp43 contains epitopes which elicit positive delayed-type hypersensitivity in human PCM patients (33). Taborda et al. (37) demonstrated that a 15-amino-acid peptide (P10) present in gp43 is responsible for glycoprotein-mediated T-cell activation and protection against PCM in BALB/c mice. As a receptor for laminin (38), gp43 may also be an important virulence factor.
A strong antibody response against gp43 is observed in PCM patients and specific antibodies persist for a long time. Depending on their immune status, some diagnosed patients are serologically negative at the time of diagnosis, with others having low levels of specific antibodies for long periods of time. Thus, it is doubtful whether these patients are ever cured (12).
In some invasive fungal diseases, the detection of circulating antigen is a useful approach to the serodiagnosis (8, 11, 17, 21), and this may also be an alternative tool for the diagnosis of PCM patients. Some researchers have previously tried to detect circulating antigen in PCM patients using polyvalent antigens or antibodies in different assays such as enzyme-linked immunosorbent assay (ELISA) competition (14), immunoradiometric assay (10), immunoelectrophoresis-immunodiffusion (15), counterimmunoelectrophoresis (32), passive hemagglutination inhibition (20), inverted linear immunoelectrophoresis (19), and immunoblotting (26). Goméz et al. (16) were the first investigators to use monoclonal antibodies for the detection of an 87-kDa circulating antigen in PCM with 80.4% sensitivity. Since gp43 represents the most important antigen of the P. brasiliensis system and elicits antibodies that are useful both for diagnosis and for monitoring patients undergoing treatment, we report here the standardization of an alternative inhibition-ELISA (inh-ELISA) for the detection of specific gp43 antigen in serum, cerebrospinal fluid (CSF) and bronchoalveolar lavage (BAL) fluid using a monoclonal anti-gp43 antibody.
MATERIALS AND METHODS
Clinical samples.
A total of 81 active PCM patients with a diagnosis established by direct KOH examination, isolation by culture, and/or positive serological tests were used in this study. One serum sample was taken from each patient at the time of diagnosis. Samples were collected in Brazil between March 1990 and December 2001 at the Mycology Laboratory of Hospital São Paulo, São Paulo; at the Department of Community Health, Federal University of Paraná Medical School, Curitiba, Paraná; and at the Mycology Laboratory of UNICAMP, Campinas, São Paulo. Patients were classified according to their clinical presentations. Of the 81 patients studied (73 males and 8 females), 11 had the acute form of PCM and 70 had the chronic form (64 multifocal and 6 unifocal). The mean age of patients with the acute form of the disease was 18.81 years, whereas the mean age of patients with the chronic forms was 46.71. Serum samples (n = 53) obtained at the time of diagnosis from different patients with other mycologically and/or serologically confirmed mycoses were also evaluated. Ninety-three serum samples from healthy volunteers (blood donors) were included as negative controls. To verify cross-reactions, 33 sera from individuals with active histoplasmosis and 20 with cryptococcosis were also tested (Table 1). In addition, we also tested 14 CSF samples and 13 BAL fluid samples obtained from patients with neuroparacoccidioidomycosis and pulmonary PCM, respectively. Also, 11 sample sera from these neuroparacoccidioidomycosis were tested. Six CSF samples obtained from patients with no fungal diseases and 10 BAL fluid samples from tuberculosis were used as negative controls (Table 2).
TABLE 1.
Detection of P. brasiliensis 43-kDa circulating antigen, by inh-ELISA, in sera from various subjects
| Serum group | No. of serum samples tested | No. of reactive samplesa | No. of nonreactive samples | Reactive samples (%) | Mean antigen concn (μg/ml) |
|---|---|---|---|---|---|
| PCM | |||||
| Acute | 11 | 11 | 0 | 100 | 18.23 |
| Chronic unifocal | 6 | 6 | 0 | 100 | 7.64 |
| Chronic multifocal | 64 | 61 | 3 | 95.31 | 8.64 |
| Histoplasmosis | 33 | 0 | 33 | 0 | 0 |
| Cryptococosis | 20 | 0 | 20 | 0 | 0 |
| NHS | 93 | 3 | 90 | 3.22 | 0.08 |
A sample was considered positive (i.e., reactive) if the antigen concentration was >1.35 μg/ml.
TABLE 2.
Detection of P. brasiliensis gp43 antigen, by inh-ELISA, in various specimens obtained from patients with PCM and their respective controls
| Specimen group | No. of serum samples tested | No. of reactive samplesa | No. of nonreactive samples | Reactive samples (%) | Mean antigen concn (μg/ml) |
|---|---|---|---|---|---|
| BAL (from patients with PCM) | 13 | 13 | 0 | 100 | 16.06 |
| CSF (from patients with PCM) | 14 | 14 | 0 | 100 | 19.26 |
| Serum (from patients with neuroparacoccidioidomycosis) | 11 | 10 | 1 | 90.9 | 4.59 |
| BAL (control) | 10 | 0 | 10 | 0 | 0 |
| CSF (control) | 6 | 0 | 6 | 0 | 0 |
| NHS | 30 | 0 | 30 | 0 | 0 |
A sample was considered positive (i.e., reactive) if the antigen concentration was >0.23 μg/ml.
Fungal isolate, exoantigen preparation, and gp43 purification.
P. brasiliensis B-339 (ATCC 200273) was obtained from the culture collection of the Disciplina de Biologia Celular da Universidade Federal de São Paulo. The isolate was transformed to the yeast phase, and exoantigen was produced according to Camargo et al. (4). Gp43 was purified from this exoantigen as described elsewhere (28). Protein determination was performed by the method of Bradford (2).
MAb production.
Monoclonal antibody (MAb) was produced in our laboratory by the method of Puccia and Travassos (29). Six-week-old BALB/c mice were immunized every 3 weeks subcutaneously with 50 μg of gp43 in phosphate-buffered saline (PBS), incorporated into Freund's complete adjuvant for the first injection and in incomplete Freund's adjuvant for the subsequent ones. Injections were always made at four different sites in the axillary and inguinal regions in final volumes of 100 μl per site. Before each immunization, mice were bled through the ocular plexus, and the serum was separated by centrifugation and stored at −20°C. Final immunization (50 μg of gp43 in 100 μl of PBS, intravenously) was performed 2 days before cell fusion, according to the method of Lopes and Alves (18). Screening of positive colonies was performed by an enzyme immunoassay (EIA), as described later. After cloning by limiting dilution and expanding positive clones, large amounts of antibodies were obtained by producing ascites in BALB/c mice previously primed with Pristane (Sigma). MAbs were purified from both culture supernatants and ascites fluids by affinity chromatography on a protein A column. Immunoglobulin (Ig) isotyping was performed with the Clone Selector mouse monoclonal antibody screening kit (Bio-Rad) according to the manufacturer's instructions.
Antibody screening by EIA.
EIA was performed as described by Puccia and Travassos (30). Briefly, polyvinyl microplates (Corning Costar) were coated with 50 μl of a 2-μg/ml solution of purified gp43 in PBS for 1 h at room temperature. After blocking free sites with PBS containing 1% (vol/vol) bovine serum albumin (Sigma) (PBS-BSA), 50 μl of culture supernatant or purified MAbs was added to each well. After 1 h of incubation at 37°C, wells were thoroughly washed with PBS containing 0.5% gelatin (Difco) and 0.05% Tween 20 (Sigma) (PBS-T-G) and treated with affinity-purified peroxidase-conjugated goat anti-mouse Ig (Bio-Rad) for 1 h at 37°C, and this was followed by three washes with PBS-T-G. Reactions were developed by the addition of o-phenylenediamine in 0.1 M acetate-phosphate buffer, pH 5.8; stopped with 4 N sulfuric acid; and read in a Titertek Multiskan EIA reader at 492 nm.
Pretreatment of immune sera for use in inh-ELISA.
Aliquots of immune serum (200 μl) were mixed with an equal volume of 0.1 M EDTA (Sigma), pH 7.2, and boiled at 100°C for 3 to 5 min. The tubes were cooled and centrifuged at 13,000g for 30 min. The supernatant was used for the test.
Inh-ELISA.
The method described by Gómez et al. (16) was followed. An inh-ELISA test was developed for serum, CSF and BAL fluid samples. The diluting buffer used in the serum experiments consisted of a pool of normal human serum (NHS) 1:10 in 0.05% PBS-Tween 20 (PBS-Tween), 20 mM MgCl2, and 1% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.). The diluting buffer for experiments with CSF and BAL fluid was the same but contained a pool of CSF or BAL fluid from individuals with no microbiological disease, respectively, instead of NHS. Purified MAb 17C anti-gp43 was used at 10 μg/ml, and all samples were tested 1:2 in diluting buffer.
Inibition plate.
An inhibition standard curve was constructed by adding different concentrations of P. brasiliensis gp43 (from 1 ng to 30 μg/ml) to 100 μl of pooled NHS (or CSF or BAL) and then adding 100 μl of the standardized concentration of MAb 17C. NHS, CSF, and BAL fluid made up 1:2 in diluting buffer were used as negative controls. All standards, samples, and controls were tested in triplicate. Samples were plated onto 96-well flat microtiter plates (Corning Costar) previously blocked by incubation with 200 μl of 5% BSA per well made up in PBS-Tween, for 2 h at 37°C. Plates were mixed in a shaker for 30 min at room temperature and then incubated overnight at 4°C.
Reaction plate.
Maxisorp polystyrene plates (Corning Costar) were coated with 500 ng of gp43 in 0.06 M carbonate buffer, pH 9.6, per well (100 μl/well). The plates were left at room temperature for 30 min and then incubated overnight at 4°C. After incubation, the plates were washed three times in PBS-Tween and blocked by incubation with 200 μl of 1% BSA in PBS per well for 1 h at 37°C; after three more washes, 100 μl from each well in the inhibition plate (containing a mixture of the MAb-circulating complexes and free MAb) was transferred to the respective wells in the reaction plate and allowed to stand for 2 h at 37°C. After washing as described above, 100 μl of goat anti-mouse IgG-peroxidase (Sigma) was added and the plates were incubated for 1 h at 37°C. After further washings, the reaction was developed with a solution of o-phenylenediamine (0.5 mg/ml; Sigma) and 0.005% H2O2. The reaction was stopped with 4 N H2SO4 after 8 to 10 min of incubation in the dark. Optical densities (ODs) were measured at 490 nm with an ELISA reader (Titertek Multiskan EIA reader). The OD at 492 nm was then plotted on a standard curve constructed from the data derived from MAb titration with NHS containing known quantities of gp43 as described above. The degree of inhibition in MAb binding was shown to be reciprocal to the concentration of circulating antigen in the sample.
The cutoff point was established as the receiver operator characteristic (ROC) curve.
Statistical analysis.
Data were analyzed statistically by the Stata 7.0 version for Windows 98/95/NT (2001 release; Stata Corporation, College Station, Tex.) and specificity, sensitivity and method efficiency were analyzed by the ROC curve. Comparisons were made by one-way analysis of variance.
RESULTS
MAb production.
A panel of different hybridoma lines reactive to P. brasiliensis was produced. MAb 17C belongs to the IgG1 subclass and recognizes an antigenic determinant of P. brasiliensis by ELISA (data not shown) with a relative mass of 43 kDa by Western blotting. This MAb showed no reactivity with Histoplasma capsulatum, Aspergillus fumigatus, or Candida albicans exoantigens and was selected to develop an inh-ELISA test.
Detection of P. brasiliensis antigenemia by inh-ELISA.
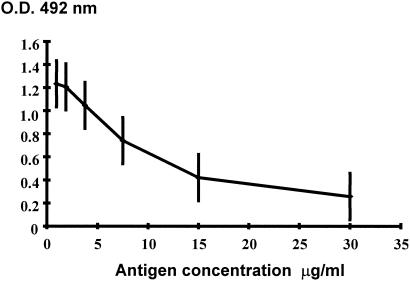
Figure 1 illustrates the standard inhibition curve obtained with known quantities of P. brasiliensis gp43. This curve was used to determine the concentration of P. brasiliensis gp43 in each sample tested. The sensitivity of the inh-ELISA ranged from 0.0053 to 30 μg of antigen per ml of serum. The cut off point was established by the ROC curve, based on the antigen concentration of PCM patients and NHS. Antigen concentrations higher than 1.35 μg/ml were considered to be a positive result.
FIG. 1.
Standard inhibition curve with MAb 17C constructed with known quantities of purified gp43. Error bars, standard deviations.
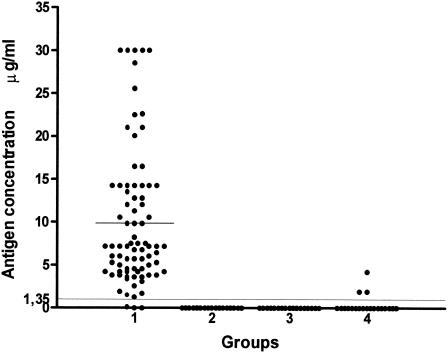
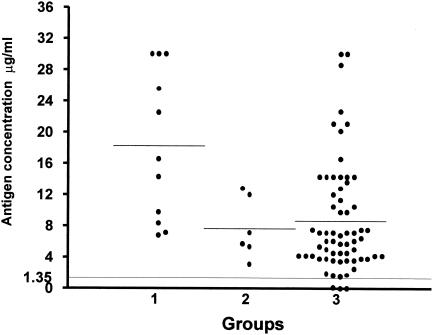
Overall, 96.29% of the 81 PCM serum samples had detectable levels of circulating gp43 antigen above the cut off point (Fig. 2), with a mean antigen concentration of 9.87 μg/ml. Table 1 and Fig. 3 show the results obtained when patients' sera were separated according to the different clinical forms. Circulating gp43 antigen was detectable in all patients with the acute form of PCM (mean, 18.23 μg/ml), and 95.71% of patients with the chronic form (mean, 8.55 μg/ml). Among the chronic form patients, those with the multifocal form (91.42%) had a mean gp43 antigen concentration of 8.64 μg/ml, and those with the unifocal form (8.57%) had a mean gp43 level of 7.64 μg/ml.
FIG. 2.
Detection of circulating antigen in sera from patients with PCM or with other mycoses and in normal human sera by inh-ELISA. Groups studied: 1, PCM (n = 81); 2, cryptococcosis (n = 20); 3, histoplasmosis (n = 33); 4, normal human sera (n = 93). Bars represent the mean antigen concentration for each group. The long, fine line represents the cutoff point (1.35 μg/ml).
FIG. 3.
Detection of circulating antigen in sera from patients with different forms of PCM by Inh-ELISA. Groups studied: 1, acute form (n = 11); 2, chronic unifocal form (n = 6); 3, chronic multifocal form (n = 64). Bars represent the mean antigen concentration for each group. The long, fine line represents the cutoff point (1.35 μg/ml).
No cross-reactions were observed in heterologous serum samples, specifically with sera from patients with histoplasmosis or cryptococcosis. False-positive reactions were observed in 3.22% (3 of 93) of the NHS samples, although the mean antigen concentration for this group was very low (0.08 μg/ml).
Detection of P. brasiliensis gp43 in CSF by the inh-ELISA.
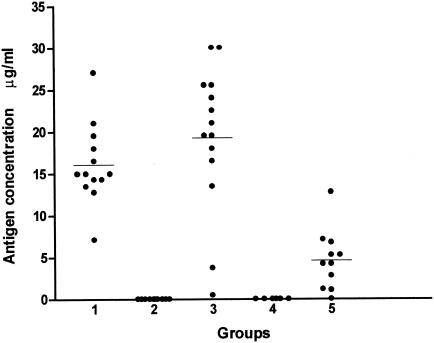
A standard inhibition curve was obtained and the cut off value was determined as 0.23 μg/ml, by the same method used for sera. The sensitivity of the test with CSF was 100% and the mean antigen concentration was equivalent to 19.26 μg/ml. CSF samples from individuals with diseases other than PCM had undetectable antigen levels (Table 2 and Fig. 4).
FIG. 4.
Detection of gp43 antigen in bronchoalveolar lavage from patients with pulmonary PCM and in cerebrospinal fluid from patients with neuroparacoccidioidomycosis by inh-ELISA. Groups studied: 1, bronchoalveolar lavage fluid (n = 13); 2, bronchoalveolar lavage fluid of patients with no fungal pulmonary disease (n = 10) (controls); 3, cerebrospinal fluid from neuroparacoccidioidomycosis patients (n = 14); 4, cerebrospinal fluid from patients with no fungal disease (n = 6); 5, serum from patients with neuroparacoccidioidomycosis (n = 11). Bars represent the average antigen concentration for each group. The cutoff point was 0.23 μg/ml.
Detection of P. brasiliensis gp43 in BAL fluid by the inh-ELISA.
A standard inhibition curve was obtained and the cut off value was determined as 0.23 μg/ml, by the same method used for sera. The sensitivity of the test for BAL fluid samples was 100% and the mean antigen concentration was equivalent to 16.06 μg/ml. BAL fluid samples from individuals with no known infectious diseases had undetectable antigen levels (Table 2 and Fig. 4).
Detection of P. brasiliensis gp43 in serum from neuroparacoccidioidomycosis patients by the inh-ELISA.
Serum samples from patients with neuroparacoccidioidomycosis showed a mean antigen concentration of 4.59 μg/ml, less than that obtained in CSF samples.
DISCUSSION
One of the main problems in the serodiagnosis of PCM based on antibody detection is the cross-reaction obtained with sera from patients with other mycoses, mainly histoplasmosis and lobomycosis. Tests based on immunodiffusion are generally considered to have high specificity in the diagnosis of PCM, but lack in sensitivity. In a previous paper we showed that lack of reactivity of sera from PCM patients in immunodiffusion tests may be related to the production of low-avidity IgG2 antibodies directed against carbohydrate epitopes (27). Estimates of the sensitivity of immunodiffusion-based tests vary from 65 to 100% (6) depending of the kind of antigen preparation used. On the other hand, tests with higher sensitivity, such as immunoenzymatic assays, present problems associated with specificity due to cross-reactivity with heterologous sera, i.e., patients with non-PCM fungal infections.
A more rational approach to the diagnosis of PCM may be the detection of P. brasiliensis-gp43 antigen in body fluids. gp43 is a Pb yeast-phase extracellular antigen and its detection in patients with proven PCM has been tested previously by many approaches. However, these tests varied in sensitivity and specificity, and in most of the studies only a small number of patients were analyzed.
Mendes-Giannini et al. (26) detected the gp43 antigen in pools of PCM patients' sera by Western blot assay, but no quantitation was attempted. Freitas Da Silva and Roque-Barreira (14), using competitive ELISA, detected circulating antigens in 33.7% of 88 PCM patients' sera, whose antigen levels varied from 0.03 to 3.4 μg/ml. However, cross-reactions were obtained with heterologous sera, such as sera from patients with aspergillosis, cryptococcosis and histoplasmosis.
The most refined study of PCM antigenemia was conducted by Gómez et al. (16) using monoclonal antibodies directed against an 87-kDa antigen of Pb (gp87) in an inh-ELISA and detected the presence of antigen in cases of PCM. In this study antigen concentrations as low as 0.0058 μg/ml of serum could be detected in sera from 46 PCM patients with PCM, showing 80.4% sensitivity. Moreover, considering sera of patients with the acute form of the disease, the inh-ELISA exhibited 100% sensitivity, with the highest antigenemia (mean, 16.25 μg/ml), while 83.6% of the patients with the multifocal chronic form of PCM showed a mean antigenemia of 14.92 μg/ml and 60% of patients with the chronic unifocal form showed a mean antigen concentration of 14.7 μg/ml. Cross-reactions with aspergillosis and histoplasmosis were also observed. Also, the authors observed cross-reactions with sera from patients with tuberculosis. They assumed that coinfection with tuberculosis and PCM was a possible explanation for these cross-reactions.
In our study, the inh-ELISA was able to detect gp43 antigen in the concentration ranging from 0.0053 to 30 μg/ml of serum. The overall sensitivity of the test among 81 PCM patients was 95.1%, with a higher sensitivity in patients with the acute form of PCM (100%) with a mean antigen concentration of 18.23 μg/ml. Among the PCM patients with the chronic form, gp43 was detected in 95.71%, with mean concentration of 8.55 μg/ml. Our results showed that the gp43 detection assay can be used for both clinical forms of the disease. Consequently, the gp43 antigen detection test may be a valuable tool in PCM diagnosis. Gp43 detection by this inh-ELISA showed 95.1% sensitivity which was higher than the 80.4% sensitivity previously reported by Gómez et al. to detect the 87-kDa antigen, and also much higher than the 33.7% sensitivity previously described by Freitas Da Silva and Roque-Barreira (14, 16).
The high percentage of positivity observed when the inh-ELISA antigen test was used for the neuroparacoccidioidomycosis group of patients, suggests that this test format may offer a more sensitive test for this difficult to diagnose form of PCM. Another interesting application for the inh-ELISA was the detection of gp43 in BAL fluid specimens showing the high sensitivity and specificity (100%) of the assay, since BAL fluid is extremely diluted. This opens a new possibility in the diagnosis of PCM, when the disease is in its initial phase in the lungs and no sputum is available for direct observation of the fungus.
Detection of low amounts of gp43 in some normal serum controls may represents PCM subclinical infection in normal people. PCM subclinical infection is defined as an asymptomatic infection caused by P. brasiliensis in normal individuals who live in an endemic area and prove reactive to the paracoccidioidin skin test (13). Positive paracoccidioidin skin testing in health population may vary from 3.70 to 62.60%, depending on the kind of antigenic preparation (9). In our previous study, using gp43 or a polysaccharide antigen (Fava Netto's antigen) as paracoccidioidin, 5% of healthy people reacted positively to both antigens (33).
Limited amounts of MAb anti-gp43 for detection of gp43 antigen can be provided by our laboratory to serve as standards for those willing to prepare it in their own laboratories. Scaling up of MAb production will depend on the number of requests addressed to our department and on the costs involved.
In conclusion, we propose the use of inh-ELISA, using a species-specific MAb, as an additional test for the diagnosis of PCM in clinical laboratory. The use of the 43-kDa antigen detection system described here will provide a rapid, sensitive, and specific mean to diagnose and differentiate between acute and chronic disease. Moreover, this test system's true potential may lie in tracking the course of antimycotic therapy by monitoring the reduction in fungal load as evidenced by a sequential reduction in antigen detected. This will be the subject of future studies.
Acknowledgments
This work was supported by a grant from FAPESP.
REFERENCES
- 1.Blotta, M. H. S. L, and Z. P. Camargo. 1993. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: relationships with clinical forms of paracoccidioidomycosis. J. Clin. Microbiol. 31:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Camargo, Z. P., J. L. Guesdon., E. Drouhet, and L. Improvisi. 1984. Enzyme-linked immunosorbent assay (ELISA) in paracoccidioidomycosis. Mycopathologia 88:31-37. [DOI] [PubMed]
- 4.Camargo, Z. P., C. Unterkircher, S. P. Campoy, and L. R. Travassos. 1988. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion test. J. Clin. Microbiol. 26:2147-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camargo, Z. P., C. S. Unterkircher, and L. R. Travassos. 1989. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J. Med. Vet. Mycol. 27:407-412. [PubMed] [Google Scholar]
- 6.Cano, L. E., and A. Restrepo. 1987. Predictive value of serologic tests in the diagnosis and follow-up pf patients with paracoccidioidomycosis. Rev. Inst. Med. Trop. São Paulo 29:276-283. [DOI] [PubMed] [Google Scholar]
- 7.Conti-Diaz, I. A., J. E. Mackinnon, L. Calegari, and S. Casserone. 1978. Estudio comparativo de la inmunoelectroforesis-immunodifusión (IEOF-I D) y de la inmunoelectroforesis (IEF) en el diagnóstico de la paracoccidioidomicosis. Mycopathologia 63:161-165. [DOI] [PubMed] [Google Scholar]
- 8.Dupont, B., M. Huber, S. J. Kim, and J. E. Bennett. 1987. Galactomannan antigenemia and antigenuria in aspergillosis studies in patients and experimentally infected rabbits. J. Infect. Dis. 155:1-11. [DOI] [PubMed] [Google Scholar]
- 9.Fava, S. C., and C. Fava Netto. 1998. Epidemiologic surveys of histoplasmin and paracoccidioidin sensitivity in Brazil. Rev. Inst. Med. Trop. São Paulo 40:155-164. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira-da-Cruz, M. F., B. Galvão-Castro, and C. T. Daniel-Ribeiro. 1991. Sensitive immunoradiometric assay for the detection of Paracoccidioides brasiliensis antigens in human sera. J. Clin. Microbiol. 29:1202-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira, R. P., B. Yu, Y. Niki, and D. Armstrong. 1990. Detection of Candida antigenuria in disseminated candidiasis by immunoblotting. J. Clin. Microbiol. 28:1075-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco, M. 1986. Host-parasite relationships in paracoccidioidomycosis. J. Med. Vet. Mycol. 25:5-18. [DOI] [PubMed] [Google Scholar]
- 13.Franco, M., Mendes, R. P., M. Moscardi-Bacchi, M. Rezkallah-Iwasso, and M. R. Montenegro. 1989. Paracoccidioidomycosis. Ballieres Clin. Trop. Commun. Dis. 4:185. [Google Scholar]
- 14.Freitas-da-Silva, G., and M. C. Roque-Barreira. 1992. Antigenemia in paracoccidioidomycosis. J. Clin. Microbiol. 30:381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia, N. M., G. B. Del Negro, H. P. Martins, and C. S. Lacaz. 1987. Detection of Paracoccidioides brasiliensis circulating antigens by immunoelectrophoresis-immunodiffusion technique. Preliminary report. Rev. Med. Trop. São Paulo 29:327-328. [DOI] [PubMed] [Google Scholar]
- 16.Gómez, B. L., J. I. Figueroa, A. J. Hamilton, B. Ortiz, M. A. Robledo, R. J. Hay, and A. Restrepo. 1997. Use of monoclonal antibody in diagnosis of paracoccidioidomycosis: new strategies for detection of circulating antigens. J. Clin. Microbiol. 35:3278-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes, K. A., J. P. Latge, and T. R. Rogers. 1990. Detection of Aspergillus antigens associated with invasive infection. J. Clin. Microbiol. 28:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes, J. D., and M. J. M. Alves. 1983. Production of monoclonal antibodies by somatic cell hybridization, p. 386-398. In C. M. Morel (ed.), Genes and parasites: a laboratory manual. Fundaçao Osvaldo Cruz, Rio de Janeiro, Brazil.
- 19.Magaldi, S. W., D. W. R. Mackenzie. 1986. Detection de antigenemia y anticuerpos de paracoccidioides mediante procedimentos electroforéticos invertidos, p. 80. In Proceedings Coloquio Internacional sobre la Paracoccidioidomicosis Corporación para Investigaciones Biológicas, Medellín, Colombia.
- 20.Magaldi, S. W., D. W. R. Mackenzie, and M. B. Albornoz. 1989. Detection of Paracoccidioides brasiliensis circulating antigen by the passive hemagglutination inhibition in patients sera, p. 18. In Proceedings Encuentro International sobre Paracoccidioidomicosis. Instituto Venezolano de Investigaciones Cientificas, Caracas, Venezuela.
- 21.Mathews, R. C. 1996. Comparative assessment of the detection of candidal antigens as a diagnostic tool. J. Med. Vet. Mycol. 34:1-10. [DOI] [PubMed] [Google Scholar]
- 22.McEwen, J. G., A. M. Garcia, B. L. Ortiz, S. Botero, and A. Restrepo. 1995. In search of the natural habitat of Paracoccidioides brasiliensis. Arch. Med. Res. 26:305-306. [PubMed] [Google Scholar]
- 23.Mendes-Giannini, M. J., M. E. Camargo, C. A. Lacaz, and A. W. Ferreira. 1984. Immunoenzymatic absorption test for serodiagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 20:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendes-Giannini, M. J., G. B. Del Negro, and A. M. Siqueira. 1994. Serodiagnosis, p. 345-363. In M. Franco, C. S. Lacaz, A. Restrepo, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, Fla.
- 25.Mendes-Giannini, M. J., J. P. Bueno, M. A. Shikanai-Yassuda, A. M. S. Stolf, A. Masuda, V. Amato-Neto, and A. W. Ferreira. 1990. Antibody responses to 43 kDa glycoprotein of Paracoccidioides brasiliensis as a marker for the evaluation of patients under treatment. Am. J. Trop. Med. Hyg. 43:200-206. [DOI] [PubMed] [Google Scholar]
- 26.Mendes-Giannini, M. J. S., J. P. Bueno, M. A. Skikanai-Yasuda, A. W. Ferreira, and A. Masuda. 1989. Detection of the 43, 000-molecular-weight glycoprotein in sera of patients with paracoccidioidomycosis. J. Clin. Microbiol. 27:2842-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neves, A. R., R. L. Mamoni, Z. P. Camargo, and M. H. S. L. Blotta. Negative immunodiffusion test results obtained with sera of paracoccidioidomycosis patients may be related to low-avidity immunoglobulin G2 antibodies directed against carbohydrate epitopes. Clin. Diagn. Lab. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 28.Puccia, R., S. Schenkman, P. A. J. Gorin, and L. R. Travassos. 1986. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect. Immun. 53:199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puccia, R., and L. R. Travassos. 1991. The 43-kDa glycoprotein from the human pathogen Paracoccidioides brasiliensis and its deglycosylated form: excretion and susceptibility to proteolysis. Arch. Biochem. Biophys. 289:298-302. [DOI] [PubMed] [Google Scholar]
- 30.Puccia, R., and L. R. Travassos. 1991. 43-Kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis or Jorge Lobo's disease. J. Clin. Microbiol. 29:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Restrepo, A. 1966. La prueba de inmunodifusíon en el diagnóstico de la paracoccidioidomicosis. Sabouraudia 4:223-230. [PubMed] [Google Scholar]
- 32.Rodrigues, M. C., C. M. Cassaguerra, and C. S. Lacaz. 1984. Antigenemia in paracoccidioidomycosis. Probable demonstration of circulating antigen by counterimmunoelectrophoresis test. Rev. Inst. Med. Trop. São Paulo 26:285-287. [DOI] [PubMed] [Google Scholar]
- 33.Saraiva, E. C. O., A. Altemani, M. Franco, C. S. Unterkircher, and Z. P. Camargo. 1986. Paracoccidioides brasiliensis-gp43 used as paracoccidioidin. J. Med. Vet. Mycol. 34:155-161. [DOI] [PubMed] [Google Scholar]
- 34.Stambuk, B. U., R. Puccia, M. L. C. Almeida, L. R. Travassos, and S. Schenkman. 1988. Secretion of the 43 kDa glycoprotein antigen by Paracoccidioides brasileinsis. J. Med. Vet. Mycol. 26:367-373. [DOI] [PubMed] [Google Scholar]
- 35.Taborda, C. P., and Z. P. Camargo. 1993. Diagnosis of paracoccidioidomycosis by passive haemagglutination assay of antibody using a purified and specific antigen—gp43. J. Med. Vet. Mycol. 31:155-160. [DOI] [PubMed] [Google Scholar]
- 36.Taborda, C. P., and Z. P. Camargo. 1994. Diagnosis of paracoccidioidomycosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J. Clin. Microbiol. 32:554-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taborda, C. P., M. A. Juliano, R. Puccia, M. Franco, and L. R. Travassos. 1998. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect Immun. 66:786-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vicentini, A. P., J-L. Gesztesi, M. F. Franco, W. Souza, J. Z. Moraes, L. R. Travassos, and J. D. Lopes. 1994. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect. Immun. 62:1465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanke, B., and A. T. Londero. 1994. Epidemiology and paracoccidioidomycosis infection, p. 109-120. In M. Franco, C. S. Lacaz, A. Restrepo, and G. Del Negro (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, Fla.