Abstract
A number of anticancer and antiparasitic drugs are postulated to target the polyamine biosynthetic pathway and polyamine function, but the exact mode of action of these compounds is still being elucidated. To establish whether polyamine analogs specifically target enzymes of the polyamine pathway, a model was developed using strains of the protozoan parasite Leishmania donovani that overproduce each of the polyamine biosynthetic enzymes. Promastigotes overexpressing episomal constructs encoding ornithine decarboxylase (ODC), S-adenosylmethionine decarboxylase (ADOMETDC), or spermidine synthase (SPDSYN) revealed robust overproduction of the corresponding polyamine biosynthetic enzyme. Polyamine pools, however, were either unchanged or only marginally affected, implying that regulatory mechanisms must exist. The ODC, ADOMETDC, and SPDSYN overproducer strains exhibited a high level of resistance to difluoromethylornithine, 5′-{[(Z)-4-amino-2-butenyl]methylamino}-5′-deoxyadenosine, and n-butylamine, respectively, confirming previous observations that these agents specifically target polyamine enzymes. Conversely, augmented levels of polyamine biosynthetic enzymes did not affect the sensitivity of L. donovani promastigotes to pentamidine, berenil, and mitoguazone, drugs that were postulated to target the polyamine pathway, implying alternative and/or additional targets for these agents. The sensitivities of wild-type and overproducing parasites to a variety of polyamine analogs were also tested. The polyamine enzyme-overproducing lines offer a rapid cell-based screen for assessing whether synthetic polyamine analogs exert their mechanism of action predominantly on the polyamine biosynthetic pathway in L. donovani. Furthermore, the drug resistance engendered by the amplification of target genes and the overproduction of the encoded protein offers a general strategy for evaluating and developing therapeutic agents that target specific proteins in Leishmania.
Polyamines are ubiquitous organic cations that play critical but still not completely defined roles in key cellular processes such as cell proliferation, differentiation, and nucleic acid synthesis (6, 17, 19, 34, 35, 59, 65). Since polyamines are especially important to rapidly growing cells, the polyamine pathway has been targeted in a multiplicity of antineoplastic and antiparasitic drug regimens. Most notably, dl-α-difluoromethylornithine (DFMO), an irreversible inhibitor of ornithine decarboxylase (ODC), the first enzyme in the polyamine biosynthesis pathway, is effectively curative against late-stage African sleeping sickness caused by the protozoan parasite Trypanosoma brucei gambiense (3, 14, 57). Interestingly, the selectivity of DFMO for the metabolic machinery of the parasite is not brought about by differential sensitivities of the parasite and human ODC enzymes to inactivation by DFMO but is rather due to a novel mechanism involving disparities in ODC turnover rates between T. brucei and the mammalian host (22, 23). DFMO is also active against other trypanosome species in mouse models and has proven effective against other genera of protozoan parasites including Plasmodium species (2, 12, 13), Giardia (24), and Leishmania (44, 52). Other inhibitors of enzymes involved in polyamine biosynthesis have also shown efficacy against parasites. For instance, 5′-{[(Z)-4-amino-2-butenyl]methylamino}-5′-deoxyadenosine (MDL73811), an inhibitor of S-adenosylmethionine decarboxylase (ADOMETDC) whose product provides the aminopropyl moieties for spermidine and spermine synthesis, is effective at eradicating T. brucei infections in rodent models (4). Many other polyamine synthesis inhibitors and antimetabolites have been synthesized, but the mechanisms of action of these compounds are mostly unknown and not easily ascertained (6, 17, 19, 34, 35, 59, 65).
Due to the absence of effective vaccines, chemotherapy has offered the only avenue for treating and preventing parasitic diseases. Unfortunately, the current arsenal of antiparasitic drugs is far from ideal, mainly because the drugs exhibit cytotoxicity due to a lack of target specificity. Thus, the need for more selective and efficacious drugs to treat or prevent parasitic diseases is imperative. The success of DFMO against African trypanosomiasis has stimulated considerable interest in the polyamine pathways of parasites and in evaluating other biosynthesis inhibitors as well as polyamine analogs as potential antiparasitic drugs.
Leishmania donovani, a diploid protozoan parasite that is closely related to T. brucei, is the causative agent of visceral leishmaniasis, a devastating and invariably fatal disease if untreated. The parasite exhibits a digenetic life cycle, with the extracellular promastigote residing in the sandfly vector and the intracellular amastigote form inhabiting the phagolysosome of mammalian macrophages. The polyamine biosynthetic pathway of L. donovani consists of three enzymes: ODC, ADOMETDC, and spermidine synthase (SPDSYN). There is no spermine synthase encoded in the leishmanial genome (L. major GeneDB), and the parasites lack spermine (36), a major polyamine in the mammalian host. The polyamine auxotrophy exhibited by L. donovani promastigotes in which both copies of ODC, ADOMETDC, or SPDSYN have been genetically eliminated has established the essential roles of these enzymes in parasite viability and proliferation (36, 53, 54). Moreover, the phenotypic characterization of these null mutants revealed significant differences between the polyamine pathways of the parasite and humans (36, 53, 54), implying that these enzymes in Leishmania and possibly other parasites have potential as targets for antiparasitic drugs (5, 16, 21, 31, 51).
Although many polyamine and ornithine analogs display antiparasitic effects, their mechanisms of action have not been demonstrated, with the single exception of DFMO (22, 23). In principle, polyamine analogs could inhibit the polyamine biosynthetic pathway, displace polyamines from performing their functions, or disrupt unrelated cellular processes. In a previous study, L. donovani ADOMETDC was overexpressed in L. donovani promastigotes, and this overexpression conferred profound resistance to MDL73811 but not to pentamidine, berenil, and mitoguazone (MGBG) (54). Those studies suggested that polyamine biosynthetic enzyme-overproducing strains could be useful in identifying whether various polyamine analogs exert their cytotoxicity predominantly by inhibiting specific enzymes of the polyamine biosynthetic pathway. We now report the generation and characterization of L. donovani lines that overexpress ODC and SPDSYN. The polyamine biosynthetic enzyme-overproducing strains were then exploited to ascertain the mode of action of a battery of polyamine analogs in L. donovani promastigotes.
MATERIALS AND METHODS
Materials, chemicals, and reagents.
DFMO and MDL73811 were obtained from Marion Merrell Dow Research Institute (Cincinnati, OH). The polyamine analogs were generated by Cellgate Inc. (Redwood City, CA). All restriction enzymes were purchased from either Invitrogen Corp. (Carlsbad, CA), Gibco-BRL Life Technologies Inc. (Gaithersburg, MD), or New England Biolabs, Inc. (Beverly, MA). Synthetic oligonucleotides were acquired from Invitrogen Corp. (Carlsbad, CA). Advantage HF2 DNA polymerase was purchased from BD Bioscience (Palo Alto, CA), Pfu Turbo DNA polymerase was acquired from Stratagene (La Jolla, CA), and Taq DNA polymerase was purchased from Promega Corp. (Madison, WI). Geneticin (G418) was procured from BioWhitaker (Walkersville, MD). Putrescine, spermidine, and n-butylamine were purchased from Sigma-Aldrich Corp. (St. Louis, MO). The transfection vectors pSNBR and pSNAR were constructed by and obtained from Stephen M. Beverley (Washington University, St. Louis, MO) (40).
Cell culture.
Wild-type L. donovani clone DI700, originally derived from the 1S Sudanese strain, was the recipient strain for transfections. Parasites were cultivated in DME-L, a completely defined Dulbecco's modified Eagle-based medium that was specifically developed for growing Leishmania promastigotes (33). Parasites grown in the presence of putrescine or spermidine were maintained in a modified DME-L medium, DME-L-CS, in which the bovine serum albumin component of DME-L was replaced with 10% chicken serum to avert polyamine oxidase-mediated polyamine toxicity (37). Transfected parasites were maintained in 100 μg/ml G418 but were cultured in media without G418 for growth experiments. To assess the effects of drugs on parasite growth, 1.0-ml volumes in 24-well plates were seeded at 1.0 × 105 parasites/ml, and parasites were enumerated after 5 days by Coulter Counter model ZF as described previously (36, 37).
Overexpression vectors.
The construction of the ADOMETDC overexpression vector pXNEO[ADOMETDC] has been described previously (54). To construct the ODC overexpression vector pSNBR[ODC], a 15-kb HindIII-EcoRI ODC-containing fragment from the ODC-5L1 cosmid (36) was inserted into the polylinker site of the pSNBR vector. The SPDSYN overexpression vector pSNAR[SPDSYN] was created by cloning a 4.5-kb BamHI-HindIII genomic fragment containing the SPDSYN coding region (53) into the polylinker site of pSNAR.
Transfections.
The construction of the ADOMETDC-overproducing L. donovani strain (DI700[pADOMETDC]) has been described previously (54). The ODC or SPDSYN overproducer strains (DI700[pODC] and DI700[pSPDSYN]) were made by transfecting wild-type L. donovani parasites with pSNBR[ODC] or pSNAR[SPDSYN], respectively, using previously reported electroporation conditions (36). Parasites harboring episomal constructs were selected with 100 μg/ml G418.
Immunoblotting.
The sources of rabbit polyclonal antibodies to purified L. donovani ODC, SPDSYN, and ADOMETDC have been reported previously (36, 53, 54). Promastigote lysates were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (38, 56), blotted onto nitrocellulose membranes using a Semi-Dry Electrophoresis Transfer Cell (Bio-Rad), and subjected to Western blot analysis according to standard protocols (56). Band intensities were quantitated by densitometry using an Epson Perfection 2400 PHOTO scanner (Epson America Inc., Long Beach, CA), and the intensity of each band was measured by the Alpha Ease FC software program (Alpha Innotech Corp., San Leandro, CA).
Polyamine pool analysis.
Polyamine pools were measured as reported previously (54). Briefly, wild-type and transfected parasites were grown for 3 days, and 1 × 107 parasites were harvested in the mid-logarithmic growth phase. Acid-soluble extracts were analyzed for putrescine and spermidine contents by reverse-phase high-performance liquid chromatography.
RESULTS
Generation of polyamine biosynthetic enzyme overproducer lines.
Parasites that overproduce ODC and SPDSYN were created by transfecting pSNBR[ODC] and pSNAR[SPDSYN], respectively, into wild-type L. donovani DI700. The episomes in the DI700[pODC] and DI700[pSPDSYN] transfectants were then amplified by selection in 100 μg/ml G418, and Southern blot analysis confirmed the presence of the plasmids in the selected parasites (data not shown). These analyses revealed that the transfectants harbored several hundred copies of the corresponding episome (data not shown). The construction of an ADOMETDC expression plasmid, transfection, and selection of episome-bearing parasites have been described previously (54).
Phenotypic characterization of polyamine biosynthetic enzyme overproducer strains.
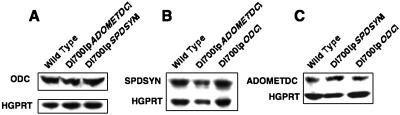
None of the three L. donovani strains transfected with plasmids encoding polyamine biosynthetic enzymes exhibited any growth defects, and their doubling times were equivalent to that of wild-type parasites (data not shown). Western blot analysis revealed robust overexpression of ODC, SPDSYN, and ADOMETDC in the relevant DI700[pODC], DI700[pSPDSYN], and DI700[pADOMETDC] transfectants, respectively (Fig. 1). Quantification of enzyme expression by densitometry indicated that levels of ODC, SPDSYN, and ADOMETDC in lysates from the transfectants were elevated ∼46-fold, ∼60-fold, and ∼25-fold compared to the nontransfected parental cell extracts. Levels of the other polyamine biosynthesis enzymes in the DI700[pODC], DI700[pSPDSYN], and DI700[pADOMETDC] transfectants were virtually unchanged compared to the wild-type line (Fig. 2).
FIG. 1.
Western blot analysis of ODC, SPDSYN, and ADOMETDC protein levels in wild-type and genetically altered L. donovani parasites. Cell lysates from wild-type (WT) cells or polyamine enzyme-overproducing strains (DI700[pODC], DI700[pSPDSYN], and DI700[pADOMETDC]) undiluted (1:1) or in various dilutions as indicated were probed with polyclonal antiserum against ODC, SPDSYN, or ADOMETDC.
FIG. 2.
Western blot analysis of polyamine pathway protein expression in wild-type and genetically altered parasites. (A to C) Lysates from wild-type, DI700[pODC], DI700[pSPDSYN], and DI700[pADOMETDC] strains were applied onto sodium dodecyl sulfate-polyacrylamide gels and probed with polyclonal antiserum against ODC (A), SPDSYN (B), or ADOMETDC (C). Each blot was also probed with an antiserum against hypoxanthine-guanine phosphoribosyltransferase (HGPRT) protein to demonstrate equal loading of parasite lysates.
The consequences of increased enzyme levels on intracellular polyamine pools were also examined (Fig. 3). The DI700[pODC] line exhibited a sevenfold increase in putrescine pools and a twofold increase in spermidine levels compared to its wild-type counterpart, while a threefold augmentation in putrescine levels was observed in the DI700[pSPDSYN] transfectant. DI700[pADOMETDC] ADOMETDC overproducers exhibited putrescine and spermidine levels similar to those of wild-type parasites.
FIG. 3.
Polyamine pool measurements in wild-type and genetically altered parasites. The cellular contents of putrescine (black columns) and spermidine (white columns) were measured in wild-type (WT), DI700[pODC], DI700[pSPDSYN], and DI700[pADOMETDC] parasites in triplicate.
Effect of DFMO, MDL73811, and n-butylamine on wild-type and overproducer strains.
DFMO, MDL73811, and n-butylamine are known to inhibit the enzymes ODC, ADOMETDC, and SPDSYN, respectively, in vitro (15, 20, 25, 26, 50). Thus, it was conjectured that these agents will also specifically target these enzymes in vivo and that augmented levels of ODC, ADOMETDC, and SPDSYN will protect the parasites from the toxicity of the compounds. As shown in Table 1, ODC, ADOMETDC, and SPDSYN overproducer strains were markedly resistant to DFMO, MDL73811, and n-butylamine, respectively, compared to wild-type parasites. Likewise, putrescine supplementation protected wild-type parasites from DFMO toxicity, and spermidine supplementation shielded the parasites from the inhibitory effects of MDL73811 and n-butylamine. It should be noted that the ODC overproducer also exhibited an increased resistance to n-butylamine. Thus, n-butylamine may not be a specific inhibitor of SPDSYN but may also act on ODC. These assays were performed as proof of concept that polyamine biosynthetic enzyme overproducer strains can be exploited to determine target specificities of synthetic polyamine analogs.
TABLE 1.
EC50 values for DFMO, MDL 73811, and n-butylaminea
| Strain | Mean EC50 (mM) ± SD
|
||
|---|---|---|---|
| DFMO | MDL73811 | n-Butylamine | |
| Wild type | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.4 ± 0.3 |
| DI700[pODC] | >1 | 2.7 ± 0.5 | 2.1 ± 0.4 |
| DI700[pSPDSYN] | 0.04 ± 0.01 | 0.2 ± 0.1 | 3.3 ± 0.8 |
| DI700[pADOMETDC] | 0.03 ± 0.02 | >10 | 0.2 ± 0.07 |
| Wild type + putrescine | >1 | 0.8 ± 0.2 | 2.0 ± 0.4 |
| Wild type + spermidine | 0.13 ± 0.08 | >10 | >10 |
The EC50 values for wild-type parasites, wild-type parasites grown in the presence of 100 μM putrescine or 100 μM spermidine, and ADOMETDC-, ODC-, or SPDSYN-overproducing parasites were determined. Each value is the mean and standard deviation of three independent experiments.
Effect of pentamidine, berenil, and MGBG on wild-type and polyamine biosynthetic enzyme overproducer strains.
Pentamidine, berenil, and MGBG have previously been postulated to exert their toxicity by inhibiting the polyamine biosynthetic pathway (7, 11, 45). However, other cellular targets have also been proposed (8, 9, 41, 43, 62). To determine whether these compounds specifically target the polyamine biosynthetic enzymes or interact with other cellular functions in intact organisms, the proliferation of wild-type and overproducer strains in the presence of these drugs was compared. The 50% effective concentration (EC50) values for wild-type parasites, parasites supplemented with putrescine or spermidine, and ODC, ADOMETDC and SPDSYN overproducer strains were nearly equivalent (Table 2). This result suggests that although pentamidine, berenil, and MGBG may inhibit polyamine biosynthetic enzymes in vivo, their primary cellular toxicity is mediated through other cellular interactions.
TABLE 2.
EC50 values for pentamidine, berenil, and MGBGa
| Strain | Mean EC50 ± SD
|
||
|---|---|---|---|
| Pentamidine (μM) | Berenil (μM) | MGBG (mM) | |
| Wild type | 1.9 ± 0.2 | 9.5 ± 3.9 | 1.8 ± 0.4 |
| DI1700[pODC] | 2.6 ± 0.3 | 8.7 ± 1.2 | 2.2 ± 0.3 |
| DI700[pSPDSYN] | 1.7 ± 0.2 | 8.0 ± 3.0 | 1.4 ± 0.3 |
| DI700[pADOMETDC] | 1.7 ± 0.2 | 9.7 ± 2.4 | 1.5 ± 0.6 |
| Wild type + putrescine | 1.9 ± 0.2 | 6.1 ± 1.5 | 2.6 ± 0.1 |
| Wild type + spermidine | 2.0 ± 0.4 | 9.7 ± 2.4 | 1.8 ± 0.5 |
The EC50 values for wild-type parasites, wild-type parasites grown in the presence of 100 μM putrescine or 100 μM spermidine, and ADOMETDC-, ODC-, or SPDSYN-overproducing parasites were established. Each value is the mean and standard deviation of three independent experiments.
Screening of polyamine analogs.
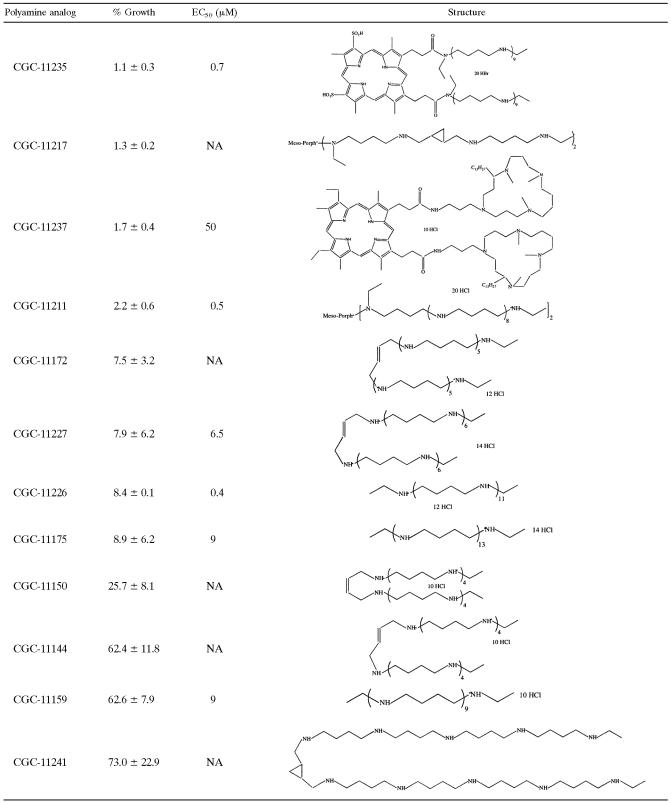
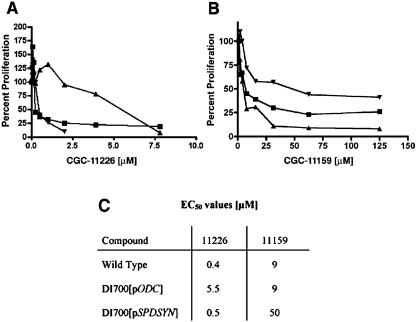
To assess whether the polyamine overproducer lines could be exploited as a cell-based screen to evaluate the cellular targets of synthetic polyamine analogs, a pilot test was performed with 25 compounds provided by Cellgate on wild-type L. donovani promastigotes. Of the 25 Cellgate compounds, 8 were cytotoxic and inhibited growth of the parasite by >90% at a concentration of 100 μM, 4 inhibited growth by 25 to 75%, and the remainder exhibited no inhibitory effect or even resulted in a higher cell density than the no-drug controls (Table 3). The potencies of some of the most cytotoxic reagents were then further evaluated at lower doses. The effective concentrations of polyamine analogs CGC-11235, CGC-11237, CGC-11211, CGC-11227, CGC-11226, CGC-11175, and CGC-11159 that inhibited cell growth by 50% (EC50 values), a practical measure of drug potency, ranged from 0.4 to 50 μM (Table 3). The eight most cytotoxic compounds were also tested against the DI700[pODC] and DI700[pSPDSYN] overproducer strains. EC50 values of 0.4 and 0.5 μM for wild-type and DI700[pSPDSYN] promastigotes were determined for CGC-11226, while for DI700[pODC] cells, the EC50 value was increased by an order of magnitude to 5.5 μM (Fig. 4A and C). In contrast, EC50 values of 9 μM for CGC-11159 were obtained for wild-type L. donovani and the DI700[pODC] overproducer, whereas an EC50 value of 50 μM was calculated for the DI700[pSPDSYN] strain.
TABLE 3.
Toxicity of synthetic polyamine analogs to wild-type parasitesaa
FIG. 4.
Growth curves of wild-type and genetically altered parasites in the presence of polyamine analogs. Wild-type (▪), DI700[pODC] (▴), and DI700[pSPDSYN] (▾) parasites were seeded at 5 × 105 cells/ml in serial dilutions of compound CGC-11226 (A) or compound CGC-11159 (B). After 5 days, cell numbers were enumerated using a Coulter Counter. Cell numbers obtained in media without compound was taken as 100% proliferation. (C) EC50 values for compounds CGC-11226 and CGC-11159 are given.
DISCUSSION
The critical role that polyamines play in cell proliferation, differentiation, and development (6, 17, 19, 34, 35, 59, 65) coupled with the success of DFMO in the treatment of African trypanosomiasis (3, 14, 57) have stimulated considerable interest in the polyamine pathway as a target for potential antiparasitic chemotherapies. Each of the three enzymes of the leishmanial polyamine biosynthetic pathway has been genetically validated as a prospective drug target via the creation of Δodc, Δspdsyn, and Δadometdc knockouts in L. donovani by double-targeted gene replacement that all exhibited polyamine auxotrophy (36, 53, 54). Leishmania promastigotes and axenic amastigotes (10) are able to transport polyamines, and a polyamine permease has been identified at the molecular level in Leishmania major (28). It is unknown, however, whether Leishmania in the amastigote form is capable of scavenging polyamines from the host phagolysosome.
Despite the plethora of drugs that have been reported to target polyamine biosynthesis in protozoan parasites, there is little evidence, other than that obtained with DFMO (22, 23), that establishes the polyamine biosynthetic machinery as the true primary intracellular target of these antiparasitic agents. Polyamine analogs, antimetabolites, or related drugs could also be cytotoxic by displacing natural polyamines or by disrupting unrelated cellular functions. In order to establish whether polyamine analogs actually target the pathway, we have created individual transgenic L. donovani strains that have been transfected with episomal constructs harboring each one of the polyamine biosynthesis genes and that consequently overproduce the encoded protein by 25- to 60-fold compared to wild-type parasites (Fig. 1). Each of these transfectant lines, DI700[pODC], DI700[pSPDSYN], and DI700[pADOMETDC], is theoretically isogeneic with their wild-type parent except for the episomal sequences, thereby facilitating target identification through phenotypic discrepancies. Our reverse genetic stratagem clearly indicates that the primary intracellular targets of DFMO and MDL73811 are ODC and ADOMETDC, respectively. Conversely, the cellular toxicities of pentamidine, berenil, and MGBG, all drugs postulated to inhibit polyamine metabolism (7, 11, 45), do not appear to be mediated through the targeting of the polyamine biosynthetic machinery in L. donovani promastigotes since the cytotoxicity profiles of the overproducer strains are virtually identical to those of wild-type parasites. These results reinforce previous observations that pentamidine, berenil, and MGBG have other cellular targets (8, 9, 41, 62).
It should be noted that the generation of some ODC and any ADOMETDC or SPDSYN overexpressor strains has been difficult to achieve in mammalian cell lines, presumably due to their sensitivity to internal polyamine levels (32, 42, 46, 60). An increase in polyamines is often associated with cancer or apoptosis, depending on the cell and tissue type (59, 63, 64, 66, 68, 70). Mammalian ODC and ADOMETDC are subjected to an intricate regulatory network that includes the regulation of expression and degradation (18, 48, 49, 58, 61, 67). Indeed, ODC and ADOMETDC are among the most labile mammalian proteins known (29, 30, 47, 69). Intriguingly, the leishmanial polyamine biosynthetic enzymes are stable proteins that show no signs of degradation after 24 h (27, 54), suggesting that different regulatory mechanisms maintain a stable intracellular polyamine level in these parasites. Thus, it may not be surprising that L. donovani strains that robustly overproduce ODC, SPDSYN, or ADOMETDC but that maintained relatively stable polyamine levels in all three cell lines could be generated.
Our inhibition studies with DFMO, MDL73811, and n-butylamine confirm the proof of principle that polyamine biosynthetic enzyme overproducer strains can be exploited to identify compounds that specifically inhibit the polyamine pathway enzymes in vivo. A pilot screen in which 25 polyamine analogs were tested against the overproducer strains ascertained that the polyamine biosynthetic enzyme-overproducing L. donovani strain can serve as a foundation for a cell-based screening assay to identify specific inhibitors of the polyamine biosynthetic enzymes. While this assay was performed with the extracellular promastigote form of the parasite, it can be extended to evaluate the effects of polyamine analogs on axenic amastigotes and intracellular amastigotes in macrophages as well. Furthermore, this screening assay can presumably be optimized by transfecting wild-type parasites and overproducer strains with a luciferase expression construct, thereby enabling the rapid and automated detection of bioluminescent signals as proliferation indicators (1, 39, 55). A battery of polyamine analogs has already been synthesized by industry and academia as antiproliferative agents, and a systematic testing of these compounds as antiparasitic agents is greatly overdue and desirable in view of the dire need of new therapeutic agents against leishmaniasis. Significantly, our approach using polyamine biosynthetic enzyme-overproducing strains presents a generally applicable strategy that can be used to screen for specific inhibitors of any protein that can be overproduced in Leishmania. In addition, the Leishmania system may provide a powerful tool for the heterologous expression of foreign genes that would enable direct target comparisons between parasites and the mammalian host. For example, our group has established that both human ADOMETDC and SPDSYN are functional in Leishmania, since transfection with their cDNAs rescued Δadometdc and Δspdsyn parasites, respectively, from polyamine auxotrophy (data not shown). Thus, this system has the potential to identify polyamine analogs that specifically target the parasite enzyme and not the human counterpart, thereby creating a therapeutic paradigm that offers selectivity.
TABLE 3.
Continued
Wild-type parasites were incubated without drug or in a 100 μM concentration of each test compound. Proliferation of wild-type parasites was enumerated after 5 days. Growth in medium without drug was taken as 100% growth. Each percent value is the mean and standard deviation of two independent experiments. Some compounds were evaluated at a range of concentrations from 0.001 to 1000 μM to determine the EC50 values.
Acknowledgments
This work was supported in part by grants AI10096 (to S.C.R.) and AI41622 (to B.U.) from the National Institute of Allergy and Infectious Disease, the Swedish Research Council (to O.H.), the J. C. Kempe Memorial Foundation (to O.H.), and the Royal Physiographic Society in Lund (to O.H.).
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Ashutosh, S. Gupta, Ramesh, S. Sundar, and N. Goyal. 2005. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrob. Agents Chemother. 49:3776-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assaraf, Y. G., J. Golenser, D. T. Spira, G. Messer, and U. Bachrach. 1987. Cytostatic effect of DL-alpha-difluoromethylornithine against Plasmodium falciparum and its reversal by diamines and spermidine. Parasitol. Res. 73:313-318. [DOI] [PubMed] [Google Scholar]
- 3.Bacchi, C. J., and P. McCann. 1987. Parasitic protozoa and polyamines, p. 317-344. In P. McCann, A. E. Pegg, and A. Sjoerdsma (ed.), Inhibition of polyamine metabolism: biological significance and basis for new therapies. Academic Press, Orlando, FL.
- 4.Bacchi, C. J., H. C. Nathan, N. Yarlett, B. Goldberg, P. P. McCann, A. J. Bitonti, and A. Sjoerdsma. 1992. Cure of murine Trypanosoma brucei rhodesiense infections with an S-adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 36:2736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacchi, C. J., and N. Yarlett. 2002. Polyamine metabolism as chemotherapeutic target in protozoan parasites. Mini Rev. Med. Chem. 2:553-563. [DOI] [PubMed] [Google Scholar]
- 6.Bachrach, U. 2005. Naturally occurring polyamines: interaction with macromolecules. Curr. Protein Pept. Sci. 6:559-566. [DOI] [PubMed] [Google Scholar]
- 7.Basselin, M., M. A. Badet-Denisot, F. Lawrence, and M. Robert-Gero. 1997. Effects of pentamidine on polyamine level and biosynthesis in wild-type, pentamidine-treated, and pentamidine-resistant Leishmania. Exp. Parasitol. 85:274-282. [DOI] [PubMed] [Google Scholar]
- 8.Basselin, M., M. A. Badet-Denisot, and M. Robert-Gero. 1998. Modification of kinetoplast DNA minicircle composition in pentamidine-resistant Leishmania. Acta Trop. 70:43-61. [DOI] [PubMed] [Google Scholar]
- 9.Basselin, M., H. Denise, G. H. Coombs, and M. P. Barrett. 2002. Resistance to pentamidine in Leishmania mexicana involves exclusion of the drug from the mitochondrion. Antimicrob. Agents Chemother. 46:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basselin, M., G. H. Coombs, and M. P. Barrett. 2000. Putrescine and spermidine transport in Leishmania. Mol. Biochem. Parasitol. 109:37-46. [DOI] [PubMed] [Google Scholar]
- 11.Bitonti, A. J., J. A. Dumont, and P. P. McCann. 1986. Characterization of Trypanosoma brucei brucei S-adenosyl-L-methionine decarboxylase and its inhibition by Berenil, pentamidine and methylglyoxal bis(guanylhydrazone). Biochem. J. 237:685-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitonti, A. J., J. A. Dumont, T. L. Bush, M. L. Edwards, D. M. Stemerick, P. P. McCann, and A. Sjoerdsma. 1989. Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with alpha-difluoromethylornithine cure murine malaria. Proc. Natl. Acad. Sci. USA 86:651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitonti, A. J., P. P. McCann, and A. Sjoerdsma. 1987. Plasmodium falciparum and Plasmodium berghei: effects of ornithine decarboxylase inhibitors on erythrocytic schizogony. Exp. Parasitol. 64:237-243. [DOI] [PubMed] [Google Scholar]
- 14.Burri, C., and R. Bruni. 2003. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 90:S49-S52. [DOI] [PubMed] [Google Scholar]
- 15.Casara, P., P. Marchal, J. Wagner, and C. Danzin. 1989. 5′-{[(Z)-4-Amino-2-butenyl]methylamino}-5′-deoxyadenosine: a potent enzyme-activated irreversible inhibitor of S-adenosyl-L-methionine decarboxylase from Escherichia coli. J. Am. Chem. Soc. 111:9111-9113. [Google Scholar]
- 16.Chaudhary, K., L. M. Ting, K. Kim, and D. S. Roos. 2006. Toxoplasma gondii purine nucleoside phosphorylase: biochemical characterization, inhibitor profiles and comparison with the Plasmodium falciparum ortholog. J. Biol. Chem. 281:25652-25658. [DOI] [PubMed] [Google Scholar]
- 17.Childs, A. C., D. J. Mehta, and E. W. Gerner. 2003. Polyamine-dependent gene expression. Cell. Mol. Life Sci. 60:1394-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffino, P. 2001. Regulation of cellular polyamines by antizyme. Nat. Rev. Mol. Cell Biol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 19.Cohen, S. S. 1998. A guide to the polyamines, p. 443-480, 481-511, 512-538. Oxford University Press, New York, NY.
- 20.Danzin, C., P. Marchal, and P. Casara. 1990. Irreversible inhibition of rat S-adenosylmethionine decarboxylase by 5′-([(Z)-4-amino-2-butenyl]methylamino)-5′-deoxyadenosine. Biochem. Pharmacol. 40:1499-1503. [DOI] [PubMed] [Google Scholar]
- 21.Didier, E. S., J. A. Maddry, P. J. Brindley, M. E. Stovall, and P. J. Didier. 2005. Therapeutic strategies for human microsporidia infections. Exp. Rev. Anti Infect. Ther. 3:419-434. [DOI] [PubMed] [Google Scholar]
- 22.Ghoda, L., M. A. Phillips, K. E. Bass, C. C. Wang, and P. Coffino. 1990. Trypanosome ornithine decarboxylase is stable because it lacks sequences found in the carboxyl terminus of the mouse enzyme which target the latter for intracellular degradation. J. Biol. Chem. 265:11823-11826. [PubMed] [Google Scholar]
- 23.Ghoda, L., T. van Daalen Wetters, M. Macrae, D. Ascherman, and P. Coffino. 1989. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science 243:1493-1495. [DOI] [PubMed] [Google Scholar]
- 24.Gillin, F. D., D. S. Reiner, and P. P. McCann. 1984. Inhibition of growth of Giardia lamblia by difluoromethylornithine, a specific inhibitor of polyamine biosynthesis. J. Protozool. 31:161-163. [DOI] [PubMed] [Google Scholar]
- 25.Goda, H., T. Watanabe, N. Takeda, M. Kobayashi, M. Wada, H. Hosoda, A. Shirahata, and K. Samejima. 2004. Mammalian spermidine synthase—identification of cysteine residues and investigation of the putrescine binding site. Biol. Pharm. Bull. 27:1327-1332. [DOI] [PubMed] [Google Scholar]
- 26.Grishin, N. V., A. L. Osterman, H. B. Brooks, M. A. Phillips, and E. J. Goldsmith. 1999. X-ray structure of ornithine decarboxylase from Trypanosoma brucei: the native structure and the structure in complex with alpha-difluoromethylornithine. Biochemistry 38:15174-15184. [DOI] [PubMed] [Google Scholar]
- 27.Hanson, S., J. Adelman, and B. Ullman. 1992. Amplification and molecular cloning of the ornithine decarboxylase gene of Leishmania donovani. J. Biol. Chem. 267:2350-2359. [PubMed] [Google Scholar]
- 28.Hasne, M. P., and B. Ullman. 2005. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J. Biol. Chem. 280:15188-15194. [DOI] [PubMed] [Google Scholar]
- 29.Heby, O., I. Holm, and L. Persson. 1990. Polyamine-mediated control of ornithine decarboxylase and S-adenosylmethionine decarboxylase expression in mammalian cells. Biochem. Soc. Trans. 18:1084-1087. [DOI] [PubMed] [Google Scholar]
- 30.Heby, O., and L. Persson. 1990. Molecular genetics of polyamine synthesis in eukaryotic cells. Trends Biochem. Sci. 15:153-158. [DOI] [PubMed] [Google Scholar]
- 31.Heby, O., S. C. Roberts, and B. Ullman. 2003. Polyamine biosynthesis enzymes as drug targets in parasitic protozoa. Biochem. Soc. Trans. 31:415-419. [DOI] [PubMed] [Google Scholar]
- 32.Ikeguchi, Y., X. Wang, D. E. McCloskey, C. S. Coleman, P. Nelson, G. Hu, L. M. Shantz, and A. E. Pegg. 2004. Characterization of transgenic mice with widespread overexpression of spermine synthase. Biochem. J. 381:701-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iovannisci, D. M., and B. Ullman. 1983. High efficiency plating method for Leishmania promastigotes in semidefined or completely-defined medium. J. Parasitol. 69:633-636. [PubMed] [Google Scholar]
- 34.Janne, J., L. Alhonen, T. A. Keinanen, M. Pietila, A. Uimari, E. Pirinen, M. T. Hyvonen, and A. Jarvinen. 2005. Animal disease models generated by genetic engineering of polyamine metabolism. J. Cell. Mol. Med. 9:865-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janne, J., L. Alhonen, M. Pietila, and T. A. Keinanen. 2004. Genetic approaches to the cellular functions of polyamines in mammals. Eur. J. Biochem. 271:877-894. [DOI] [PubMed] [Google Scholar]
- 36.Jiang, Y., S. C. Roberts, A. Jardim, N. S. Carter, S. Shih, M. Ariyanayagam, A. H. Fairlamb, and B. Ullman. 1999. Ornithine decarboxylase gene deletion mutants of Leishmania donovani. J. Biol. Chem. 274:3781-3788. [DOI] [PubMed] [Google Scholar]
- 37.Kaur, K., K. Emmett, P. P. McCann, A. Sjoerdsma, and B. Ullman. 1986. Effects of DL-alpha-difluoromethylornithine on Leishmania donovani promastigotes. J. Protozool. 33:518-521. [DOI] [PubMed] [Google Scholar]
- 38.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 39.Lang, T., S. Goyard, M. Lebastard, and G. Milon. 2005. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell. Microbiol. 7:383-392. [DOI] [PubMed] [Google Scholar]
- 40.LeBowitz, J. H., C. M. Coburn, D. McMahon-Pratt, and S. M. Beverley. 1990. Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc. Natl. Acad. Sci. USA 87:9736-9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leon, W. 1978. Selective inhibition of k-DNA synthesis of Trypanosoma cruzi and Leishmania tarentolae by berenil and ethidium bromide. An. Acad. Bras. Cienc. 50:597-598. [PubMed] [Google Scholar]
- 42.Morris, D. R. 1991. A new perspective on ornithine decarboxylase regulation: prevention of polyamine toxicity is the overriding theme. J. Cell. Biochem. 46:102-105. [DOI] [PubMed] [Google Scholar]
- 43.Mukherjee, A., P. K. Padmanabhan, M. H. Sahani, M. P. Barrett, and R. Madhubala. 2006. Roles for mitochondria in pentamidine susceptibility and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 145:1-10. [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay, R., and R. Madhubala. 1993. Effect of a bis(benzyl)polyamine analogue, and DL-alpha-difluoromethylornithine on parasite suppression and cellular polyamine levels in golden hamster during Leishmania donovani infection. Pharmacol. Res. 28:359-366. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay, R., and R. Madhubala. 1995. Antileishmanial activity of berenil and methylglyoxal bis(guanylhydrazone) and its correlation with S-adenosylmethionine decarboxylase and polyamines. Int. J. Biochem. Cell Biol. 27:55-59. [DOI] [PubMed] [Google Scholar]
- 46.Nisenberg, O., A. E. Pegg, P. A. Welsh, K. Keefer, and L. M. Shantz. 2006. Overproduction of cardiac S-adenosylmethionine decarboxylase in transgenic mice. Biochem. J. 393:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pegg, A. E. 1984. The role of polyamine depletion and accumulation of decarboxylated S-adenosylmethionine in the inhibition of growth of SV-3T3 cells treated with alpha-difluoromethylornithine. Biochem. J. 224:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pegg, A. E., H. Xiong, D. J. Feith, and L. M. Shantz. 1998. S-Adenosylmethionine decarboxylase: structure, function and regulation by polyamines. Biochem. Soc. Trans. 26:580-586. [DOI] [PubMed] [Google Scholar]
- 49.Pegg, A. E. 2006. Regulation of ornithine decarboxylase. J. Biol. Chem. 281:14529-14532. [DOI] [PubMed] [Google Scholar]
- 50.Poulin, R., L. Lu, B. Ackermann, P. Bey, and A. E. Pegg. 1992. Mechanism of the irreversible inactivation of mouse ornithine decarboxylase by alpha-difluoromethylornithine. Characterization of sequences at the inhibitor and coenzyme binding sites. J. Biol. Chem. 267:150-158. [PubMed] [Google Scholar]
- 51.Reguera, R. M., R. B. Fouce, J. C. Cubria, M. L. Bujidos, and D. Ordonez. 1995. Fluorinated analogues of L-ornithine are powerful inhibitors of ornithine decarboxylase and cell growth of Leishmania infantum promastigotes. Life Sci. 56:223-230. [DOI] [PubMed] [Google Scholar]
- 52.Reguera, R. M., B. L. Tekwani, and R. Balana-Fouce. 2005. Polyamine transport in parasites: a potential target for new antiparasitic drug development. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 140:151-164. [DOI] [PubMed] [Google Scholar]
- 53.Roberts, S. C., Y. Jiang, A. Jardim, N. S. Carter, O. Heby, and B. Ullman. 2001. Genetic analysis of spermidine synthase from Leishmania donovani. Mol. Biochem. Parasitol. 115:217-226. [DOI] [PubMed] [Google Scholar]
- 54.Roberts, S. C., J. Scott, J. E. Gasteier, Y. Jiang, B. Brooks, A. Jardim, N. S. Carter, O. Heby, and B. Ullman. 2002. S-Adenosylmethionine decarboxylase from Leishmania donovani. Molecular, genetic, and biochemical characterization of null mutants and overproducers. J. Biol. Chem. 277:5902-5909. [DOI] [PubMed] [Google Scholar]
- 55.Roy, G., C. Dumas, D. Sereno, Y. Wu, A. K. Singh, M. J. Tremblay, M. Ouellette, M. Olivier, and B. Papadopoulou. 2000. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol. Biochem. Parasitol. 110:195-206. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 57.Schechter, P. J., J. L. R. Barlow, and A. Sjoerdsma. 1987. Clinical aspects of inhibition of ornithine decarboxylase with emphasis on therapeutic trials of eflornithine (DFMO) in cancer and protozoan diseases, p. 345-364. In P. McCann, A. E. Pegg, and A. Sjoerdsma (ed.), Inhibition of polyamine metabolism: biological significance and basis for new therapies. Academic Press, Orlando, FL.
- 58.Seiler, N. 2004. Catabolism of polyamines. Amino Acids 26:217-233. [DOI] [PubMed] [Google Scholar]
- 59.Seiler, N., and F. Raul. 2005. Polyamines and apoptosis. J. Cell. Mol. Med. 9:623-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shantz, L. M., and A. E. Pegg. 1994. Overproduction of ornithine decarboxylase caused by relief of translational repression is associated with neoplastic transformation. Cancer Res. 54:2313-2316. [PubMed] [Google Scholar]
- 61.Shantz, L. M., and A. E. Pegg. 1999. Translational regulation of ornithine decarboxylase and other enzymes of the polyamine pathway. Int. J. Biochem. Cell Biol. 31:107-122. [DOI] [PubMed] [Google Scholar]
- 62.Singh, R., K. A. Siddiqui, M. S. Valenzuela, and H. K. Majumder. 1995. Kinetoplast DNA minicircle binding proteins in a Leishmania Spp: interference of protein DNA interaction by berenil. Ind. J. Biochem. Biophys. 32:437-441. [PubMed] [Google Scholar]
- 63.Tabib, A., and U. Bachrach. 1999. Role of polyamines in mediating malignant transformation and oncogene expression. Int. J. Biochem. Cell Biol. 31:1289-1295. [DOI] [PubMed] [Google Scholar]
- 64.Takao, K., M. Rickhag, C. Hegardt, S. Oredsson, and L. Persson. 2006. Induction of apoptotic cell death by putrescine. Int. J. Biochem. Cell Biol. 38:621-628. [DOI] [PubMed] [Google Scholar]
- 65.Thomas, T., and T. J. Thomas. 2001. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 58:244-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian, H., Q. Huang, L. Li, X. X. Liu, and Y. Zhang. 2006. Gene expression of ornithine decarboxylase in lung cancers and its clinical significance. Acta Biochim. Biophys. Sin. (Shanghai) 38:639-645. [DOI] [PubMed] [Google Scholar]
- 67.Wallace, H. M., A. V. Fraser, and A. Hughes. 2003. A perspective of polyamine metabolism. Biochem. J. 376:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wallon, U. M., and T. G. O'Brien. 2005. Polyamines modulate carcinogen-induced mutagenesis in vivo. Environ. Mol. Mutagen. 45:62-69. [DOI] [PubMed] [Google Scholar]
- 69.White, M. W., and D. R. Morris. 1989. S-Adenosylmethionine decarboxylase: genes and expression, p. 331-343. In U. Bachrach and Y. M. Heimer (ed.), The physiology of polyamines. CRC Press, Boca Raton, FL.
- 70.Young, L., R. Salomon, W. Au, C. Allan, P. Russell, and Q. Dong. 2006. Ornithine decarboxylase (ODC) expression pattern in human prostate tissues and ODC transgenic mice. J. Histochem. Cytochem. 54:223-229. [DOI] [PubMed] [Google Scholar]