Abstract
The most common mechanism by which Staphylococcus aureus gains resistance to vancomycin is by adapting its physiology and metabolism to permit growth in the presence of vancomycin. Several studies have examined the adaptive changes occurring during the transition to vancomycin-intermediate resistance, leading to a model of vancomycin resistance in which decreased cell wall turnover and autolysis result in increased cell wall thickness and resistance to vancomycin. In the present study, we identified metabolic changes common to vancomycin-intermediate S. aureus (VISA) strains by assessing the metabolic and growth characteristics of two VISA strains (vancomycin MICs of 8 μg/ml) and two isogenic derivative strains with vancomycin MICs of 32 μg/ml. Interestingly, we observed the parental strains had impaired catabolism of nonpreferred carbon sources (i.e., acetate), and this impairment became more pronounced as vancomycin resistance increased. To determine if acetate catabolism impairment is common to VISA strains, we assessed the ability of VISA and vancomycin-sensitive S. aureus (VSSA) clinical isolates to catabolize acetate. As expected, a significantly greater percentage of VISA strains (71%) had impaired acetate catabolism relative to VSSA (8%). This is an important observation because staphylococcal acetate catabolism is implicated in growth yield and antibiotic tolerance and in regulating cell death and polysaccharide intercellular adhesin synthesis.
The emergence of methicillin-resistant Staphylococcus aureus as a major cause of hospital- and community-acquired infections (32) often necessitates the use of vancomycin to treat these infections. However, the effectiveness of vancomycin as a treatment option is diminishing due to the emergence of vancomycin-resistant S. aureus (VRSA). Staphylococci demonstrating an intermediate level of resistance to vancomycin (MIC of 8 μg/ml) first emerged in Japan in 1996 (13, 14). The following year in the United States, S. aureus with a similarly elevated vancomycin MIC was isolated (2). These strains have adapted to grow in the presence of intermediate levels of vancomycin and are collectively referred to as vancomycin-intermediate S. aureus (VISA). In addition to physiological adaptation, S. aureus strains have been described that have acquired the van genes, encoding high-level resistance to vancomycin (1, 3, 4, 37). These two mechanisms of antibiotic resistance highlight a recurring theme; bacteria primarily gain resistance to antibiotics by acquisition of a genetic element conferring high-level resistance or by adapting their physiology and metabolism to life in the presence of antibiotics.
Most studies that have attempted to ascertain the adaptive changes occurring during the transition to growth in the presence of vancomycin have focused on alterations in cell wall synthesis and morphology (8, 11, 21, 27), autolysis (11, 28, 35), and global transcriptional changes (7, 18, 19, 34). These studies have led to a model of vancomycin resistance in which decreased cell wall turnover and autolysis result in increased cell wall thickness and resistance to vancomycin. Mechanistically, the thickened cell wall is thought to limit the access of vancomycin to its target (6, 9, 11, 12, 26). Because this model of vancomycin resistance relies on an alteration in cell wall thickness, most research has focused on adaptations affecting cell wall thickening.
Transcriptional profiling experiments have identified a number of physiological adaptations in VISA strains. Kuroda et al. described increases in fructose utilization, fatty acid metabolism, and putative ABC transporter genes in VISA strains compared to their more susceptible counterparts (16). These and other physiological changes have led to speculation that to create a thickened cell wall, VISA strains increase glucose utilization and decrease the intracellular glutamine concentration (6, 9). This decrease in glutamine availability is thought to increase the proportion of glutamine nonamidated muropeptides, which increase the affinity of vancomycin for the cell wall at sites away from the lethal target, specifically, the nascent cell wall at the cell membrane.
One barrier to expanding and improving the understanding of S. aureus vancomycin resistance is the paucity of isogenic pairs of vancomycin-sensitive S. aureus (VSSA) and VISA strains. To circumvent this barrier, Mongodin et al. grew two VISA strains (Mu50 and HIP5827) in medium containing increasing concentrations of vancomycin until the derivative strains had vancomycin MICs of 32 μg/ml (strains Mu50-32 and VP-32), thus providing an alternative to isogenic VSSA and VISA pairs. Using these isogenic strain pairs of low and high vancomycin resistance provided insight into the transcriptional changes that occur during adaptation to growth in the presence of vancomycin (19). In the present study, we used these isogenic pairs as a means to identify common physiological differences and then examined if these differences were present in naturally occurring VISA strains.
MATERIALS AND METHODS
Bacterial strains, materials, and growth conditions.
Strains used in this study are listed in Table 1. S. aureus strains were grown in tryptic soy broth containing 0.25% glucose (TSB; BD Biosciences) or on TSB containing 1.5% agar. Vancomycin (Sigma) was used at concentrations between 4 and 32 μg/ml. Unless otherwise stated, all bacterial cultures were inoculated 1:200 from an overnight culture (normalized for growth) into TSB, incubated at 37°C, and aerated at 225 rpm with a flask-to-medium ratio of 10:1. Bacterial growth was assessed by measuring the optical density at 600 nm (OD600).
TABLE 1.
Strains used in this study
| Strain designation | spa profile (spa type) | Source or reference |
|---|---|---|
| Mu50-32 | TJMBMDMGMK (2) | 19 |
| VP-32 | TJMGMK (12) | 19 |
| NRS12 | TJMBMDMGMK (2) | NARSAa |
| NRS17 | TJMBMDMGMK (2) | NARSA |
| NRS56 | WGKAOMQ (3) | NARSA |
| NRS63 | WGKAOMQ (3) | NARSA |
| NRS118 | YHFGFMBQBLO (4) | NARSA |
| Mu50 | TJMBMDMGMK (2) | 14 |
| HIP5827 | TJMGMK (12) | 33 |
| BK3570 | YHGFMBQBLO (1) | B. Kreiswirth collection |
| BK3701 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK4916 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK5464 | TJMBMDMGMM (731) | B. Kreiswirth collection |
| BK5465 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK5466 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK5467 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK5468 | TJMEMDMGMK (24) | B. Kreiswirth collection |
| BK5469 | TJMBMDMGMM (731) | B. Kreiswirth collection |
| BK5473 | TMBMDMGMK (230) | B. Kreiswirth collection |
| BK11211 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK11216 | YHGFMBQBLO (1) | B. Kreiswirth collection |
| BK11228 | YHGFMBQBLO (1) | B. Kreiswirth collection |
| BK11229 | YHGCMBQBLO (7) | B. Kreiswirth collection |
| BK11270 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK11275 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK11276 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK11429 | YHGFMBQBLO (1) | B. Kreiswirth collection |
| BK11472 | YHGCMBQBLO (7) | B. Kreiswirth collection |
| BK11478 | YHGFMBQBLO (1) | B. Kreiswirth collection |
| BK11490 | YHGFMBQBLO (1) | B. Kreiswirth collection |
| BK15641 | TJMBMDMGMK (2) | B. Kreiswirth collection |
| BK15681 | YHGCMBQBLO (7) | B. Kreiswirth collection |
| BK792 | YHGFMBQBLO (1) | B. Kreiswirth collection |
NARSA, Network on Antimicrobial Resistance in Staphylococcus aureus.
spa typing.
spa typing was performed essentially as described elsewhere (25) with the noted exception that the PCR primers listed in Table 2 were used. The spa type was assigned using eGenomics software (http://tools.egenomics.com).
TABLE 2.
Primers used in this study
| Primer name | Nucleotide sequence (5′ → 3′) |
|---|---|
| spa forward | GCCAAAGCGCTAACCTTTTA |
| spa reverse | TCCAGCTAATAACGCTGCAC |
| CidA1-F | CCCCATATGCACAAAGTCCAATTA |
| CidA1-R | CCCCTCGAGTTCATAAGCGTCTACACC |
| CidB1-F | TGATTTTGTTGACTGTCGTT |
| CidB1-R | TCATGTGACACTTCGATACC |
| LrgA1-F | CCCCATATGGTCGTGAAACAACAAAAAGACGC |
| LrgA1-R | CCCCTCGAGATCATGAGCTTGTGCCTCCTC |
Measurement of acetate, glucose, and ammonia in culture supernatants.
Aliquots of bacteria (1.5 ml) were centrifuged for 5 min at 20,800 × g at 4°C, and supernatants were removed and stored at −20°C until use. Acetate, glucose, and ammonia concentrations were determined with kits purchased from R-Biopharm, Inc., and used according to the manufacturer's directions.
Aconitase activity assay.
Cell-free lysates of S. aureus were prepared as follows. Aliquots (3 ml) were harvested at the indicated times, suspended in 1.5 ml of lysis buffer containing 90 mM Tris (pH 8.0), 100 μM fluorocitrate, and 50 μg/ml lysostaphin (AMBI). The samples were incubated at 37°C for 10 min and passed through a French press (two times at 15,000 lb/in2). The lysate was centrifuged for 5 min at 20,800 × g at 4°C. Aconitase activity was assayed in the resulting cell-free lysate by the method described by Kennedy et al. (15). One unit of aconitase activity is defined as the amount of enzyme necessary to give a ΔA240 min−1 of 0.0033 (5). Protein concentrations were determined by the Lowry method (17).
Northern blot analysis.
Bacterial cultures (25 ml) were grown in TSB incubated at 37°C and aerated at 250 rpm with a flask-to-medium ratio of 10:1. Aliquots of bacteria (10 ml) were harvested by centrifugation when the growth was between 3.0 and 4.0 A600 units. Total RNA was extracted using an RNeasy RNA isolation kit (QIAGEN) and a Fastprep FP120 instrument (Bio 101 Thermo Savant) essentially as described previously (20).
Total RNA (5 μg) was electrophoresed through a 0.9% agarose gel containing 0.66 M formaldehyde in morpholinepropanesulfonic acid (MOPS) running buffer (20 mM MOPS, 10 mM sodium acetate, 2 mM EDTA, pH 7.0). The gel was then subjected to capillary transfer to a nylon membrane (Micron Separations) using 20× SSC (0.3 M Na3-citrate, 3.0 M NaCl, pH 7.0). RNA was fixed to the nylon membrane by cross-linking in a UV Stratalinker 1800 (Stratagene). Hybridization and processing of blots was carried out using the DIG system (Roche Applied Science) according to the manufacturer's recommendations. Approximate transcript sizes were determined by comparison to an RNA molecular mass ladder (Invitrogen). Probes were synthesized using the PCR-based DIG probe synthesis kit (Roche) following the manufacturer's protocol and using the primers listed in Table 2.
PIA immunoblotting.
Polysaccharide intercellular adhesin (PIA) was extracted from 109 bacteria, grown to an OD600 of 1.0, by boiling in 0.5 M EDTA (pH 8.0) for 5 min. Aliquots of PIA were applied to an Immobilon-P membrane (Millipore) and blocked with 5% skim milk for 3 h. The membrane was incubated overnight with anti-PIA antiserum (a kind gift of D. Mack) and, subsequently, for 2 h with a goat anti-rabbit horseradish peroxidase conjugate (Bio-Rad). The presence of PIA was detected by autoradiography using enhanced chemiluminescence reagents (GE Healthcare Life Sciences).
Statistical analysis.
Statistical significance was assessed with Student's t test. To determine if a correlation existed between two parameters, a Pearson's correlation coefficient was calculated.
RESULTS
Physiological changes associated with increasing vancomycin resistance.
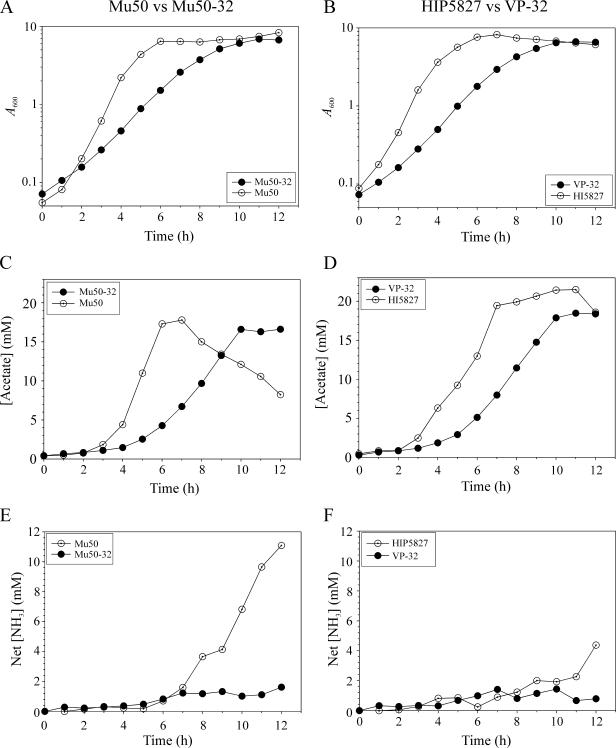
Increased resistance to vancomycin is associated with changes in bacterial morphology, physiology, and growth characteristics (8, 19, 27). In particular, increased resistance to vancomycin decreased the growth rate and cell size relative to isogenic strains of lower resistance (19). This change in growth rate and cell size is analogous to wild-type S. aureus entering into the post-exponential growth phase after exhausting a nutrient (e.g., carbon or phosphate) (30). In staphylococci, entry into the post-exponential growth phase usually coincides with the catabolism of nonpreferred carbon sources and induction of the tricarboxylic acid (TCA) cycle, leading us to hypothesize that increased resistance to vancomycin alters intermediary metabolism; specifically, we were interested in the ability of parental and derivative strains to catabolize acetate. The rationale for examining acetate catabolism is that staphylococci growing aerobically in a medium containing glucose will accumulate a high concentration of acetate in the culture medium; however, when the concentration of glucose is insufficient to permit rapid growth, the staphylococci will undergo a diauxic shift and catabolize the acetate. To test this hypothesis, the growth of vancomycin-resistant strains Mu50-32 and VP-32 (vancomycin resistance of ≥32 μg/ml) was compared to isogenic parental strains Mu50 and HIP5827 (vancomycin resistance of 8 μg/ml). As previously described (19), the growth rate of the vancomycin-resistant strains Mu50-32 and VP-32 was dramatically slower than the isogenic parental strains (Fig. 1A and B). The decreased growth rate was reflected in the slower depletion of glucose from the culture medium (data not shown) and in the delay in achieving the maximal acetate concentration in the culture medium (Fig. 1C and D). Interestingly, only strain Mu50 significantly extracted acetate from the culture medium during 12 h of growth, suggesting that it alone was capable of catabolizing nonpreferred carbon sources. Acetate catabolism requires a fully functioning tricarboxylic acid cycle and is linked to amino acid catabolism (31). Ammonia production is an indicator of amino acid catabolism; hence, we measured the concentration of ammonia in the culture medium (Fig. 1E and F). As expected, strain Mu50 accumulated significantly more ammonia in the culture medium relative to strain Mu50-32. In contrast, strain HIP5827 accumulated an equivalent amount of ammonia in the culture medium relative to strain VP-32 until the end of the 12-h growth experiment. (To compare and contrast these results with those of VSSA strains, please refer to reference 31.) Taken together, these data suggested that strains HIP5827, Mu50-32, and VP-32 had impaired intermediary metabolism, specifically, in TCA cycle function.
FIG. 1.
Growth and physiological characteristics of low-level vancomycin-resistant parental strains (Mu50 and HIP5827) and high-level resistant isogenic derivative strains (Mu50-32 and VP-32). (A and B) Growth curves of Mu50 and Mu50-32 (A) and HIP5827 and VP-32 (B). (C and D) Acetate accumulation and depletion in the culture supernatants of isogenic strain pairs Mu50 and Mu50-32 (C) and HIP5827 and VP-32 (D). (E and F) Net ammonia accumulation in the culture supernatants of strain pairs Mu50 and Mu50-32 (E) and HIP5827 and VP-32 (F). The results presented are representative of at least two independent experiments. Symbols are defined in the figure insets.
Aconitase activity of VISA parental strains and VRSA derivative strains.
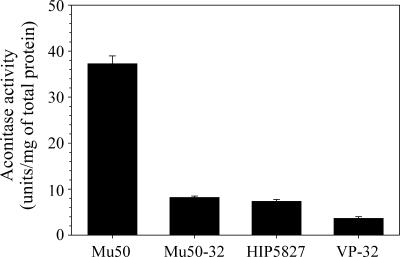
In contrast to previous speculation (16, 19), the physiological data (Fig. 1) suggested that increased vancomycin resistance correlated with decreased carbon flow through the TCA cycle. To test this hypothesis, we assessed post-exponential-growth-phase (12-h) TCA cycle activity by measuring aconitase enzymatic activity in the parental strains Mu50 and HIP5827 and the derivative strains Mu50-32 and VP-32 (Fig. 2). As expected, TCA cycle activity was significantly lower in strains Mu50-32 and VP-32 relative to the parental strains (Mu50 versus Mu50-32, P = 0.000004; HIP5827 versus VP-32, P = 0.0005). Surprisingly, parental strain HIP5827 had very low aconitase activity relative to Mu50 or other S. aureus strains (29), confirming that its TCA cycle activity was also impaired.
FIG. 2.
Post-exponential-growth-phase (12-h) aconitase activity of parental and derivative strain pairs. Aconitase activities were determined in triplicate from two independent cultures during the exponential phase of growth. The results are presented as the mean and standard error of the mean.
Acetate catabolism of VISA clinical isolates.
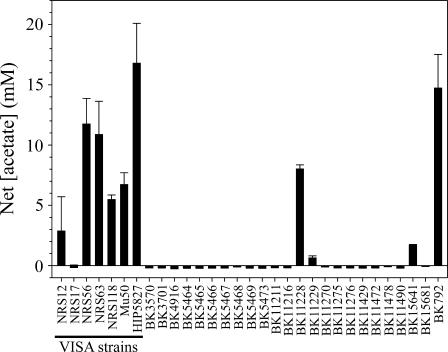
The unexpected observations that highly vancomycin-resistant derivative strains Mu50-32 and VP-32 had significantly decreased aconitase activities relative to the vancomycin-intermediate resistant parental strains (Fig. 2) and that strains HIP5827, Mu50-32, and VP-32 had impaired acetate catabolism (Fig. 1) led us to speculate that impaired acetate catabolism might be a phenotype common to VISA strains. To address this possibility, we determined the concentration of acetate in the culture supernatants of 7 VISA strains and 24 VSSA strains (Table 1) after they had entered into the stationary phase (12 h) (Fig. 3). Five of seven VISA strains (71%) had a concentration of acetate greater than 5 mM in the culture supernatant after 12 h of growth, whereas only 2 out of 24 VSSA strains (8%) had an equivalent concentration of acetate in the culture medium. Because the growth rate of VISA strains is slightly slower than VSSA strains (31), the more likely explanation for the dramatic difference in acetate catabolism between the VISA and VSSA strains is a difference in the growth rate; that is to say, organisms growing slowly will catabolize acetate slowly. However, this possibility is inconsistent with the observation that the seven VISA strains have equivalent growth rates (data not shown), yet strain NRS 17 completely catabolized acetate within 12 h while all other VISA strains had varying degrees of acetate catabolism impairment (Fig. 3). Similarly, the growth rates of VSSA acetate-catabolizing strains and VSSA acetate catabolism-impaired strains were equivalent (data not shown). Taken together, these data suggest that the difference in acetate catabolism between VISA and VSSA strains is not growth related. Additionally, the genetic diversity of the VSSA and VISA strains (Table 1) strongly suggests the acetate catabolism impairment was independent of genetic lineage.
FIG. 3.
Net acetate concentration in the culture media of VISA and VSSA strains after 12 h of growth. The results are presented as the mean and standard error of the mean from three independent cultures.
Physiological consequences of impaired acetate catabolism.
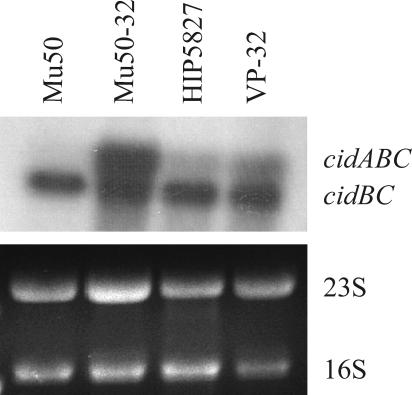
Previously, we observed that TCA cycle inactivation inhibited S. aureus acetate catabolism and enhanced long-term survival (30). Furthermore, it was determined the cid and lrg operons (murein hydrolase activity regulatory operons) were regulated by the concentration of acetic acid in the culture medium (22). Taken together, these observations led us to speculate that derivative strains Mu50-32 and VP-32 would have altered cid and lrg mRNA transcription relative to the isogenic parental strains Mu50 and HIP5827. Northern blot analysis of bacterial cultures harvested late in the exponential phase of growth revealed that derivative strains Mu50-32 and VP-32 had a greater amount of cidABC mRNA relative to the parental strains (Fig. 4). (The cid operon is comprised of two overlapping transcripts [cidABC and cidBC].) Consistent with previous results (19), the amount of lrgAB mRNA was either lower in the derivative strains relative to the parental strains or approximately equivalent (data not shown). These data led us to speculate that the impaired acetate catabolism alters cell death by altering transcription of the cid and lrg murein hydrolase regulatory loci.
FIG. 4.
Northern blot assay demonstrating increased expression of cidABC in highly vancomycin-resistant derivative strains. Total RNA was isolated from strains Mu50, Mu50-32, HIP5827, and VP-32 when the optical density was between 3 and 4 A600 units, transferred to a charged nylon membrane, and probed with a cidB-specific probe. The results are representative of three independent experiments.
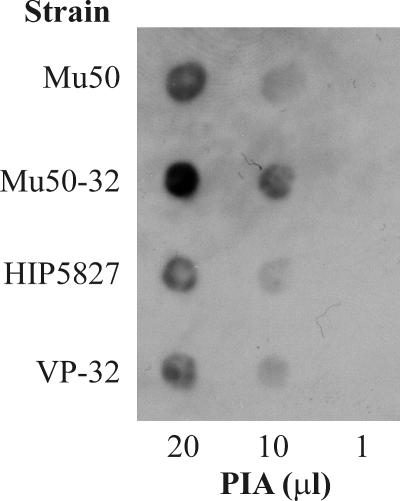
Recently, we demonstrated that increased PIA synthesis is associated with decreased TCA activity (36). The observations that the VRSA derivative strains had impaired acetate catabolism (Fig. 1C and D) and decreased aconitase activity relative to the VISA parental strains led us to hypothesize that as vancomycin resistance increased, the synthesis of PIA would increase. This possibility is consistent with transcriptional profiling data that demonstrated that transcription of icaR, a repressor of PIA synthesis, was decreased in a VISA strain relative to a VSSA strain (18). To test this hypothesis, the amount of PIA produced by the parental and derivative strains was assessed by immunoblotting. As expected, PIA synthesis was increased in strain Mu50-32 relative to Mu50 (Fig. 5) but was unchanged in strain VP-32 relative to HIP5827. The absence of an increase in PIA synthesis in the latter strain pair is likely because there is only a minimal change in TCA cycle activity between the parental and derivative strains.
FIG. 5.
PIA immunoblot assay of parental and derivative strain pairs. PIA was extracted from 109 bacteria grown to an A600 of 1.0, and 20-, 10-, and 1-μl volumes were transferred to an Immobilon-P membrane for immunoblotting. The results are representative of multiple (n > 3) independent experiments.
DISCUSSION
VISA strains Mu50 and HIP5827, chosen by Mongodin et al. for in vitro serial passage, represent genetically (Table 1) and phenotypically (Fig. 1) diverse S. aureus strains, an advantage when attempting to identify phenotypes common to VISA isolates.
Using these strains and their corresponding derivative strains (Mu50-32 and VP-32), we found the transition from low-level vancomycin resistance to high-level resistance was accompanied by a decrease in TCA cycle activity and an exacerbation of an existing acetate catabolism impairment (Fig. 1 and 2). The acetate catabolism impairment of the parental VISA strains led us to speculate this phenotype would be common to VISA strains. Indeed, we found a strong correlation between impaired acetate catabolism and vancomycin-intermediate resistance in a genetically diverse group of S. aureus strains (Fig. 3 and Table 1). The remainder of this discussion will focus on a potential cause of impaired acetate catabolism in VISA strains and the implications of this impairment.
Possible basis for impaired acetate catabolism.
Increased resistance to vancomycin is associated with changes in bacterial morphology, physiology, and growth characteristics, most notably, an increase in the thickness of the cell wall (8, 11, 21, 27). The increased thickness of the cell wall correlates with an increase in genes involved in cell wall biosynthesis and regulation (18); hence, there is likely an increased demand for cell wall biosynthetic components N-acetyl-glucosamine and N-acetyl-muramic acid. Both N-acetyl-glucosamine and N-acetyl-muramic acid are synthesized from the glycolytic intermediate β-d-fructose 6-phosphate, which will be abundant when readily catabolizable carbohydrates are available. Alternatively, when carbohydrates are limiting, β-d-fructose 6-phosphate can be synthesized by gluconeogenesis from the TCA cycle intermediate oxaloacetate, resulting in the withdrawal of carbon from the TCA cycle. The first step of the TCA cycle is the assimilation of acetyl coenzyme A into citric acid, a step requiring the four-carbon intermediate oxaloacetate. If oxaloacetate is withdrawn for gluconeogenesis, then anaplerotic reactions are required to maintain TCA cycle function, a potential problem when carbon is limiting. Depending on the severity of the four- or five-carbon TCA cycle intermediate limitation, the assimilation of acetyl coenzyme A will proceed more slowly, or not at all; thus, acetate will remain in the culture medium.
Implications of acetate catabolism impairment.
Irrespective of the cause of impaired acetate catabolism in VISA strains, the impairment establishes a condition that can affect several physiological traits important in virulence. Acetate catabolism in staphylococci is required for maximal growth yield (31) and antibiotic tolerance (22) and is involved in regulating cell death (22) and PIA synthesis (36). Consistent with these observations, we found the transition from low-level to high-level vancomycin resistance was accompanied by increased cidABC transcription and PIA synthesis. Predictably, the differences in the severity of acetate catabolism impairment in the parental strains (Mu50 and HIP6827) relative to the derivative strains (Mu50-32 and VP-32) are in accordance with the differences in PIA synthesis and transcription of cidABC in these same strains. Specifically, there are dramatic differences in acetate catabolism, PIA synthesis, and cidABC expression between Mu50 and Mu50-32 but only minimal differences between HIP5827 and VP-32. The extent to which these changes contribute to vancomycin resistance and pathogenesis is unknown; however, PIA is an important virulence factor and likely contributes to in vivo survival (10, 23, 24).
A very small percentage of VSSA strains are impaired in their ability to utilize nonpreferred carbon sources, such as acetate (31; G. Somerville and B. Kreiswirth, unpublished observations) (Fig. 3). Interestingly, in the present study, we found the majority of VISA strains are impaired in acetate catabolism, raising several interesting and potentially important possibilities. First, does acetate catabolism impairment confer a competitive advantage versus nonimpaired strains when grown in the presence of vancomycin? If so, do acetate catabolism-impaired VSSA strains serve as a reservoir for the generation of VISA strains? Second, can acetate catabolism be used as a predictor of vancomycin treatment failure? That is to say, if acetate catabolism-impaired S. aureus were isolated from a patient during vancomycin treatment, then could this be an early indicator of the emergence of vancomycin resistance? Third, is there a direct connection between acetate catabolism and increased cidABC transcription and PIA synthesis? To address these and other questions requires defined mutations in isogenic pairs of VSSA and VISA strains.
Acknowledgments
We are indebted to D. Mack for providing anti-PIA antiserum. We also thank the Network on Antimicrobial Resistance in Staphylococcus aureus for providing the VISA isolates used in this study.
The manuscript is a contribution of the University of Nebraska Agricultural Research Division, supported in part by funds provided through the Hatch Act. Additional support was provided by grant number P20 RR-17675 from the National Center for Research Resources, a component of the National Institutes of Health, to G.S. and NIH grant R01AI038901 and DOD grant DAAD 19-03-1-0191 to K.W.B.
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print on 27 November 2006.
REFERENCES
- 1.Anonymous. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 2.Anonymous. 1997. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morb. Mortal. Wkly. Rep. 46:765-766. [PubMed] [Google Scholar]
- 3.Anonymous. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 4.Anonymous. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 5.Baughn, A. D., and M. H. Malamy. 2002. A mitochondrial-like aconitase in the bacterium Bacteroides fragilis: implications for the evolution of the mitochondrial Krebs cycle. Proc. Natl. Acad. Sci. USA 99:4662-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui, L., A. Iwamoto, J. Q. Lian, H. M. Neoh, T. Maruyama, Y. Horikawa, and K. Hiramatsu. 2006. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui, L., J. Q. Lian, H. M. Neoh, E. Reyes, and K. Hiramatsu. 2005. DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3404-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui, L., X. Ma, K. Sato, K. Okuma, F. C. Tenover, E. M. Mamizuka, C. G. Gemmell, M. N. Kim, M. C. Ploy, N. El-Solh, V. Ferraz, and K. Hiramatsu. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui, L., H. Murakami, K. Kuwahara-Arai, H. Hanaki, and K. Hiramatsu. 2000. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 44:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fluckiger, U., M. Ulrich, A. Steinhuber, G. Döring, D. Mack, R. Landmann, C. Goerke, and C. Wolz. 2005. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect. Immun. 73:1811-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanaki, H., K. Kuwahara-Arai, S. Boyle-Vavra, R. S. Daum, H. Labischinski, and K. Hiramatsu. 1998. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J. Antimicrob. Chemother. 42:199-209. [DOI] [PubMed] [Google Scholar]
- 12.Hanaki, H., H. Labischinski, Y. Inaba, N. Kondo, H. Murakami, and K. Hiramatsu. 1998. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J. Antimicrob. Chemother. 42:315-320. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 14.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy, M. C., M. H. Emptage, J. L. Dreyer, and H. Beinert. 1983. The role of iron in the activation-inactivation of aconitase. J. Biol. Chem. 258:11098-11105. [PubMed] [Google Scholar]
- 16.Kuroda, M., K. Kuwahara-Arai, and K. Hiramatsu. 2000. Identification of the up- and down-regulated genes in vancomycin-resistant Staphylococcus aureus strains Mu3 and Mu50 by cDNA differential hybridization method. Biochem. Biophys. Res. Commun. 269:485-490. [DOI] [PubMed] [Google Scholar]
- 17.Lowry, O. H., N. J. Rosebrough, L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:267-275. [PubMed] [Google Scholar]
- 18.McAleese, F., S. W. Wu, K. Sieradzki, P. Dunman, E. Murphy, S. Projan, and A. Tomasz. 2006. Overexpression of genes of the cell wall stimulon in clinical isolates of Staphylococcus aureus exhibiting vancomycin-intermediate S. aureus-type resistance to vancomycin. J. Bacteriol. 188:1120-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mongodin, E., J. Finan, M. W. Climo, A. Rosato, S. Gill, and G. L. Archer. 2003. Microarray transcription analysis of clinical Staphylococcus aureus isolates resistant to vancomycin. J. Bacteriol. 185:4638-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patton, T. G., K. C. Rice, M. K. Foster, and K. W. Bayles. 2005. The Staphylococcus aureus cidC gene encodes a pyruvate oxidase that affects acetate metabolism and cell death in stationary phase. Mol. Microbiol. 56:1664-1674. [DOI] [PubMed] [Google Scholar]
- 21.Reipert, A., K. Ehlert, T. Kast, and G. Bierbaum. 2003. Morphological and genetic differences in two isogenic Staphylococcus aureus strains with decreased susceptibilities to vancomycin. Antimicrob. Agents Chemother. 47:568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice, K. C., J. B. Nelson, T. G. Patton, S. J. Yang, and K. W. Bayles. 2005. Acetic acid induces expression of the Staphylococcus aureus cidABC and lrgAB murein hydrolase regulator operons. J. Bacteriol. 187:813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shopsin, B., M. Gomez, M. Waddington, M. Riehman, and B. N. Kreiswirth. 2000. Use of coagulase gene (coa) repeat region nucleotide sequences for typing of methicillin-resistant Staphylococcus aureus strains. J. Clin. Microbiol. 38:3453-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieradzki, K., M. G. Pinho, and A. Tomasz. 1999. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J. Biol. Chem. 274:18942-18946. [DOI] [PubMed] [Google Scholar]
- 27.Sieradzki, K., and A. Tomasz. 2003. Alterations of cell wall structure and metabolism accompany reduced susceptibility to vancomycin in an isogenic series of clinical isolates of Staphylococcus aureus. J. Bacteriol. 185:7103-7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieradzki, K., and A. Tomasz. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somerville, G. A., S. B. Beres, J. R. Fitzgerald, F. R. DeLeo, R. L. Cole, J. S. Hoff, and J. M. Musser. 2002. In vitro serial passage of Staphylococcus aureus: changes in physiology, virulence factor production, and agr nucleotide sequence. J. Bacteriol. 184:1430-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somerville, G. A., M. S. Chaussee, C. I. Morgan, J. R. Fitzgerald, D. W. Dorward, L. J. Reitzer, and J. M. Musser. 2002. Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival. Infect. Immun. 70:6373-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somerville, G. A., B. Saïd-Salim, J. M. Wickman, S. J. Raffel, B. N. Kreiswirth, and J. M. Musser. 2003. Correlation of acetate catabolism and growth yield in Staphylococcus aureus: implications for host-pathogen interactions. Infect. Immun. 71:4724-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Styers, D., D. J. Sheehan, P. Hogan, and D. F. Sahm. 2006. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann. Clin. Microbiol. Antimicrob. 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenover, F. C., M. V. Lancaster, B. C. Hill, C. D. Steward, S. A. Stocker, G. A. Hancock, C. M. O'Hara, S. K. McAllister, N. C. Clark, and K. Hiramatsu. 1998. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J. Clin. Microbiol. 36:1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Utaida, S., P. M. Dunman, D. Macapagal, E. Murphy, S. J. Projan, V. K. Singh, R. K. Jayaswal, and B. J. Wilkinson. 2003. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 149:2719-2732. [DOI] [PubMed] [Google Scholar]
- 35.Utaida, S., R. F. Pfeltz, R. K. Jayaswal, and B. J. Wilkinson. 2006. Autolytic properties of glycopeptide-intermediate Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 50:1541-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuong, C., J. B. Kidder, E. R. Jacobson, M. Otto, R. A. Proctor, and G. A. Somerville. 2005. Staphylococcus epidermidis polysaccharide intercellular adhesin production significantly increases during tricarboxylic acid cycle stress. J. Bacteriol. 187:2967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weigel, L. M., D. B. Clewell, S. R. Gill, N. C. Clark, L. K. McDougal, S. E. Flannagan, J. F. Kolonay, J. Shetty, G. E. Killgore, and F. C. Tenover. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302:1569-1571. [DOI] [PubMed] [Google Scholar]