Abstract
The activity of different analogs of pyrazinamide on Mycobacterium tuberculosis fatty acid synthase type I (FASI) in replicating bacilli was studied. Palmitic acid biosynthesis was diminished by 96% in bacilli treated with n-propyl pyrazinoate, 94% in bacilli treated with 5-chloro-pyrazinamide, and 97% in bacilli treated with pyrazinoic acid, the pharmacologically active agent of pyrazinamide. We conclude that the minimal structure of pyrazine ring with an acyl group is sufficient for FASI inhibition and antimycobacterial activity.
Pyrazinamide (PZA), a first-line sterilizing drug in tuberculosis chemotherapy (2, 6), is the prodrug of the pharmacologically active agent pyrazinoic acid (POA). POA is released by amide hydrolysis of PZA (Fig. 1) (7, 11). Mycobacterium tuberculosis is the only mycobacterial species that is susceptible to both PZA and POA, while Mycobacterium bovis is susceptible only to POA (5, 12, 14). Interestingly, the antimycobacterial activity of these compounds takes place only in an acidic medium (9, 14), which allows for the intracellular accumulation of POA. In addition, M. tuberculosis is uniquely susceptible to PZA among mycobacteria species due to a deficient POA efflux mechanism (14). Despite the proven efficacy of PZA in clinical use, PZA activity in vitro is not only pH dependent but also very low compared to other highly effective tuberculosis drugs, such as isoniazid and rifampin, and depends significantly on the use of a small inoculum (15, 16).
FIG. 1.
Pyrazinamide analogs with antimycobacterial activity. Enzymes that could convert PZA or n′PPA to POA inside the mycobacterial cell are indicated. The amidase, pyrazinamidase/nicotinamidase (PncA), is encoded by the pncA gene.
PZA analogs, such as pyrazinoic acid esters (PAEs), N-acylpyrazinamide, and 5-chloro-pyrazinamide (5-Cl-PZA), are more potent antimycobacterial drugs than PZA (4, 5, 8, 13). 5-Cl-PZA inhibits M. tuberculosis fatty acid synthase type I (FASI) (3, 18), whereas FASI inhibition by PZA has been challenged (3). PAEs, such as n-propyl pyrazinoate (n′PPA), can release POA by ester hydrolysis (Fig. 1) (4, 13), and it is assumed that their mode of action is identical to that of PZA and POA (16). Importantly, n′PPA and other PAEs were also shown to possess a broader spectrum of activity that includes other mycobacterial species, such as the POA-resistant M. avium and M. kansasii (1, 4, 13).
Our study aimed to test (i) whether n′PPA activity, like POA, depends on acidified media and, by inference, depends on POA accumulation; (ii) whether n′PPA, like 5-Cl-PZA, inhibits FASI activity in replicating tuberculosis bacilli; and (iii) whether the antimycobacterial activity of POA correlates with FASI inhibition only in acidic media.
The MIC of POA and n′PPA against M. tuberculosis was determined by the broth macrodilution test using an inoculum of 105 CFU/ml in Middlebrook 7H9 medium supplemented with 10% (vol/vol) OADC enrichment (Difco), 0.2% (vol/vol) glycerol, and 0.05% (vol/vol) Tween 80 (the pH of the medium was adjusted to 6 or 6.8 with an aqueous solution of 4.25% phosphoric acid or 5% dibasic potassium phosphate). The MICs of POA were 300 μg/ml at pH 6.8 and 100 μg/ml at pH 6, as previously described (9, 10). Surprisingly, the MICs of n′PPA were 60 μg/ml at pH 6 and 80 μg/ml at pH 6.8.
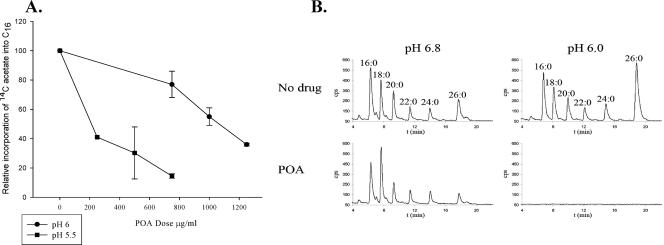
FASI inhibition by n′PPA, 5-Cl-PZA, and PZA was assayed by measuring the 14C incorporation into mycobacterial fatty acids. Basically, tuberculous bacilli from a frozen stock were grown in 7H12 media to an optical density at 600 nm (OD600) of 0 0.3 to 0.4 and then diluted 10 times into fresh medium for another 48 to 72 h before 10-ml aliquots of this culture (OD600 = 0.2 to 0.25) were spun down in 50-ml conical tubes. The spun cells were resuspended in freshly made 10 ml of drug-containing medium with either PZA (1,200 μg/ml at pH 6), 5-Cl-PZA (250 μg/ml), or n′PPA (800 μg/ml) for 12 h and then labeled with [1-14C]acetate (1 μCi/ml) for 6 h. The culture was then spun, and the soluble lipids were extracted and saponified from the drug-treated labeled bacilli (18). The resulting free fatty acids were derivatized to their UV-absorbing p-bromophenacyl esters and separated and quantified by reversed-phase high-pressure liquid chromatography (HPLC) using one-seventh of the derivatization reaction (18). The HPLC mobile phase was acetonitrile, and the flow rate was 1 ml/min for 5 min, followed by a linear increase to 2 ml/min over 1 min; this was then maintained at 2 ml/min for 14 min. The UV-absorbing p-bromophenacyl fatty acid esters and the 14C-labeled fatty acid esters were detected as described previously (18). The chromatograms peaks were identified by comparison with chromatograms of p-bromophenacyl fatty acid ester standards. Labeled palmitic acid ([14C]C16) was quantified and compared to the level of [14C]C16 of the untreated sample to yield relative incorporation of [14C]acetate into C16, as a measure of FASI inhibition. PZA, 5-Cl-PZA, and n′PPA application resulted in a severe decrease in fatty acid biosynthesis in M. tuberculosis (Fig. 2). The biosynthesis of palmitic acid (C16), the principle product of FASI, was inhibited on average by 88% with PZA, by 94% with 5-Cl-PZA, and by 96% with n′PPA. With POA, the C16 biosynthesis inhibition was greatly affected by the pH and POA concentration (Fig. 3A). For example, a 60% inhibition of C16 biosynthesis was achieved at pH 5.5 with only 250 μg of POA/ml, whereas it required 1,250 μg of POA/ml to reach the same inhibition at pH 6.0. Importantly, acidic medium by itself even induced an increase in fatty acid biosynthesis in tuberculous bacilli, as described previously (3, 18). The same pH effect was also observed in M. bovis, where POA (1,500 μg/ml) inhibited C16 biosynthesis by 97% at pH 6 but only by 30% at pH 6.8 (Fig. 3B).
FIG. 2.
HPLC chromatograms of extracted fatty acids after n′PPA or 5-Cl-PZA treatment of M. tuberculosis bacilli. The 1-14C-labeled fatty acids chromatograms (lower panel) are coupled to the UV absorbency chromatograms (upper panel) showing nonlabeled fatty acids, which reflect the amount of lipid extract injected in the HPLC column.
FIG. 3.
(A) Quantitative analysis of 1-14C-labeled C16 (y axis) from M. tuberculosis bacilli treated with different concentrations of POA (x axis) at pH 5.5 or 6. (B) HPLC chromatogram of extracted 1-14C-labeled fatty acids from M. bovis BCG bacilli treated with POA (1,500 μg/ml) at either pH 6 or 6.8.
The pH independence of n′PPA activity compared to POA can be ascribed to either increased diffusion by the more lipophilic n′PPA or to an intrinsic activity of the ester, or both. It has yet to be established that hydrolysis of n′PPA to POA is required for its antimycobacterial activity. The intrinsic activity of PAEs can be argued based on the facts that more stable pyrazinoate esters still possess good antimycobacterial activity (1) and that PAEs are active against POA-resistant M. avium (1, 13). The possibility of intrinsic activity of PAEs and the fact that 5-chloro pyrazinoic acid is a stronger acid but a weaker antimycobacterial agent than POA (5) argues strongly against a mechanism of protonated POA causing cytoplasmic acidification and membrane potential collapse affecting transport, which was suggested to be the mechanism of activity of POA (16, 17).
The main findings of the present study are that n′PPA inhibited fatty acid biosynthesis as well as 5-Cl-PZA and that its antimycobacterial activity did not depend on pH as much as POA. The only common structural motif between n′PPA and 5-Cl-PZA is the pyrazinoyl nucleus, suggesting that the 5-chloro substitution is not a prerequisite for FASI inhibition as was suggested by Boshoff et al. (3). Furthermore, POA application resulted in C16 biosynthesis inhibition in M. bovis BCG at pH 6 and in M. tuberculosis, where a relationship between pH, POA dose, and C16 biosynthesis inhibition was observed.
In summary, by using a set of related compounds, we have deduced that the minimal structure of pyrazine ring with an acyl group is sufficient to confer both FASI inhibition and antimycobacterial activity. Therefore, FASI inhibition cannot be excluded as a mechanism for antimycobacterial activity of PZA and related analogs.
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Bergmann, K. E., M. H. Cynamon, and J. T. Welch. 1996. Quantitative structure-activity relationship for the in vitro antimycobacterial activity of pyrazinoic acid esters. J. Med. Chem. 16:3394-3400. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, and A. A. Vernon. 2004. American Thoracic Society, Centers for Disease Control and Prevention, and the Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. [DOI] [PubMed] [Google Scholar]
- 3.Boshoff, H. I., V. Mizrahi, and C. E. Barry III. 2002. Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase. I. J. Bacteriol. 184:2167-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cynamon, M. H., R. Gimi, F. Gyenes, C. A. Sharpe, K. E. Bergmann, H. J. Han, L. B. Gregor, R. Rapuolu, G. Luciano, and J. T. Welch. 1995. Pyrazinoic acid esters with broad spectrum in vitro antimycobacterial activity. J. Med. Chem. 38:3902-3907. [DOI] [PubMed] [Google Scholar]
- 5.Cynamon, M. H., R. J. Speirs, and J. T. Welch. 1998. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob. Agents Chemother. 42:462-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosset, J. 1978. The sterilizing value of rifampicin and pyrazinamide in experimental short course chemotherapy. Bull. Int. Union Tuberc. 53:5-12. [PubMed] [Google Scholar]
- 7.Konno, K., F. M. Feldman, and W. McDermott. 1967. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am. Rev. Respir. Dis. 95:461-469. [DOI] [PubMed] [Google Scholar]
- 8.Liu, Z. Z., X. D. Guo, L. E. Straub, G. Erdos, R. J. Prankerd, R. J. Gonzalez-Rothi, and H. Schreier. 1991. Lipophilic N-acylpyrazinamide derivatives: synthesis, physicochemical characterization, liposome incorporation, and in vitro activity against Mycobacterium avium-intracellulare. Drug Des. Discov. 8:57-67. [PubMed] [Google Scholar]
- 9.McDermott, W., and R. Tompsett. 1954. Activation of pyrazinamide and nicotinamide in acidic environment in vitro. Am. Rev. Tuberc. 70:748-754. [DOI] [PubMed] [Google Scholar]
- 10.Salfinger, M., and L. B. Heifets. 1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pH by the radiometric method. Antimicrob. Agents Chemother. 32:1002-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salfinger, M., A. J. Crowle, and L. B. Reller. 1990. Pyrazinamide and pyrazinoic acid activity against tubercle bacilli in cultured human macrophages and in the BACTEC system. J. Infect. Dis. 162:201-207. [DOI] [PubMed] [Google Scholar]
- 12.Scorpio, A., and Y. Zhang. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 13.Speirs, R. J., J. T. Welch, and M. H. Cynamon. 1995. Activity of n-propyl pyrazinoate against pyrazinamide-resistant Mycobacterium tuberculosis: investigations into mechanism of action of and mechanism of resistance to pyrazinamide. Antimicrob. Agents Chemother. 39:1269-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang, Y., A. Scorpio, H. Nikaido, and Z. H. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in the unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 181:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, Y., S. Permar, and S. Sun. 2002. Conditions that may affect the results of Mycobacterium tuberculosis susceptibility testing to pyrazinamide. J. Med. Microbiol. 51:42-49. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Y., and D. Mitchison. 2003. The curious characteistics of pyrazinamide: a review. Int. J. Tuberc. Lung Dis. 7:6-21. [PubMed] [Google Scholar]
- 17.Zhang, Y., M. M. Wade, A. Scorpio, H. Zhang, and Z. Sun. 2003. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 52:790-795. [DOI] [PubMed] [Google Scholar]
- 18.Zimhony, O., J. S. Cox, J. T. Welch, C. Vilcheze, and W. R. Jacobs, Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 6:1043-1047. [DOI] [PubMed] [Google Scholar]