Abstract
The gram-negative soil bacillus Burkholderia pseudomallei is the causative agent of melioidosis, a severe and potentially fatal septicemic disease that is endemic to Southeast Asia and northern Australia. Its intrinsic resistance to many antibiotics is attributed mainly to the presence of several drug efflux pumps, and therefore, inhibitors of such pumps are expected to restore the activities of many clinically important antimicrobial agents that are the substrates of these pumps. The phenothiazine antipsychotic and antihistaminic drugs prochlorperazine, chlorpromazine, and promazine have a synergistic interaction with a wide spectrum of antimicrobial agents, thereby enhancing their antimicrobial potency against B. pseudomallei. Antimicrobial agents that interacted synergistically with the phenothiazines include streptomycin, erythromycin, oleandomycin, spectinomycin, levofloxacin, azithromycin, and amoxicillin-clavulanic acid. The MICs of these antibiotics were reduced as much as 8,000-fold in the presence of the phenothiazines. Antimicrobial agents which did not interact synergistically with the phenothiazines include gentamicin, amoxicillin, and ampicillin. Omeprazole, a proton pump inhibitor, provided an augmentation of antimicrobial activities similar to that of the phenothiazines, suggesting that the phenothiazines might have interfered with the proton gradient at the inner membrane. B. pseudomallei cells accumulated more erythromycin in the presence of the phenothiazines, an effect similar to that of carbonyl cyanide m-chlorophenylhydrazone, a proton gradient uncoupler. In the presence of the phenothiazines, a much reduced concentration of erythromycin (0.06× MIC) also protected human lung epithelial cells and macrophage cells from B. pseudomallei infection and attenuated its cytotoxicity.
Melioidosis is an infectious disease that is caused by the gram-negative soil bacillus Burkholderia pseudomallei, which is endemic in Southeast Asia and northern Australia. Infection may be acquired through direct skin contact with contaminated soil or surface water or by ingestion of such contaminated water or dust. Clinical symptoms depend upon the route of infection, but four clinical forms are generally described: localized infection, pulmonary infection, septicemia, and chronic suppurative infections of the skin. The disease ranges from unsuspected asymptomatic infection to overwhelming and fatal septicemia. Prostate, liver, and spleen abscesses are common presentations in infected adults, while acute suppurative parotitis is observed in almost 40% of pediatric cases in Thailand (7, 24, 25).
B. pseudomallei is intrinsically resistant to many common antibiotics, including β-lactams, penicillins, narrow-spectrum and expanded-spectrum cephalosporins, most aminoglycosides, macrolides, rifampin, and polymyxins (5). Ceftazidime is the drug of choice for the treatment of severe melioidosis, and co-amoxiclav or a combination of co-trimoxazole and doxycycline is used for maintenance therapy, which is typically over 20 weeks. B. pseudomallei is also classified as a potential agent for bioterrorism, for which co-trimoxazole is recommended for postexposure prophylaxis in the event of a biological attack, as there is no melioidosis vaccine available for humans (2). It has become apparent that efflux-related multidrug resistance (MDR) is a significant complicating factor in the chemotherapy of bacterial infections. The B. pseudomallei genome encodes several three-component efflux pumps, comprising an integral cytoplasmic membrane drug proton antiporter of the resistance-nodulation-division (RND) family and a channel-forming outer membrane protein linked together by a periplasmic protein. The majority of these pumps remain uncharacterized, but the BpeAB-OprB and AmrAB-OprA efflux pumps are known to be responsible for resistance to aminoglycosides and macrolides through proton gradient-dependent efflux of the drugs (4, 17).
Inhibitors of MDR efflux pumps would be expected to restore the activities of antimicrobial agents that are substrates of these pumps. In B. pseudomallei, inhibition of BpeAB-OprB would provide an additional benefit of attenuating virulence, because of the involvement of the pump in the efflux of quorum-sensing autoinducers (3). The search for candidate efflux pump inhibitors (EPIs) can be costly and time-consuming, so it is reasonable to examine selected drugs that are already in clinical use as potential inhibitors. Phenothiazines are dopamine receptor antagonists which are used clinically as antihistaminic agents or neuroleptics for the management of psychosis and have been shown to have modest antimicrobial activities against a wide array of microorganisms (13). When combined at one-fourth their MICs with common antimicrobial substrates of MDR pumps of Staphylococcus aureus, phenothiazines augmented the antimicrobial activities of these substrates (11). Phenothiazines also potentiated the activities of some antitubercular drugs against multidrug-resistant Mycobacterium tuberculosis strains (26). In this regard, phenothiazines could be used as adjuncts to antibiotics where resistance is noted. Phenothiazines are hypothesized to inhibit the proton motive force-dependent pumps, possibly via their direct interaction with the pump and, to a lesser extent, a reduction in the transmembrane potential (11).
In this study, we addressed the effect of the phenothiazines prochlorperazine (PCPZ), chlorpromazine (CPZ), and promazine (PMZ) on the augmentation of antimicrobial activities of several antibiotics, especially the aminoglycosides and macrolides, which are the substrates of the B. pseudomallei BpeAB-OprB and AmrAB-OprB efflux pumps. We showed that phenothiazines produced a synergistic interaction with the antibiotics similar to that of omeprazole (OPZ), a proton pump inhibitor, thus suggesting that phenothiazines might disrupt the proton gradient required by the B. pseudomallei RND efflux pumps. This was verified by an impaired efflux of erythromycin by B. pseudomallei in the presence of the phenothiazines PCPZ and CPZ. Additionally, we showed that the inclusion of a phenothiazine as an adjunct treatment, together with a subinhibitory concentration of erythromycin, protected human lung epithelial cells and macrophage cells from B. pseudomallei infection and its cytotoxicity.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Burkholderia pseudomallei KHW is a virulent clinical isolate which we have described and used previously in our studies (6). For routine maintenance cultures and an erythromycin accumulation assay, B. pseudomallei KHW was cultured on Luria-Bertani (LB) agar or LB broth (Becton Dickinson, Cockeysville, MD). For determination of antimicrobial susceptibility and a checkerboard titration assay, B. pseudomallei KHW was cultured in Mueller-Hinton broth (Becton Dickinson).
Determination of antimicrobial susceptibilities.
MICs were assayed using 96-well microtiter plates with the standard broth microdilution method as described previously (4, 18). All antibiotics used were purchased from Sigma (St. Louis, MO), except amoxicillin-clavulanic acid (AMC), levofloxacin (LVX), and azithromycin (AZM), which were purchased from Beecham Pharmaceuticals (Brentford, England), Aventis Pharma Deutschland GmbH (Frankfurt, Germany), and Pfizer S.A. (New York, NY), respectively. The checkerboard titration assay used for assessing interaction between PCPZ, CPZ, PMZ, or OPZ and an antibiotic was performed in 96-well microtiter plates according to the protocol described by Lomovskaya et al. (16). The antibiotics tested included streptomycin, gentamicin, erythromycin (ERY), oleandomycin, amoxicillin, ampicillin, spectinomycin, AMC, LVX, and AZM. MICs of the antimicrobial agents were determined either alone or together with the phenothiazines or omeprazole at concentrations ranging from 0.98 to 1,000 μM. Prochlorperazine dimaleate salt, chlorpromazine hydrochloride, promazine hydrochloride, and omeprazole were purchased from Sigma.
[14C]erythromycin accumulation assay.
Efflux of erythromycin was monitored by measuring the amount of [14C]erythromycin in intact B. pseudomallei KHW cells according to the procedure described previously (4). Briefly, 5 ml of LB medium was inoculated (1:50) with an overnight culture of B. pseudomallei KHW and incubated for 2 h at 37°C with shaking until the optical density at 600 nm was ∼0.5. PCPZ, CPZ, or carbonyl cyanide m-chlorophenylhydrazone (CCCP; Sigma) was added to a final concentration of 250 μM, 500 μM, or 20 μM, respectively. After 10 min, [14C]erythromycin was added to a final concentration of 0.1 μg/ml. One-milliliter aliquots were removed at the beginning of the assay and 4 h later, and the amount of [14C]erythromycin in the cells was measured using an LS6500 multipurpose liquid scintillation counter (Beckman Instruments Inc., Fullerton, CA).
Cell invasion and cytotoxicity assays.
Invasion of A549 human lung epithelial cells and THP-1 human macrophage cells with wild-type B. pseudomallei KHW was performed as described previously (4), with the following modifications. The bacteria were added to the mammalian cells in 1.5 ml of culture medium containing one of the following and incubated for 4 h: PCPZ (250 μM), CPZ (500 μM), or PCPZ (250 μM) and erythromycin (8 μg/ml); CPZ (500 μM) and erythromycin (8 μg/ml); erythromycin (8 μg/ml) only; or erythromycin (128 μg/ml) only. No erythromycin or phenothiazine was added to the positive control. Human lung epithelial cells (A549) and macrophage cells (THP-1) were cultured using Dulbecco's modified Eagle medium or RPMI 1640 medium, respectively, which were supplemented with 10% (vol/vol) fetal bovine serum (Sigma). The assay was performed in triplicate.
The cytotoxicity of B. pseudomallei on A549 and THP-1 cells was determined by measuring the release of lactate dehydrogenase enzyme using a cytotoxicity detection kit (Roche, Mannheim, Germany) as described previously (3). Briefly, mid-log-phase B. pseudomallei KHW cells were added at a multiplicity of infection of 100 to the wells of a 24-well microtiter plate containing A549 or THP-1 cells (105 cells/well) in culture medium containing PCPZ (250 μM), CPZ (500 μM), PCPZ (250 μM), and erythromycin (8 μg/ml); CPZ (500 μM) and erythromycin (8 μg/ml); erythromycin (8 μg/ml) alone; or erythromycin (128 μg/ml) alone. The cells were incubated for 4 h. Only PCPZ (250 μM) or CPZ (500 μM), but no B. pseudomallei, was added to the cells in the negative controls. The culture plate was then centrifuged and 100 μl of the culture supernatant from each well was used for the lactate dehydrogenase assay. The assay was performed in triplicate.
RESULTS
Effect of phenothiazines on antimicrobial properties of antibiotics.
In the checkerboard titration assays, none of the phenothiazines alone exhibited an antimicrobial effect on B. pseudomallei, even at concentrations up to 1,000 μM (data not shown). PCPZ, CPZ, and PMZ potentiated the antimicrobial properties of a wide spectrum of antibiotics against B. pseudomallei, including aminoglycosides, macrolides, β-lactams, and a fluoroquinolone. Each of the phenothiazines interacted synergistically with streptomycin, spectinomycin, erythromycin, oleandomycin, azithromycin, amoxicillin-clavulanic acid, and levofloxacin, but the most pronounced synergistic interactions were with the aminoglycoside and macrolide antibiotics (Table 1). PCPZ (500 μM) produced the greatest synergistic interaction with spectinomycin, resulting in a >1,000-fold reduction in spectinomycin's MIC for B. pseudomallei, but it had very little synergistic interaction with spectinomycin when added at concentrations lower than 500 μM (Table 1). In comparison, CPZ and PMZ were both more potent than PCPZ in their synergistic interactions with the aminoglycoside and macrolide antibiotics. For example, CPZ and PMZ reduced the MIC of streptomycin for B. pseudomallei KHW >4,000-fold and >2,000-fold, respectively, compared to a reduction of only 4-fold by PCPZ. Similarly, CPZ and PMZ reduced the MIC of oleandomycin >8,000-fold and 4,000-fold, respectively, compared to the >30-fold reduction by PCPZ. CPZ and PMZ were also more effective in the reduction of the MIC of erythromycin to >500-fold compared to only 16-fold for PCPZ (Table 1). There were also significant differences in the synergistic interactions between each of the phenothiazines and erythromycin. The checkerboard titration assays showed that significant synergistic interaction between a phenothiazine and an antibiotic was achievable mostly with phenothiazine concentrations above 250 μM, and there was a distinct concentration threshold below which such synergistic interaction was not evident.
TABLE 1.
Synergistic activities in vitro of phenothiazines and omeprazole with antibiotics against B. pseudomallei KHW
| Antibiotic | EPI | MIC of antibiotic (μg/ml) at indicated concn of phenothiazine or omeprazole (μM)a:
|
Maximum decrease in MIC (n-fold) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1,000 | 500 | 250 | 125 | 62.5 | 31.3 | 15.6 | 7.8 | 3.9 | |||
| Aminoglycoside | ||||||||||||
| Streptomycin | PCPZ | 1,024 | 256 | 256 | 512 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 4 |
| CPZ | 1,024 | <0.25 | <0.25b | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | >4,000 | |
| PMZ | 1,024 | 0.5 | 516 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | >2,000 | |
| OPZ | 1,024 | <0.25b | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | >4,000 | |
| Spectinomycin | PCPZ | 256 | <0.25 | <0.25b | 32 | 32 | 128 | 256 | 256 | 256 | 256 | >1,000 |
| CPZ | 256 | <0.25 | 32 | 128 | 256 | 256 | 256 | 256 | 256 | 256 | >1,000 | |
| PMZ | 256 | <0.25b | 64 | 64 | 256 | 256 | 256 | 256 | 256 | 256 | >1,000 | |
| OPZ | 256 | 32 | 64 | 64 | 256 | 256 | 256 | 256 | 256 | 256 | 8 | |
| Macrolide | ||||||||||||
| ERY | PCPZ | 128 | 8 | 8 | 8 | 32 | 32 | 64 | 64 | 128 | 128 | 16 |
| CPZ | 128 | <0.25 | 8 | 32 | 128 | 128 | 128 | 128 | 128 | 128 | >500 | |
| PMZ | 128 | <0.25 | 32 | 64 | 128 | 128 | 128 | 128 | 128 | 128 | >500 | |
| OPZ | 128 | <0.25 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | >500 | |
| Oleandomycin | PCPZ | >2,000 | 64 | 64 | 128 | 256 | 256 | 512 | 512 | >2,000 | >2,000 | >30 |
| CPZ | >2,000 | <0.25 | 128 | 256 | 512 | 1,024 | >2,000 | >2,000 | >2,000 | >2,000 | >8,000 | |
| PMZ | >2,000 | 0.5 | 256 | >2,000 | >2,000 | >2,000 | >2,000 | >2,000 | >2,000 | >2,000 | >4,000 | |
| OPZ | >2,000 | <0.25 | >2,000 | >2,000 | >2,000 | >2,000 | >2,000 | >2,000 | >2,000 | >2,000 | >8,000 | |
| AZM | PCPZ | 32 | <0.25 | <0.25 | <0.25b | 16 | 32 | 32 | 32 | 32 | 32 | >128 |
| CPZ | 32 | <0.25 | 8 | 16 | 16 | 32 | 32 | 32 | 32 | 32 | >128 | |
| PMZ | 32 | <0.25 | 16 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | >128 | |
| OPZ | 32 | 16 | 16 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 2 | |
| β-Lactam | ||||||||||||
| AMC | PCPZ | 16 | <0.25 | <0.25 | <0.25b | 16 | 16 | 16 | 16 | 16 | 16 | >64 |
| CPZ | 16 | 0.5 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 32 | |
| PMZ | 16 | 0.5 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 32 | |
| OPZ | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 1 | |
| Fluoroquinolone | ||||||||||||
| LVX | PCPZ | 2 | <0.25 | <0.25b | 2 | 2 | 2 | 2 | 2 | 2 | 2 | >8 |
| CPZ | 2 | <0.25 | <0.25 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | >8 | |
| PMZ | 2 | <0.25 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | >8 | |
| OPZ | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
Values in boldface denote synergistic interaction between each respective phenothiazine, or omeprazole, and antibiotic.
Denotes the lowest concentration of antibiotic used in this assay.
There were no synergistic interactions between the phenothiazines and the β-lactam antibiotics amoxicillin and ampicillin, probably because these antibiotics are not actively effluxed by the B. pseudomallei RND pumps (data not shown). However, each of the phenothiazines tested increased the susceptibility of B. pseudomallei to amoxicillin-clavulanic acid at least 32-fold, with PCPZ (250 μM) being the most effective, reducing the MIC of amoxicillin-clavulanic acid >64-fold, from 16 μg/ml to less than 0.25 μg/ml (Table 1). There was also no synergistic interaction between the phenothiazines and gentamicin (data not shown). This was unexpected, as the aminoglycoside is also a substrate of both the BpeAB-OprB and the AmrAB-OprB RND efflux pumps.
Omeprazole provided synergistic interaction with antibiotics similar to those of the phenothiazines.
The proton pump inhibitor OPZ provided reductions in the MICs of the antibiotics similar to those of PCPZ, CPZ, and PMZ. The addition of 1,000 μM OPZ reduced the MICs of streptomycin, oleandomycin, and erythromycin >2,000-fold, >8,000-fold, and >500-fold, respectively (Table 1). This suggests that the phenothiazines probably interfered with the membrane proton gradient that is required for active efflux of the susceptible antibiotics. In contrast to the phenothiazines, OPZ produced a slightly different effect, in that it interacted only minimally with spectinomycin and azithromycin and showed no interaction at all with amoxicillin-clavulanic acid (Table 1).
Prochlorperazine and chlorpromazine inhibit efflux of [14C]erythromycin.
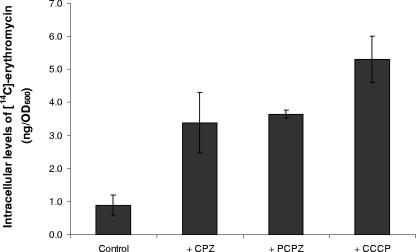
Erythromycin is a substrate of the RND efflux pumps BpeAB-OprB and AmrAB-OprA, and hence, only low levels of [14C]erythromycin were detected in the B. pseudomallei KHW cells when they were exposed to a subinhibitory concentration (0.1 μg/ml) of erythromycin (Fig. 1) (4, 17). Almost 4-fold-more [14C]erythromycin accumulated in the B. pseudomallei KHW cells in the presence of either 250 μM PCPZ or 500 μM CPZ, as a result of reduced erythromycin efflux (Fig. 1). PCPZ and CPZ yielded the same effects on the intracellular accumulation of erythromycin as that obtained when B. pseudomallei was exposed to a subinhibitory concentration (20 μM) of CCCP, a proton gradient uncoupler, thus suggesting that the phenothiazines might have disrupted the membrane proton gradient, directly interacted with the pump to inhibit the efflux of erythromycin, or both.
FIG. 1.
Effects of PCPZ, CPZ, and CCCP on the intracellular accumulation of [14C]erythromycin in B. pseudomallei KHW. The amount of [14C]erythromycin retained in intact B. pseudomallei KHW cells 4 h after exposure to exogenous [14C]erythromycin was determined as described in Materials and Methods. B. pseudomallei KHW cells were treated with 500 μM CPZ, 250 μM PCPZ, or 20 μM CCCP for 4 h. The control experiment used untreated B. pseudomallei KHW cells. The assay was performed in triplicate. OD600, optical density at 600 nm. Error bars indicate standard deviations.
Synergistic activities of the phenothiazines and erythromycin protect A549 and THP-1 cells from B. pseudomallei.
We have shown previously that any impairment to the BpeAB-OprB efflux pump function would result in an attenuation of cell invasion and the cytotoxicity of B. pseudomallei KHW (3). The similar effects of the phenothiazines and CCCP on antibiotic accumulation in B. pseudomallei suggest that synergistic interactions between phenothiazines and erythromycin could also protect human lung epithelial and macrophage cells from invasion and the cytotoxic effects of B. pseudomallei. Invasion of both human lung epithelial cells (A549) and human macrophage cells (THP-1) by B. pseudomallei KHW was significantly attenuated by adding a subinhibitory concentration of erythromycin (0.06× MIC, 8 μg/ml) to the culture medium together with either PCPZ (250 μM) or CPZ (500 μM) (Fig. 2A and B). Without phenothiazines, invasion of A549 and THP-1 cells by B. pseudomallei KHW was attenuated only by adding erythromycin at its MIC (128 μg/ml), and not at the subinhibitory concentration of 8 μg/ml. Similarly, 250 μM PCPZ and 500 μM CPZ alone afforded no protection against cell invasion by B. pseudomallei KHW (Fig. 2A and B).
FIG. 2.
Synergistic and protective effects of phenothiazines and erythromycin against invasion of human lung epithelial cells and macrophages by B. pseudomallei KHW. Invasion of A549 human lung epithelial cells (A) and THP-1 human macrophage cells (B) by B. pseudomallei KHW was performed as described in Materials and Methods. PCPZ250, 250 μM final concentration of PCPZ; CPZ500, 500 μM final concentration of CPZ; ERY8, concentration of erythromycin at 0.06× MIC (8 μg/ml); ERY128, concentration of erythromycin at MIC (128 μg/ml); +, present; −, absent. Error bars indicate standard deviations.
The cytotoxicities of B. pseudomallei KHW on A549 and THP-1 cells were attenuated about fourfold and threefold, respectively, in the presence of erythromycin at its MIC of 128 μg/ml but not in the presence of 250 μM PCPZ or 500 μM CPZ alone. However, in the presence of either 250 μM PCPZ or 500 μM CPZ, the subinhibitory concentration of erythromycin (0.06× MIC, 8 μg/ml) was equally effective in attenuating the cytotoxicity of B. pseudomallei KHW for A549 and THP-1 cells, thus affirming the synergistic interaction between the phenothiazines and erythromycin (Fig. 3A and B).
FIG. 3.
Synergistic and protective effects of phenothiazines and erythromycin against the cytotoxicity of B. pseudomallei KHW. The cytotoxicities of B. pseudomallei KHW for A549 (A) and THP-1 (B) cells were determined by measuring the release of lactate dehydrogenase from the cells as described in Materials and Methods. PCPZ250, 250 μM final concentration of PCPZ; CPZ500, 500 μM final concentration of CPZ; ERY8, 8-μg/ml final concentration of erythromycin; ERY128, 128-μg/ml final concentration of erythromycin; +, present; −, absent. Error bars indicate standard deviations.
DISCUSSION
The intrinsic resistance of B. pseudomallei to many antibiotics, especially aminoglycosides and macrolides, is largely attributed to the activities of the RND efflux pumps BpeAB-OprB and AmrAB-OprA (4, 17). The efflux is an active process which is dependent on the membrane proton gradient (19). Efflux pumps can be considered potentially effective antibacterial targets, and the resultant EPI-antibiotic combination drug should exhibit increased potency, an enhanced spectrum of antimicrobial activity, and a reduced propensity for the bacterium to acquire resistance. The EPIs that have been identified function as competitive or noncompetitive substrate inhibitors, prevent ATP binding, or disturb the proton gradient. These include the synthetic dipeptide amide l-Phe-l-Arg-β-napthylamide (MC-207,110), which has been shown to significantly decrease the level of intrinsic resistance of Pseudomonas aeruginosa to fluoroquinolones, reverse the acquired resistance due to the overexpression of efflux pumps, and reduce the emergence of P. aeruginosa strains that are highly resistant to fluoroquinolones. Such EPIs can cause increased accumulation of substrates without disrupting the proton gradient (16).
Phenothiazines belong to a class of nonantibiotic drugs that could inhibit efflux pumps that confer resistance to fluoroquinolones in S. aureus (11, 12). We showed that the phenothiazines PCPZ, CPZ, and PMZ did not exhibit any antimicrobial activities on B. pseudomallei KHW at concentrations up to 1 mM. However, when used together with antibiotics, these phenothiazines interacted synergistically with streptomycin, erythromycin, oleandomycin, spectinomycin, levofloxacin, azithromycin, and amoxicillin-clavulanic acid, albeit to various degrees, to enhance their antimicrobial potencies against B. pseudomallei. The synergistic interactions between the phenothiazines and antibiotics were most pronounced for the aminoglycosides (streptomycin and spectinomycin) and macrolides (erythromycin, oleandomycin, and azithromycin) which are also substrates of two B. pseudomallei RND efflux pumps, BpeAB-OprB and AmrAB-OprA (4, 17). The inhibitory effects of phenothiazines on multidrug efflux pumps have been reported previously, including augmentation of the potency of common efflux pump substrates against S. aureus strains possessing different efflux-related MDR mechanisms and the inhibition of NorA pump function and non-NorA-related efflux phenotypes in a concentration-dependent manner. The mechanism of inhibition of efflux pumps by phenothiazines and thioxanthenes appears most likely to involve direct interactions of these compounds with the pump, and disruption of the proton gradient is involved to a somewhat lesser extent (11). The lack of significant cytotoxicity effects despite the relatively high concentrations of drugs used in our experiments is also an argument against their role as proton gradient inhibitors. It is also generally acknowledged that proton motive force inhibitors would not make good EPIs because they would exhibit significant cytotoxicity effects. Phenothiazines have also been shown to inhibit the function of eukaryotic MDR efflux pumps (10). CPZ and PMZ were the most potent of the phenothiazines tested, achieving at least a 500-fold-higher augmentation of the antimicrobial activity of streptomycin against B. pseudomallei than a similar concentration of PCPZ. Similar variations in efficacy were observed for the interactions between the different phenothiazines and oleandomycin. We attribute these variations in potency of the phenothiazines to their different charges at neutral pH. CPZ, PMZ, and PCPZ each contain a cationic N-CH3 group, which could potentially interact with the membrane to alter its permeability (22). The pKa values of CPZ, PMZ, and PCPZ are 9.3, 9.4, and 8.1, respectively, suggesting that CPZ and PMZ would be more positively charged than PCPZ at pH 7 and thus were expected to be more effective in altering the outer membrane permeability to dissipate the proton gradient (8).
One of the targets which gives rise to the synergistic interaction between the phenothiazines and the susceptible antibiotics seems to be the proton gradient. CPZ has been shown to affect ion flux across the membrane in S. aureus and Saccharomyces cerevisiae and alter the transmembrane potential in Leishmania donovani (9, 13, 27). Thus, we expect the B. pseudomallei RND efflux pumps, which depend on the membrane proton gradient to energize substrate translocation of antibiotics, to be affected by phenothiazines. Such pumps would include the B. pseudomallei BpeAB-OprB and AmrAB-OprB pumps which actively efflux aminoglycosides and macrolides (4, 17). Indeed, our data showed that the addition of phenothiazines rendered B. pseudomallei more susceptible not only to the aminoglycosides and macrolides but also to levofloxacin and the β-lactam amoxicillin-clavulanic acid. It is interesting to note that the phenothiazines had no effect on the MIC of amoxicillin alone, but a synergistic effect was observed between the phenothiazines and amoxicillin-clavulanic acid, suggesting that clavulanic acid could be a substrate of a yet-uncharacterized RND efflux pump in B. pseudomallei. In P. aeruginosa, β-lactamase inhibitors are substrates of the MexAB-OprM and MexEF-OprN efflux pumps (15). Phenothiazines have also rendered methicillin-resistant S. aureus more susceptible to oxacillin, probably also due to their effect on a similar efflux pump (14). The ability to reproduce similar synergistic interactions between the same antibiotics and omeprazole, a proton pump inhibitor, also supports the notion that the phenothiazines might affect the functions of the B. pseudomallei RND efflux pumps through changes in the proton gradient. Omeprazole also inhibited the activity of the S. aureus NorA pump, but as in B. pseudomallei, the concentration of omeprazole required was above what is clinically attainable (1). In this respect, however, we are unable to explain why the MIC of gentamicin, which is also a substrate of the BpeAB-OprB multidrug efflux pump in B. pseudomallei, was unaffected by the phenothiazines (4). Gentamicin, like streptomycin, spectinomycin, and azithromycin, is cationic and could disrupt an otherwise intact and impermeable lipopolysaccharide layer by cationic binding, and streptomycin, with two extra amine groups, is more cationic and hence is more disruptive to the lipopolysaccharide layer than gentamicin (23). However, if the mechanism of inhibition of efflux pumps by phenothiazines could be attributed principally to their direct interaction with the pump, then a plausible explanation would be that the phenothiazines interfered with the binding of streptomycin and spectinomycin to the efflux pumps, but not gentamicin. Moreover, as the relatively large concentrations of phenothiazines used in this study did not exhibit any significant cytotoxicity effects, it is plausible that these drugs do not function primarily as proton gradient inhibitors (Fig. 3). We have ascertained that there was no change in the MIC of gentamicin in the presence or absence of omeprazole (data not shown). Thus, we believe that the mechanism of synergistic interaction between phenothiazines and antibiotics is likely to involve direct interference with the antibiotic-pump interaction, as well as a limited disruption of the proton motive force.
We have previously shown that the disruption of BpeAB-OprB function could attenuate B. pseudomallei virulence in cell invasion and cytotoxicity assays (3). In this study, we showed that the use of a subinhibitory concentration (0.06× MIC) of erythromycin, together with a phenothiazine as an adjunct treatment, not only inhibited B. pseudomallei growth but also protected the mammalian cells from infection by B. pseudomallei and its cytotoxicity. Erythromycin is an effective therapy against infection by intracellular pathogens, as it is able to enter eukaryotic cells and was shown to be markedly concentrated within polymorphonuclear leukocytes (21). CPZ is also concentrated by human macrophages, where it exerted bactericidal properties against S. aureus phagocytosed by human monocyte-derived macrophages (20). The successful attenuation of cell invasion and killing by B. pseudomallei by using a combination of a phenothiazine and a subinhibitory concentration of erythromycin might be the result of an accumulation or concentration of erythromycin and the phenothiazine within the mammalian cells, thus enhancing their bacteriostatic and bactericidal properties. Alternatively, the combination of a phenothiazine and erythromycin could also exert an inhibitory effect on the efflux pump and consequently reduce its virulence by inhibiting the efflux of a key metabolite(s) that is required for expression of virulence in B. pseudomallei.
The data support the potential role of phenothiazines as EPIs in B. pseudomallei. Although we acknowledge that the concentrations (250 μM to 1 mM) at which phenothiazines have such beneficial effects on the antimicrobial agents are not clinically achievable, there is nevertheless a basis for the future development of compounds that can either inhibit the multidrug efflux pumps or dissipate the proton motive force required for active efflux of antimicrobial agents. It is also possible that maximal bacterial pump inhibition may not be required to achieve a beneficial effect in vivo, but this would require verification using animal studies. It is envisaged that chemical modification of the phenothiazines could result in compounds with less central nervous system toxicity but improved inhibitory activity towards the bacterial efflux pumps. The wide spectrum of antibiotics which interact synergistically with the phenothiazines lends further support to the idea of developing these drugs into EPIs, as phenothiazines could augment the antimicrobial activities of nearly all clinically used antibiotics. Although B. pseudomallei KHW is a virulent clinical isolate that has been well characterized in our laboratory, we are cognizant that the extent of the synergistic interactions between phenothiazines and antibiotics could vary from strain to strain and recommend further studies to include more B. pseudomallei isolates.
Acknowledgments
This work was supported by the National Medical Research Council of Singapore (grant NMRC 1012/2005).
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Aeschlimann, J. R., L. D. Dresser, G. W. Kaatz, and M. J. Rybak. 1999. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossi, P., A. Tegnell, A. Baka, F. Van Loock, J. Hendriks, A. Werner, H. Maidhof, and G. Gouvras. 2004. Bichat guidelines for the clinical management of glanders and melioidosis and bioterrorism-related glanders and melioidosis. Euro Surveill. 9:E17-E18. [PubMed] [Google Scholar]
- 3.Chan, Y. Y., and K. L. Chua. 2005. The Burkholderia pseudomallei BpeAB-OprB efflux pump: expression and impact on quorum sensing and virulence. J. Bacteriol. 187:4707-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, Y. Y., T. M. Tan, Y. M. Ong, and K. L. Chua. 2004. BpeAB-OprB, a multidrug efflux pump in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 48:1128-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaowagul, W. 2000. Recent advances in the treatment of severe melioidosis. Acta Trop. 74:133-137. [DOI] [PubMed] [Google Scholar]
- 6.Chua, K. L., Y. Y. Chan, and Y. H. Gan. 2003. Flagella are virulence determinants of Burkholderia pseudomallei. Infect. Immun. 71:1622-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dance, D. A., T. M. Davis, Y. Wattanagoon, W. Chaowagul, P. Saiphan, S. Looareesuwan, V. Wuthiekanun, and N. J. White. 1989. Acute suppurative parotitis caused by Pseudomonas pseudomallei in children. J. Infect. Dis. 159:654-660. [DOI] [PubMed] [Google Scholar]
- 8.de Boer, T., R. Bijma, and K. Ensing. 1998. Tuning of the selectivity in capillary electrophoresis by cyclodextrins illustrated by the separation of some structurally related phenothiazines. J. Capillary Electrophor. 5:65-71. [PubMed] [Google Scholar]
- 9.Eilam, Y. 1983. Membrane effects of phenothiazines in yeasts. I. Stimulation of calcium and potassium fluxes. Biochim. Biophys. Acta 733:242-248. [DOI] [PubMed] [Google Scholar]
- 10.Ford, J. M., W. C. Prozialeck, and W. N. Hait. 1989. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol. Pharmacol. 35:105-115. [PubMed] [Google Scholar]
- 11.Kaatz, G. W., V. V. Moudgal, S. M. Seo, and J. E. Kristiansen. 2003. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:719-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaatz, G. W., S. M. Seo, L. O'Brien, M. Wahiduzzaman, and T. J. Foster. 2000. Evidence for the existence of a multidrug efflux transporter distinct from NorA in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristiansen, J. E., and L. Amaral. 1997. The potential management of resistant infections with non-antibiotics. J. Antimicrob. Chemother. 40:319-327. [DOI] [PubMed] [Google Scholar]
- 14.Kristiansen, M. M., C. Leandro, D. Ordway, M. Martins, M. Viveiros, T. Pacheco, J. Molnar, J. E. Kristiansen, and L. Amaral. 2006. Thioridazine reduces resistance of methicillin-resistant Staphylococcus aureus by inhibiting a reserpine-sensitive efflux pump. In Vivo 20:361-366. [PubMed] [Google Scholar]
- 15.Li, X. Z., L. Zhang, R. Srikumar, and K. Poole. 1998. β-Lactamase inhibitors are substrates for the multidrug efflux pumps of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore, R. A., D. DeShazer, S. Reckseidler, A. Weissman, and D. E. Woods. 1999. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 43:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7-A6 and MIC testing supplemental tables M100-S13, 6th ed., vol. 23, no. 2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 19.Nikaido, H. 2001. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin. Cell Dev. Biol. 12:215-223. [DOI] [PubMed] [Google Scholar]
- 20.Ordway, D., M. Viveiros, C. Leandro, M. Jorge Arroz, J. Molnar, J. E. Kristiansen, and L. Amaral. 2002. Chlorpromazine has intracellular killing activity against phagocytosed Staphylococcus aureus at clinical concentrations. J. Infect. Chemother. 8:227-231. [DOI] [PubMed] [Google Scholar]
- 21.Prokesch, R. C., and W. L. Hand. 1982. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 21:373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajyaguru, J. M., and M. J. Muszynski. 1997. Enhancement of Burkholderia cepacia antimicrobial susceptibility by cationic compounds. J. Antimicrob. Chemother. 40:345-351. [DOI] [PubMed] [Google Scholar]
- 23.Rivera, M. H., R. E. W. Hancock, J. G. Sawyer, A. Haug, and E. J. McGroarty. 1988. Enhanced binding of polycationic antibiotics to lipopolysaccharide from an aminoglycoside-supersusceptible, tolA mutant strain of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 32:649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srirompotong, S., and S. Saeng-Sa-Ard. 2004. Acute suppurative parotitis. J. Med. Assoc. Thai. 87:694-696. [PubMed] [Google Scholar]
- 25.Vatcharapreechasakul, T., Y. Suputtamongkol, D. A. Dance, W. Chaowagul, and N. J. White. 1992. Pseudomonas pseudomallei liver abscesses: a clinical, laboratory, and ultrasonographic study. Clin. Infect. Dis. 14:412-417. [DOI] [PubMed] [Google Scholar]
- 26.Viveiros, M., and L. Amaral. 2001. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int. J. Antimicrob. Agents 17:225-228. [DOI] [PubMed] [Google Scholar]
- 27.Zilberstein, D., V. Liveanu, and A. Gepstein. 1990. Tricyclic drugs reduce proton motive force in Leishmania donovani promastigotes. Biochem. Pharmacol. 39:935-940. [DOI] [PubMed] [Google Scholar]