Abstract
Posaconazole is a triazole antifungal for prophylaxis of invasive fungal infection and treatment of oropharyngeal candidiasis. We evaluated the effects of gender, age, and race/ethnicity (black or white) on the steady-state pharmacokinetics of posaconazole in two studies on healthy adult subjects (≥18 years of age). Additionally, we explored the effect of P-glycoprotein expression and MDR1 genotype on posaconazole pharmacokinetics in black and white subjects. Age, gender, and race/ethnicity had no clinically relevant effects on posaconazole pharmacokinetics. No association was observed between any MDR1 single-nucleotide polymorphism and the area under the concentration-time curve for posaconazole. Posaconazole was safe and well tolerated regardless of age, gender, or race/ethnicity. In conclusion, age, gender, and race/ethnicity have no clinically relevant effects on the steady-state pharmacokinetics of posaconazole in healthy adults; therefore, dosage adjustments based on these covariates are unnecessary.
Elderly persons can experience compromised gastrointestinal, hepatic, and renal function (17, 23), which influences drug absorption, metabolism, and elimination. In addition, gender-related differences, such as body size and muscle mass, may result in pharmacokinetic differences (1), and genetic variations among racial/ethnic groups also can alter drug disposition. For example, genetic polymorphism of the human multidrug resistance (MDR1) gene has been shown to cause significant variability in P-glycoprotein (P-gp) expression between racial groups (22). White persons, who predominantly carry the T/T or C/T genotype, express less P-gp in intestinal epithelial cells than do black persons, who predominantly carry the C/C or C/T genotype (2, 14, 22). Thus, these factors must be considered potential sources of variability in drug pharmacokinetics parameters.
Posaconazole is a triazole antifungal for prophylaxis of invasive fungal infection and treatment of oropharyngeal candidiasis. The agent has potent in vitro and in vivo activity against a wide spectrum of clinically important yeasts and molds (3, 4, 13, 18-20, 25). Moreover, posaconazole has proven effective as a prophylaxis for high-risk patients (4a, 27) and as salvage therapy for patients with invasive fungal infections who were intolerant of, or who had disease refractory to, other antifungal therapies (26, 28) (data on file; Schering-Plough Research Institute [SPRI], Kenilworth, NJ). Posaconazole has also conferred a significant survival benefit on patients with aspergillosis (28).
Posaconazole pharmacokinetics are well characterized for healthy adults. After single or multiple doses are increased, concentrations in plasma increase in a dose-proportional manner up to 800 mg/day (5). Posaconazole oral suspension has a terminal elimination half-life (t1/2) of approximately 35 h (G. Krishna and A. Sansone-Parsons, presented at the 41st American Society of Health-System Pharmacists Midyear Clinical Meeting and Exhibition, 2006), making once-daily administration possible; exposure is significantly enhanced by twice-daily dosing and coadministration with food or a nutritional supplement (8, 10, 21). Unlike other mold-active azoles, posaconazole is not extensively metabolized by CYP450 isoenzymes (16). Instead, most (77%) of the oral dose is fecally eliminated as the parent compound, with the remainder excreted as glucuronidated derivatives in urine (16). Posaconazole inhibits hepatic CYP3A4 but has no significant effect on CYP2C8/9, CYP1A2, CYP2D6, or CYP2E1 activity (30).
Given the potentially important effects that age, gender, and race/ethnicity may have on posaconazole disposition, two independent studies were undertaken to determine if these factors alter the pharmacokinetic profile. In addition, because posaconazole is an inhibitor and substrate of P-gp (data on file; SPRI, Kenilworth, NJ), it was important to determine whether pharmacokinetics differed among racial/ethnic groups. The first study evaluated the effects of age and gender on posaconazole pharmacokinetics. The second study assessed the pharmacokinetics of posaconazole in white or black subjects and explored the effect of P-gp expression and MDR1 genotype on posaconazole pharmacokinetics in these racial/ethnic populations.
MATERIALS AND METHODS
Subjects.
Men and women aged 18 to 45 years or 65 years and older with a body mass index (BMI) between 19 and 29 kg/m2 (age and gender study), and 18 to 45 years with a BMI between 19 and 27 kg/m2 (race/ethnicity study), were eligible for enrollment if they were in good health as determined by medical history, physical examination, electrocardiography, and routine laboratory test findings. In the race/ethnicity study, both parents of each subject must have been of the same racial/ethnic background.
In both studies, subjects were excluded if they used acetaminophen within 72 h, any other over-the-counter or prescription drug within 14 days, or alcohol- or xanthine-containing substances within 72 h of study drug administration. Subjects were also excluded if they had local or systemic infections within 4 weeks of study drug administration, if they had been treated with an investigational drug within 30 days of study initiation, or if they had undergone previous treatment with posaconazole. Women were required either not to have childbearing potential or to be using effective methods of contraception.
The studies were conducted in accordance with the Declaration of Helsinki and were approved by an accredited institutional review board. All subjects gave their written informed consent before study entry.
Study design and treatment regimen.
The age and gender study was a randomized, placebo-controlled, third-party, blinded study, and the race/ethnicity study was an open-label, parallel-group study. Each had a multiple-dose, single-center design.
In the age and gender study, subjects were divided into four groups: group 1, men aged 18 to 45 years, inclusive; group 2, women aged 18 to 45 years, inclusive; group 3, men 65 years old or older; group 4, women 65 years old or older. Subjects were randomly assigned within each group to receive either posaconazole oral suspension (400 mg) twice daily or a matching placebo in a 3:1 ratio (drug/placebo). In the race/ethnicity study, subjects were divided into two groups: group 1, black subjects; group 2, white subjects. All subjects received posaconazole oral suspension (400 mg) twice daily.
In both studies, subjects received the study drug in the morning and evening on days 1 through 7 and received only the morning dose on day 8. Each dose was administered with approximately 200 ml noncarbonated, room temperature water. After administration of the posaconazole dose, the oral cavity was inspected to ensure that the dose was completely swallowed. To ensure complete dose intake, the dosing container was rinsed twice with 50 ml tap water and the contents were administered to the subject. Because previous studies showed that food, especially food with a high fat content, increases the bioavailability of posaconazole (6, 8), the study medication was administered within 10 min of the subject's completing a high-fat meal. The morning dose was administered at approximately 8 a.m., after breakfast, and the evening dose was administered at approximately 8 p.m., after dinner.
Subjects were confined to the study center during both studies, from day −2 to day 11 of the age and gender study and from day −2 to day 10 of the race/ethnicity study. No strenuous physical activity was permitted for 24 h before day −2 and for the duration of the confinement period. Subjects returned to the study center for an additional pharmacokinetic sample on the morning of day 12 and for a follow-up visit on day 30 of the age and gender study. Subjects returned for additional pharmacokinetic and safety evaluations on the mornings of days 11 and 12 of the race/ethnicity study.
The relationship between MDR1 mRNA expression and the area under the concentration-time curve (AUC) for posaconazole over the dosing interval (AUCτ) was also explored.
Sample collection and assay for pharmacokinetic assessments.
In both studies, blood samples (4 ml each) for the determination of posaconazole plasma concentrations were collected on day −1 (blank sample); on day 1 at 2, 4, 5, 6, 8, and 12 h after the morning dose; and beginning on day 8 at 0 (predose), 2, 4, 5, 6, 8, 12, 24, 48, 72, and 96 h after the morning dose. Trough samples were collected on day 6 before the evening dose and on day 7 before the morning and evening doses. All blood samples were collected into heparinized tubes and centrifuged within 30 min of collection to obtain plasma.
Plasma posaconazole concentrations were determined using a validated method of liquid chromatography with tandem mass spectrometry with a lower limit of quantitation of 1 ng/ml and a calibration range of 1 to 4,000 ng/ml. Analyses were performed at PPD Development Inc. (Richmond, VA).
Pharmacokinetic analysis.
Concentration-time data for posaconazole in plasma were used to calculate the pharmacokinetic parameters using model-independent methods (12). In both studies, the primary pharmacokinetic end points were the AUC and the maximum observed concentration (Cmax). Cmax, minimum concentration (Cmin), and time of maximum concentration (Tmax) were the observed values. The terminal-phase rate constant (k), terminal-phase half-life, and apparent total clearance (CL/F) on day 8 were determined (12).
In both studies, summary statistics (mean, standard deviation, and coefficient of variation [CV]) were provided for the posaconazole concentration data and the pharmacokinetic parameters at each time point.
In the age and gender study, the primary pharmacokinetic parameters (log-transformed AUC and Cmax) for the subset of subjects who received posaconazole were analyzed using an analysis of variance (ANOVA) model to evaluate the main effects of age and gender. The relative bioavailability of posaconazole for age and gender was assessed by the mean difference of the log-transformed AUC from 0 to 12 h (AUC0-12) and Cmax values. Ninety percent confidence intervals (90% CIs) of these estimates were calculated.
In the race/ethnicity study, the pharmacokinetic parameters were analyzed using a one-way ANOVA model for racial/ethnic group. Means for subjects administered posaconazole across both racial/ethnic groups, expressed as a ratio of the log-transformed data, were calculated for AUC0-12 and Cmax. Two-sided 90% CI estimates of the mean ratios were calculated using the pooled data.
To assess steady state, ANOVA was performed on the posaconazole Cmin data for day 6, day 7, and day 8. A subject was considered to have achieved steady state if there was no systematic increase in Cmin.
Pharmacogenetic analysis. (i) MDR1 SNP analysis.
On day −2, 6 ml whole blood was collected from participants in the race/ethnicity study to obtain genomic DNA. Samples were frozen at −20°C, shipped to DNA Sciences (now Genaissance Pharmaceuticals, Inc., Morrisville, NC) for DNA purification, and sent to Schering-Plough Research Institute for analysis. Genotyping of previously identified single-nucleotide polymorphisms (SNPs) and novel SNP identification were performed by direct cycle sequencing of PCR products amplified from the genomic DNA of study subjects. Forward and reverse primer sequences for PCR were 5′ tailed with universal sequencing primers (−21M13 [5′-TGTAAAACGACGGCCAGT] and M13REV [CAGGAAACAGCTATGACC]) and were designed using Primer3 software to amplify MDR1 coding regions.
After DNA amplification, cycle sequencing was performed using ABI PRISM BigDye Primer Cycle Sequencing DNA sequencing kits (Applied Biosystems, Foster City, CA). A, C, G, and T reaction products were pooled, purified, resuspended in water, and resolved by capillary gel electrophoresis on an ABI 3700 DNA analyzer (Applied Biosystems). Chromatograms were transferred to a Unix workstation, base-called with Phred (version 0.990722.g), assembled with Phrap (version 3.01), and scanned with Polyphred (version 3.5), and the results were viewed with Consed (version 9.0).
(ii) MDR1 mRNA expression analysis.
Total RNA was extracted from whole blood obtained from subjects at baseline (day −2) and after treatment (day 8) and was then frozen at −20°C until processing. TaqMan primers (forward sequence, 5′-AGGAAGCCAATGCCTATGACTTTA-3′; reverse sequence, 5′-CAACTGGGCCCCTCTCTCTC-3′) and probes (sequence, 5′-ATGAAACTGCCTCATAAATTTGACACCCTGG-3′) were designed with ABI Primer Express software (Applied Biosystems).
Quantitative PCR was carried out with an ABI Prism 7900HT sequence detection system (Applied Biosystems). PCR mixtures were prepared using the components from the Invitrogen (Carlsbad, CA) Platinum quantitative reverse transcription-PCR one-step kit. Reverse transcription-PCR was performed in a single 384-well plate; separate plates of the same RNAs were used to quantitate 18S RNA as an internal control for RNA quality, and a primer/probe set for the CD4 promoter was used to check the RNAs for genomic contamination. PCR data were quantitated based on a standard curve generated using serial dilutions of a fragment of the MDR1 gene generated by PCR. Fourfold dilutions began at 0.25 ng, and 8 dilutions were used to generate the standard curve. Quantities were then calculated based on the standards and were converted to femtograms per reaction.
Safety analysis.
In both studies, safety was assessed based on tabulations of adverse events and results of physical examinations, vital signs, electrocardiograms, and clinical laboratory tests (complete blood count, blood chemistry, and urinalysis). Subjects were continuously observed and questioned during the studies for the possible occurrence of adverse events. The investigators assessed the intensity (mild, moderate, severe, or life-threatening) of each adverse event and its relationship to the study medication.
RESULTS
Subject disposition and demographics.
In the age and gender study, 73 subjects were enrolled, and 69 received at least one dose of study medication (51 with posaconazole, 18 with a placebo). Of these 69 subjects, 3 subjects in the posaconazole group withdrew from the study for reasons unrelated to the assigned study treatment and 2 placebo-treated subjects discontinued early because of dietary issues. Sixty-four subjects completed the study. Plasma pharmacokinetic parameters were determined from the 48 subjects who completed dosing with posaconazole, and data from all 69 treated subjects were included in the safety analysis.
In the race/ethnicity study, 56 subjects enrolled in and completed the study. Data from all 56 subjects were used to assess the pharmacokinetic parameters of posaconazole and to evaluate its safety and tolerability. All subjects participated in the pharmacogenetic analysis of MDR1 genotype and RNA expression. In both studies, demographic and baseline characteristics were well balanced between comparator groups (Table 1).
TABLE 1.
Demographic and baseline characteristics of subjects
| Characteristic | Age and gender study
|
Race/ethnicity study
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men, 18-45 yr old (n = 18) | Women, 18-45 yr old (n = 18) | Men, ≥65 yr old (n = 17) | Women, ≥65 yr old (n = 16) | White subjects (n = 28) | Black subjects (n = 28) | Total (n = 56) | ||||||||
| Demographic characteristics
|
||||||||||||||
| Age (yr)
|
||||||||||||||
| Mean (SD) | 30.0 (7.6) | 29.8 (10.5) | 71.3 (5.6) | 71.4 (6.1) | 32.6 (8.8) | 36.7 (6.7) | 34.6 (8.0) | |||||||
| Range | 19-43 | 18-44 | 65-83 | 64-85 | 18-45 | 24-45 | 18-45 | |||||||
| Gender (no. [%])
|
||||||||||||||
| Female | 0 | 18 (100) | 0 | 16 (100) | 10 (36) | 2 (7) | 12 (21) | |||||||
| Male | 18 (100) | 0 | 17 (100) | 0 | 18 (64) | 26 (93) | 44 (79) | |||||||
| Race/ethnicity (no. [%])
|
||||||||||||||
| White | 16 (88.9) | 17 (94.4) | 16 (94.1) | 16 (100) | 28 (100) | 0 | 28 (50) | |||||||
| Black | 1 (5.6) | 0 | 1 (5.9) | 0 | 0 | 28 (100) | 28 (50) | |||||||
| Asian | 1 (5.6) | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| Hispanic | 0 | 1 (5.6) | 0 | 0 | 0 | 0 | 0 | |||||||
| Baseline characteristics
|
||||||||||||||
| Wt (kg)
|
||||||||||||||
| Mean (SD) | 78.2 (10.7) | 69.1 (9.2) | 82.4 (11.9) | 70.8 (9.5) | 75.56 (8.78) | 76.92 (7.38) | 76.24 (8.06) | |||||||
| Range | 62.3-101.8 | 51.4-81.8 | 65.9-110.9 | 55.5-90.0 | 59.1-100.0 | 62.7-90.0 | 59.1-100.0 | |||||||
| BMI (kg/m2)
|
||||||||||||||
| Mean (SD) | 24.2 (2.4) | 24.5 (3.0) | 25.9 (2.1) | 27.0 (3.7) | NRa | NR | NR | |||||||
| Range | 19.7-28.4 | 19.0-29.0 | 22.3-29.8 | 21.7-37.5 | NR | NR | NR | |||||||
NR, not reported.
Posaconazole pharmacokinetics. (i) Age and gender study.
On day 1, there was a small increase in posaconazole exposure for elderly men compared with elderly women; however, this difference was not observed after multiple-dose administration (Tables 2 and 3). Therefore, pharmacokinetic data for men and women were combined, thereby increasing the power of the analysis for the effect of age.
TABLE 2.
Mean pharmacokinetic parameters of posaconazole on day 8 after multiple-dose administration of posaconazole at 400 mg twice a day
| Parameter | Men and women, pooled
|
Young subjects (18-45 yr old)
|
Elderly subjects (≥65 yr old)
|
|||
|---|---|---|---|---|---|---|
| Young (18-45 yr old) (n = 24) | Elderly (≥65 yr old) (n = 24) | Men (n = 13) | Women (n = 11) | Men (n = 12) | Women (n = 12) | |
| Cmax (ng/ml) (CV) | 2,607 (40) | 3,374 (38) | 2,682 (51) | 2,517 (18) | 3,250 (36) | 3,498 (40) |
| Median Tmax (h) (range) | 5 (4-8) | 5 (0-12) | 4 (4-8) | 5 (4-8) | 5 (0-6) | 6 (0-12) |
| AUC0-12 (ng · h/ml) (CV) | 26,740 (40) | 35,444 (39) | 27,784 (51) | 25,507 (20) | 32,963 (35) | 37,924 (43) |
| t&12frac; (h) (CV) | 31.6 (18)a,b | 41.8 (27)b,c | 31.3 (21)b,d | 31.9 (14)b,e | 37.7 (21)b,f | 42.4 (NC)b,g,h |
| CL/F (liters/h) (CV) | 33.1 (29) | 26.7 (45) | 33.5 (35) | 32.6 (22) | 28.1 (45) | 25.4 (46) |
n = 18.
t&12frac; could not be determined for some subjects because the number of samples collected during the terminal elimination phase was insufficient.
n = 9.
n = 10.
n = 8.
n = 6.
n = 2.
NC, not calculated. CV could not be calculated for <3 subjects.
TABLE 3.
Statistical comparisons on days 1 and 8, based on log-transformed data, expressed as the ratios of the categories of subjects compared
| Parameter and ratio | Relative bioavailability (%) | 90% CI | ||
|---|---|---|---|---|
| Day 1
|
||||
| Cmax (ng/ml)
|
||||
| Young women/young men | 101 | 79-129 | ||
| Elderly women/elderly men | 70.6 | 55-90 | ||
| Elderly/younga | 120 | 100-143 | ||
| AUC0-12 (ng · h/ml)
|
||||
| Young women/young men | 98.9 | 79-124 | ||
| Elderly women/elderly men | 76.1 | 61-95 | ||
| Elderly/younga | 113 | 96-133 | ||
| Day 8
|
||||
| Cmax (ng/ml)
|
||||
| Young women/young men | 100 | 77-129 | ||
| Elderly women/elderly men | 107 | 83-138 | ||
| Elderly/younga | 126 | 106-150 | ||
| AUC0-12 (ng · h/ml)
|
||||
| Young women/young men | 98.1 | 75-128 | ||
| Elderly women/elderly men | 112 | 87-146 | ||
| Elderly/younga | 129 | 108-155 | ||
Male and female subjects combined.
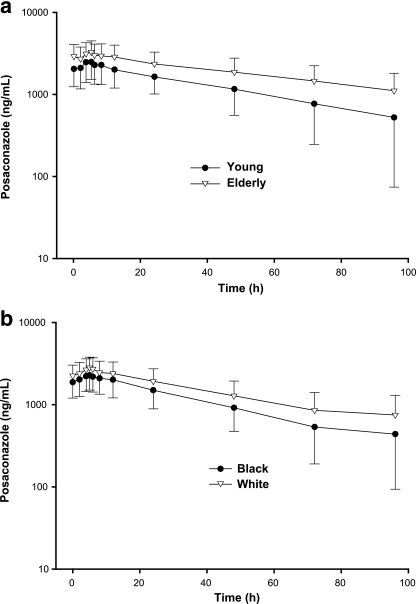
Age had a small but clinically insignificant effect on the pharmacokinetics of posaconazole at steady state (Table 2; Fig. 1a). Relative oral bioavailability estimates based on log-transformed Cmax and AUC values comparing elderly subjects with young subjects were 126% (90% CI, 106% to 150%) and 129% (90% CI, 108% to 155%), respectively (Table 3). However, because the AUC range for young (14,081 to 70,185 ng · h/ml) and elderly (14,495 to 64,675 ng · h/ml) subjects showed considerable overlap and because CVs for mean AUC and Cmax values ranged from 38% to 40%, this small difference in exposure was not considered relevant in light of the interpatient variability in posaconazole exposures seen within each group.
FIG. 1.
Mean posaconazole concentration-time plot. (a) Young and elderly subjects (men and women combined) at steady state. (b) Black subjects and white subjects at steady state.
Peak concentrations of posaconazole in plasma were attained at a median Tmax of 4 to 6 h after oral administration to young, elderly, male, and female subjects, and steady state was achieved after multiple-dose administration for 8 days. At steady state, CL/F values were similar among the four groups (Table 2).
(ii) Race/ethnicity study.
After single-dose (day 1) and multiple-dose (steady state, day 8) administration of posaconazole, no statistically significant differences in the AUC0-12 and Cmax values were noted between black subjects and white subjects (Table 4; Fig. 1b).
TABLE 4.
Mean pharmacokinetic parameters of posaconazole on day 8 after multiple-dose administration of posaconazole oral suspension at 400 mg twice a day to 56 healthy subjects of different racial/ethnic groups
| Parameter | White subjects (n = 28) | Black subjects (n = 28) |
|---|---|---|
| Cmax (ng/ml) (CV) | 2,857 (36) | 2,399 (36) |
| Median Tmax (h) (range) | 5 (4-8) | 5 (2-12) |
| AUC0-12 (ng · h/ml) (CV) | 30,091 (37) | 25,107 (36) |
| t&12frac; (h) (CV) | 37.3 (24)a,b | 32.2 (24)b,c |
| CL/F (liters/h) (CV) | 31.7 (50) | 37.0 (44) |
n = 18.
t&12frac; could not be determined for some subjects.
n = 21.
On day 1, relative oral bioavailability estimates, based on log-transformed Cmax and AUC0-12 values comparing blacks with whites, were 99.8% (90% CI, 81% to 124%) and 102% (90% CI, 82% to 126%). At steady state (day 8), there was a trend toward decreased exposure for black subjects; mean AUC0-12 values were 25,107 ng · h/ml for black subjects and 30,091 ng · h/ml for white subjects. Relative bioavailability estimates based on log-transformed Cmax and AUC0-12 values were 84.4% (90% CI, 70% to 101%) and 84.2% (90% CI, 70% to 101%), respectively. In light of the intersubject variability of Cmax and AUC values (CV range, 36% to 37%) and the considerable overlap, based on the rangse of AUC values for black subjects and white subjects (8,938 to 41,785 ng · h/ml versus 9435 to 50,080 ng · h/ml, respectively), this increase in exposure was not considered clinically relevant.
Pharmacogenetic analysis of MDR1 genotype and RNA expression.
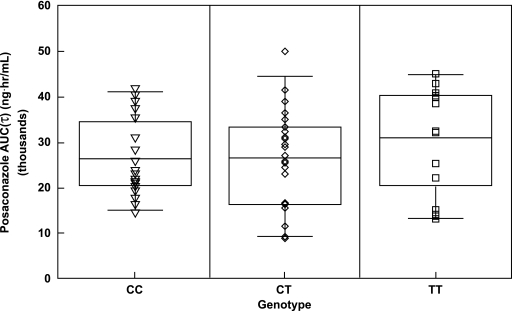
DNA sequence analysis of 9 of 27 exons of the MDR1 gene identified 41 SNPs. Fewer than half (20/41) of the identified SNPs had minor allele frequencies greater than 5% in black subjects or white subjects. Twelve of 20 SNPs had not been described previously. Of the 28 white subjects, 39% carried the T/T genotype, 50% carried the C/T genotype, and 11% carried the C/C genotype. Of the 28 black subjects, 7% carried the T/T genotype, 36% carried the C/T genotype, and 57% carried the C/C genotype. These data are consistent with those reported previously, indicating that the distribution of MDR1 genotypes in the population recruited are consistent with those of the general population (22). No association was observed between any MDR1 SNP and the AUC of posaconazole (Fig. 2). Furthermore, no correlation was noted (r2 = 0.0128) between MDR1 mRNA levels and the AUC of posaconazole.
FIG. 2.
Effects of C3435T polymorphism on the pharmacokinetics of posaconazole.
Safety and tolerability.
Safety analyses included data for 69 subjects in the age and gender study and 56 subjects in the race/ethnicity study. In both studies, posaconazole was safe and well tolerated in all groups after multiple-dose administration.
(i) Adverse events.
In the age and gender study, 43 (62%) subjects reported adverse events during the study period. Similar rates of adverse events were reported among the subjects administered posaconazole (33/51 [65%]) and placebo (10/18 [56%]). The most common treatment-emergent adverse events among posaconazole-treated subjects were headache (9/51 [18%]), musculoskeletal pain (6/51 [12%]), abdominal pain (5/51 [10%]), fever (4/51 [8%]), and pharyngitis (4/51 [8%]). Although musculoskeletal pain and pharyngitis were not considered to be related to posaconazole, approximately half the remaining treatment-emergent adverse events were considered possibly or probably related to posaconazole. The most common treatment-emergent adverse events reported for the placebo group were nausea (22%), constipation (11%), flatulence (11%), and headache (11%) (Table 5). Men reported more adverse events than women (37% versus 11%, respectively), though plasma posaconazole concentrations were similar for men and women. Additionally, there was no clear association between the incidence of adverse events among posaconazole-treated subjects and age or gender.
TABLE 5.
Most common treatment-emergent adverse events in the age and gender study
| Group (n) | No. (%) of subjects with the following adverse event:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Headache | Musculoskeletal pain | Abdominal pain | Fever | Pharyngitis | Constipation | Flatulence | Nausea | |
| Posoconazole (51) | 33 (65) | 9 (18) | 6 (12) | 5 (10) | 4 (8) | 4 (8) | 3 (6) | 3 (6) | 2 (4) |
| Placebo (18) | 10 (56) | 2 (11) | 0 | 0 | 1 (6) | 0 | 2 (11) | 2 (11) | 4 (22) |
Treatment-emergent adverse events were mild to moderate in severity, except for one report of a severe headache, which resolved in approximately 3 h after treatment with acetaminophen, for a 29-year old man receiving posaconazole. It was thought that the headache was possibly related to posaconazole treatment. Seventeen days after receiving the last dose of posaconazole, a 42-year-old man experienced a serious adverse event and was admitted to the hospital because of midsternal chest pain, elevated blood pressure, rapid heart rate, numbness in the right arm and jaw, and swelling and tingling in the hands associated with the primary symptoms. Electrocardiography revealed a normal sinus rhythm with no signs of ischemia or infarction, and cardiac profile and d-dimer test findings were negative. The symptoms resolved, and the subject was discharged from the hospital after 2 days with a prescription for atenolol. No significant changes in blood pressure resulted from the study medication, and all electrocardiograms were normal. The investigator considered all events for this patient unlikely to be related to study drug.
In the race/ethnicity study, 43 (77%) of the 56 subjects reported at least one adverse event. There was no significant difference in the incidence of treatment-emergent adverse events between white subjects (20/28 [71%]) and black subjects (23/28 [82%]). For both racial/ethnic groups, the most common adverse events were dry mouth (21/56 [38%]), headache (17/56 [30%]), dry skin (12/56 [21%]), increased frequency of micturition (11/56 [20%]), and insomnia (7/56 [13%]). All adverse events were mild to moderate in intensity, and most were considered by the investigator to be possibly related to treatment administration.
(ii) Clinical laboratory test results.
In the age and gender study, 18 of 51 posaconazole-treated subjects (33%) experienced at least one mild or moderate elevation (grade 1 or 2 by the Common Toxicity Criteria) in levels of hepatic enzymes (gamma-glutamyltranspeptidase, aspartate aminotransferase, and alanine aminotransferase). Incidences of elevated hepatic enzyme concentrations were similar across the four study groups. Elevated concentrations of one or more liver enzymes were considered adverse events by the investigator for five of these subjects. All levels returned to normal by the 30-day follow-up visit. No other clinically significant changes in laboratory parameters from baseline were reported.
In the race/ethnicity study, no clinically significant changes in laboratory parameters were observed. Specifically, no elevations in liver function tests were noted. No consistent or clinically relevant changes in blood pressure, pulse rate, or oral body temperature were observed in either study.
DISCUSSION
The results of these pharmacokinetic and pharmacogenetic studies demonstrate that age, gender, and race/ethnicity have no clinically relevant effect on posaconazole pharmacokinetics in healthy subjects. Although posaconazole exposure was increased by 26% to 29% for elderly subjects compared with younger subjects after multiple-dose administration, this modest increase in exposure was not considered clinically relevant, because the AUC range for young (14,081 to 70,185 ng · h/ml) and elderly (14,495 to 64,675 ng · h/ml) subjects showed considerable overlap and the incidence of adverse events among age groups was similar. Gender and race/ethnicity (black or white) had no clinically relevant effect on posaconazole pharmacokinetics. These observations are in agreement with the results of a previous study (R. Courtney, M. Martinho, J. Lim, et al., presented at the 13th European Congress of Clinical Microbiology and Infectious Diseases, 2003) that showed age, gender, race/ethnicity, and weight had no effect on plasma posaconazole concentrations at steady state in 46 patients with human immunodeficiency virus and oropharyngeal or esophageal candidiasis.
The lack of an age-related effect on posaconazole pharmacokinetic parameters is likely attributable to the manner in which posaconazole is eliminated from the body. Renal excretion is a minor route of posaconazole elimination (16). The results of a single-dose pharmacokinetic study of healthy volunteers with normal renal function and patients with varying degrees of renal insufficiency indicated that plasma posaconazole concentrations are not affected by renal insufficiency (7). Most (77%) of an orally administered posaconazole dose is excreted unchanged in the feces; oxidative metabolism accounts for only a minor route of elimination (16). Approximately 14% of an administered dose is renally excreted, primarily as glucuronide conjugates (11, 16). Because the phase II biotransformations are not affected by age to the same extent as the phase I biotransformations (23), it is unlikely that the terminal-phase half-life of posaconazole is prolonged in the elderly.
Although age and gender do not influence posaconazole pharmacokinetics, they have been shown to alter the disposition of other triazole antifungal agents. For example, systemic exposure to oral voriconazole is as much as twofold greater for healthy women aged 18 to 45 years than for healthy men in the same age range, although gender has no effect on voriconazole pharmacokinetics when women and men 65 years old or older are compared with each other (15) (package inserts for VFEND [voriconazole] for injection, VFEND tablets, and VFEND for oral suspension; Pfizer). Furthermore, plasma voriconazole concentrations are 80% to 90% higher in elderly patients (older than 65 years) than in younger patients, though these higher concentrations are not associated with an increased incidence of adverse events (15) (package inserts for VFEND for injection, VFEND tablets, and VFEND for oral suspension; Pfizer). Mean t1/2 values for fluconazole differ with age: 16.8 to 18.1 h for children, 25.1 h for younger women, 29 h for younger men, and 37.2 h for men and women aged 65 and older (9).
Racial/ethnic background might have been expected to influence the disposition of posaconazole because of differences in P-gp polymorphism between black subjects and white subjects. Posaconazole, though weak in comparison to ketoconazole and itraconazole, is a substrate and an inhibitor of P-gp, based on results from in vitro studies (29) (data on file; Schering-Plough Research Institute, Kenilworth, NJ). An apparent lack of any net P-gp-mediated effect on the disposition of posaconazole was further confirmed by a lack of association between any MDR1 SNP or MDR1 mRNA and the AUC of posaconazole. Based on these findings, it was concluded that the moderate variability observed in the pharmacokinetic parameters of posaconazole was probably not caused by interindividual differences in MDR1 expression and that P-gp does not contribute significantly to posaconazole disposition in an in vivo setting, even though posaconazole is a substrate for P-gp, based on in vitro data. In contrast, P-gp polymorphisms may influence itraconazole to a far greater extent. This was demonstrated in a study in which itraconazole was administered before fexofenadine, a P-gp substrate, to individuals with either the T/T or the G/C phenotype (24). The AUC of fexofenadine was found to be significantly higher and CL/F to be significantly lower in individuals with the T/T phenotype than in individuals with the G/C haplotype, thus indicating greater P-gp inhibition by itraconazole in the T/T individuals.
Of note, the CL/F values were substantially higher in these studies than in a previous study (5), in which the value of CL/F for the same dose as that used in the current studies, 400 mg twice daily, was 11.5 liters/h on day 14 of posaconazole administration. All doses in that study were administered in the tablet formulation. In the current studies, values ranged from 25.4 liters/h to 37 liters/h, and all doses were administered as the oral suspension. The dissimilarity in CL/F values among these studies may stem from differences in exposure with use of the tablet formulation versus the oral suspension. This is supported by the findings of another study, which found that posaconazole administered as a single 200-mg oral-suspension dose showed a significant increase in bioavailability compared with administration as two 100-mg tablets (137% increase in AUC0-72) (8). Based on these data, the oral suspension rather than the tablet formulation was selected for development in the posaconazole clinical program.
In the present studies, posaconazole was safe and well tolerated regardless of age, gender, or race/ethnicity. Additionally, no consistent, clinically relevant changes in blood pressure, pulse rate, or oral body temperature were observed in either study.
In conclusion, age, gender, and race/ethnicity have no clinically relevant effects on the steady-state pharmacokinetics of posaconazole. Dosage adjustments based on these covariates are, therefore, unnecessary. In all populations studied, posaconazole at 400 mg twice daily for 8 days was safe and well tolerated, consistent with the similar mean plasma posaconazole concentration-time profiles observed for each group.
Acknowledgments
We thank the staff and volunteers of Covance GFI Research (previously West Pharmaceutical Services, Inc.) and also Research Solutions, Little Rock, AR.
This work was supported by Schering-Plough Research Institute.
None of the authors have commercial affiliations, consultancies, stock or equity interests, and/or patent licensing arrangements that could be considered to pose a conflict of interest.
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Beierle, I., B. Meibohm, and H. Derendorf. 1999. Gender differences in pharmacokinetics and pharmacodynamics. Int. J. Clin. Pharmacol. Ther. 37:529-547. [PubMed] [Google Scholar]
- 2.Cascorbi, I., T. Gerloff, A. Johne, C. Meisel, S. Hoffmeyer, M. Schwab, E. Schaeffeler, M. Eichelbaum, U. Brinkmann, and I. Roots. 2001. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin. Pharmacol. Ther. 69:169-174. [DOI] [PubMed] [Google Scholar]
- 3.Connolly, P., J. Wheat, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, J. Goldberg, E. Brizendine, and D. Loebenberg. 1999. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob. Agents Chemother. 43:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly, P., L. J. Wheat, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, J. Goldberg, E. Brizendine, and D. Loebenberg. 2000. Comparison of a new triazole, posaconazole, with itraconazole and amphotericin B for treatment of histoplasmosis following pulmonary challenge in immunocompromised mice. Antimicrob. Agents Chemother. 44:2604-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Cornely, O. A., J. Maertens, D. Winston, et al. 2005. Abstr. 47th Am. Soc. Hematol., abstr 1844.
- 5.Courtney, R., S. Pai, M. Laughlin, J. Lim, and V. Batra. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtney, R., E. Radwanski, J. Lim, and M. Laughlin. 2004. Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men. Antimicrob. Agents Chemother. 48:804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtney, R., A. Sansone, W. Smith, T. Marbury, P. Statkevich, M. Martinho, M. Laughlin, and S. Swan. 2005. Posaconazole pharmacokinetics, safety, and tolerability in subjects with varying degrees of chronic renal disease. J. Clin. Pharmacol. 45:185-192. [DOI] [PubMed] [Google Scholar]
- 8.Courtney, R., D. Wexler, E. Radwanski, J. Lim, and M. Laughlin. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debruyne, D., and J. P. Ryckelynck. 1993. Clinical pharmacokinetics of fluconazole. Clin. Pharmacokinet. 24:10-27. [DOI] [PubMed] [Google Scholar]
- 10.Ezzet, F., D. Wexler, R. Courtney, G. Krishna, J. Lim, and M. Laughlin. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211-220. [DOI] [PubMed] [Google Scholar]
- 11.Ghosal, A., N. Hapangama, Y. Yuan, J. Achanfuo-Yeboah, R. Iannucci, S. Chowdhury, K. Alton, J. E. Patrick, and S. Zbaida. 2004. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil). Drug Metab. Dispos. 32:267-271. [DOI] [PubMed] [Google Scholar]
- 12.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics. Marcel Dekker, New York, NY.
- 13.Gonzalez, G. M., R. Tijerina, L. K. Najvar, R. Bocanegra, M. Rinaldi, D. Loebenberg, and J. R. Graybill. 2002. In vitro and in vivo activities of posaconazole against Coccidioides immitis. Antimicrob. Agents Chemother. 46:1352-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmeyer, S., O. Burk, O. Von Richter, H. P. Arnold, J. Brockmoller, A. Johne, I. Cascorbi, T. Gerloff, I. Roots, M. Eichelbaum, and U. Brinkmann. 2000. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 97:3473-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeu, L., F. J. Piacenti, A. G. Lyakhovetskiy, and H. B. Fung. 2003. Voriconazole. Clin. Ther. 25:1321-1381. [DOI] [PubMed] [Google Scholar]
- 16.Krieter, P., B. Flannery, T. Musick, M. Gohdes, M. Martinho, and R. Courtney. 2004. Disposition of posaconazole following single-dose oral administration in healthy subjects. Antimicrob. Agents Chemother. 48:3543-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamy, P. P. 1991. Physiological changes due to age. Pharmacodynamic changes of drug action and implications for therapy. Drugs Aging 1:385-404. [DOI] [PubMed] [Google Scholar]
- 18.Oakley, K. L., G. Morrissey, and D. W. Denning. 1997. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2001. In vitro activities of posaconazole (SCH 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:2862-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and the SENTRY Participants Group. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sansone-Parsons, A., G. Krishna, A. Calzetta, D. Wexler, B. Kantesaria, M. A. Rosenberg, and M. A. Saltzman. 2006. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob. Agents Chemother. 50:1881-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaeffeler, E., M. Eichelbaum, U. Brinkmann, A. Penger, S. Asante-Poku, U. M. Zanger, and M. Schwab. 2001. Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet 358:383-384. [DOI] [PubMed] [Google Scholar]
- 23.Schmucker, D. L. 2001. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging 18:837-851. [DOI] [PubMed] [Google Scholar]
- 24.Shon, J. H., Y. R. Yoon, W. S. Hong, P. M. Nguyen, S. S. Lee, Y. G. Choi, I. J. Cha, and J. G. Shin. 2005. Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin. Pharmacol. Ther. 78:191-201. [DOI] [PubMed] [Google Scholar]
- 25.Sun, Q. N., L. K. Najvar, R. Bocanegra, D. Loebenberg, and J. R. Graybill. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob. Agents Chemother. 46:2310-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullmann, A. J., O. A. Cornely, A. Burchardt, R. Hachem, D. P. Kontoyiannis, K. Topelt, R. Courtney, D. Wexler, G. Krishna, M. Martinho, G. Corcoran, and I. Raad. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullmann, A. J., J. H. Lipton, D. H. Vesole, et al. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M716.
- 28.Walsh, T. J., I. Raad, T. F. Patterson, P. Chandrasekar, G. R. Donowitz, R. Graybill, R. E. Greene, R. Hachem, S. Hadley, R. Herbrecht, A. Langston, A. Louie, P. Ribaud, B. H. Segal, D. A. Stevens, J.-A. H. van Burik, C. S. White, G. Corcoran, J. Gogate, G. Krishna, L. Pedicone, C. Hardalo, and J. R. Perfect. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2-12. [DOI] [PubMed] [Google Scholar]
- 29.Wang, E. J., K. Lew, C. N. Casciano, R. P. Clement, and W. W. Johnson. 2002. Interaction of common azole antifungals with P glycoprotein. Antimicrob. Agents Chemother. 46:160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wexler, D., R. Courtney, W. Richards, C. Banfield, J. Lim, and M. Laughlin. 2004. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. Eur. J. Pharm. Sci. 21:645-653. [DOI] [PubMed] [Google Scholar]