Abstract
Tolerance to vancomycin and teicoplanin in 90 clinical isolates of coagulase-negative staphylococci (CoNS) was investigated by time-kill curve methodology. Only six strains, belonging to the Staphylococcus lugdunensis species, exhibited tolerance. The seven other S. lugdunensis strains tested displayed weak susceptibility to the bactericidal activity of glycopeptides compared to the other CoNS. These phenomena are of concern, since S. lugdunensis is recognized as one of the most pathogenic CoNS.
Coagulase-negative staphylococci (CoNS) are involved in infections that require bactericidal treatment, such as indwelling foreign body-related infections, endocarditis, and meningitis (4, 10). As CoNS become more resistant to beta-lactams (2), glycopeptides are often considered to be antibiotics of last resort (12). Some investigators, however, have reported glycopeptide tolerance for sporadic CoNS (16, 23). Antibiotic tolerance describes a particular “type of resistance” in bacteria capable of surviving, but not growing, in the presence of a normally lethal dose of a given bactericidal antibiotic (20, 21). As early screenings for glycopeptide tolerance in CoNS have been performed by the controversial minimal bactericidal concentration (MBC)/MIC determinations (1, 14, 19, 21), the present study was designed to examine vancomycin and teicoplanin tolerance in a collection of clinically significant CoNS by using the killing curve method, which is considered to be the most reliable method according to the Clinical and Laboratory Standards Institute (CLSI) (formerly NCCLS) (14).
An initial set of 79 clinically significant isolates of CoNS from 79 individual patients attending the Rouen University Hospital between January 1999 and April 2001 was studied. Strains were identified to the species level with the ID32Staph system (bioMérieux, Marcy l'Etoile, France) and by a gap gene PCR-restriction fragment length polymorphism assay (24). This set reflected the current epidemiology of CoNS (11), with Staphylococcus epidermidis as a very dominant species (n = 66; 84% of the isolates) and with some less frequently encountered species, i.e., S. hominis (n = 4), S. capitis (n = 3), S. lugdunensis (n = 2), S. warneri (n = 2), S. haemolyticus (n = 1), and S. pasteuri (n = 1).
The MICs of vancomycin (Eli Lilly & Co., Saint-Cloud, France) and teicoplanin (Sanofi-Aventis, Romainville, France) were determined by the agar dilution method in accordance with CLSI guidelines (15). S. aureus ATCC 29213 was used as a reference control strain. The replicator prong delivered approximately 104 CFU per spot. All the isolates were susceptible to vancomycin (MICs, ≤4 μg/ml) according to the breakpoints of the Comité de l'Antibiogramme de la Société Française de Microbiologie (6) and according to those of the CLSI (5). Fifty-two isolates were susceptible to teicoplanin (MICs, ≤4 μg/ml), 22 isolates showed intermediate susceptibility (MICs = 8 μg/ml), and 5 isolates were resistant (MICs = 16 μg/ml) according to Comité de l'Antibiogramme de la Société Française de Microbiologie breakpoints. This categorization corresponds to 74 isolates that were susceptible to teicoplanin (MICs, ≤8 μg/ml) and 5 isolates that showed intermediate susceptibility (MICs, >8 μg/ml and ≤32 μg/ml) according to CLSI breakpoints.
Time-kill curves were performed according to CLSI guidelines (14), with a mean starting inoculum at 5.6 log10 CFU/ml (standard deviation, 0.1), flasks containing 50 ml of Mueller-Hinton broth (Becton Dickinson, Le Pont de Clayes, France), and antibiotic at 10 times the MIC. Bacterial counts were performed just before and at 6 and 24 h after the addition of antibiotics. To prevent carryover effects (14, 19), 0.5-ml samples were removed from the flasks, diluted 10-fold, and subcultured (0.1-ml aliquots in duplicate) on prewarmed blood agar plates. Tolerance was defined as a <3-log10 reduction of the bacterial count after 24 h according to CLSI guidelines (14) and also as a <1-log10 reduction of the bacterial count after 6 h, according to methods described previously by May et al. (13).
Only 2 of the 79 isolates tested were found to be tolerant to glycopeptides: S. lugdunensis 111A53, which was tolerant to vancomycin, and S. lugdunensis 111A91, which was tolerant to teicoplanin (Table 1). Of note, these two isolates were the only S. lugdunensis isolates of the 79 CoNS studied. For these two isolates, additional time-kill curves were performed using antibiotic concentrations of 5, 10, and 20 times the MIC to detect a potential Eagle (or paradoxical) effect (14, 21). The latter phenomenon was excluded for both glycopeptides (Table 2), and these additional results confirmed a glycopeptide tolerance. As tolerance has also been defined by an MBC/MIC ratio of ≥32, MBC/MIC ratios of both glycopeptides were determined for the two S. lugdunensis isolates in triplicate according to CLSI recommendations (14), with a starting inoculum of between 105 and 106 CFU/ml in Mueller-Hinton broth. The quality control strain S. aureus ATCC 25923 was tested within each assay (14). The MICs were comparable to those determined by the agar dilution procedure (data not shown). Despite disparities between the MBC/MIC ratios obtained (Table 2), the vancomycin tolerance of isolate 111A53 (MBC/MIC ratio of ≥32; two of three assays) and the teicoplanin tolerance of isolate 111A91 (MBC/MIC ratio of ≥32; three of three assays) were confirmed.
TABLE 1.
Variations in bacterial counts of 90 isolates of CoNS after 6 and 24 h of glycopeptide exposition at 10× MIC
| Strain | Mean (SDa) Δlog CFU/ml forb:
|
|||
|---|---|---|---|---|
| Vancomycin
|
Teicoplanin
|
|||
| 6 h | 24 h | 6 h | 24 h | |
| First-set strains (n = 79) | ||||
| 77 CoNS | −2.98 (1.04) | −4.80 (0.98) | −3.22 (0.98) | −5.33 (0.74) |
| S. lugdunensis 111A53 | −0.31 (0.08) | −1.94 (0.18) | −0.66 (0.16) | −3.77 (0.23) |
| S. lugdunensis 111A91 | −0.31 (0.08) | −3.53 (0.20) | −0.34 (0.10) | −2.26 (0.16) |
| Additional-set strains (n = 11) | ||||
| 7 S. lugdunensis strains | −1.17 (0.74) | −4.10 (0.70) | −0.49 (0.26) | −4.42 (0.62) |
| S. lugdunensis ATCC 49576 | −1.38 (0.23) | −3.91 (0.17) | −0.47 (0.11) | −2.40 (0.33) |
| S. lugdunensis 111A223 | −0.67 (0.23) | −1.65 (0.24) | −0.43 (0.07) | −2.81 (0.11) |
| S. lugdunensis ATCC 43809 | −0.21 (0.16) | −2.18 (0.44) | −0.12 (0.06) | −1.29 (0.33) |
| S. lugdunensis 111A229 | −2.24 (0.47) | −2.42 (0.25) | −0.31 (0.21) | −2.33 (0.31) |
Experiments were performed in duplicate for each strain.
Boldface type indicates a tolerance phenomenon according to CLSI criteria.
TABLE 2.
Evaluation of glycopeptides tolerance in two S. lugdunensis isolates by time-kill curves and MBC/MIC ratios
| Isolate | Time-kill curve
|
MBC/MIC ratioa
|
|||||
|---|---|---|---|---|---|---|---|
| Antibiotic concn | Variation in bacterial numbers (mean log CFU/ml) for:
|
||||||
| Vancomycin
|
Teicoplanin
|
Vancomycin | Teicoplanin | ||||
| 6 h | 24 h | 6 h | 24 h | ||||
| S. lugdunensis 111A53, vancomycin-tolerant isolate | 5× MIC | −0.05 | −1.72 | −0.33 | −1.55 | 64, 8, >128 | 8, 32, >512 |
| 10× MIC | −0.31 | −1.94 | −0.66 | −3.77 | |||
| 20× MIC | −0.07 | −1.74 | −0.23 | −3.32 | |||
| S. lugdunensis 111A91, teicoplanin-tolerant isolate | 5× MIC | −1.16 | −3.90 | −0.29 | −1.57 | 8, 1, 1 | 64, >512, 64 |
| 10× MIC | −0.31 | −3.53 | −0.34 | −2.26 | |||
| 20× MIC | −0.56 | −2.83 | −0.47 | −3.90 | |||
Boldface type indicates a tolerance phenomenon according to the criterion of each methodology. Ratios are in the order of first assay, second assay, third assay.
The frequency of glycopeptide tolerance observed among CoNS in this set (2/79; 2.5%) is markedly lower than those reported in two previous studies (3/10 [30%] and 17/50 [34%], respectively) (16, 23). Those studies are not, however, strictly comparable since the CoNS identification methods were not described, and tolerance screening tests consisted only of MBC/MIC ratio determinations. Furthermore, one of those studies (23), involving 50 S. epidermidis isolates, used a less stringent threshold (MBC/MIC ratio of ≥16) than that which is now recommended (MBC/MIC ratio of ≥32) (14).
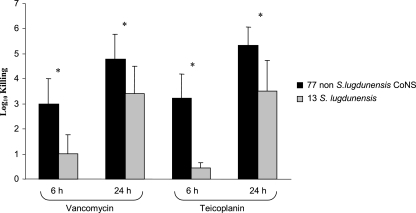
Our data prompted us to search for tolerance by killing curves among an additional set of 11 S. lugdunensis isolates, including 3 reference strains (ATCC 43809, ATCC 49576, and ATCC 700328) and 8 clinical isolates (3/8 from the Versailles General Center Hospital). Tolerance was found for four of these additional strains (Table 1). Overall, nearly half of the S. lugdunensis strains tested (6/13 strains) met the bacteriological criteria for tolerance to either vancomycin or teicoplanin. In addition, glycopeptides displayed a weaker and, above all, slower bactericidal activity against the seven other S. lugdunensis isolates than against the other CoNS tested (mainly S. epidermidis). In fact, after 6 h, the reduction in bacterial counts due to vancomycin and teicoplanin was on average 2 log10 CFU/ml weaker for the S. lugdunensis strains than for the 77 other CoNS (statistically significant difference, Mann-Whitney U test with P values of <0.05) (Fig. 1). Of note, all these 13 isolates were fully susceptible to vancomycin (MICs, 0.5 to 2 μg/ml) and to teicoplanin (MICs, 0.5 to 1 μg/ml).
FIG. 1.
Comparative killing of glycopeptides after 6 and 24 h of exposition at 10 times the MIC against two populations of coagulase-negative staphylococci: 77 non-S. lugdunensis isolates versus 13 S. lugdunensis isolates. Error bars indicate standard deviations, and asterisks indicate statistically significant differences (P < 0.05).
This study shows a defect in the bactericidal activity of glycopeptides against CoNS of the S. lugdunensis species. Since its description in 1988 (8), this species, shown to be part of the normal skin flora, has been described as being one of the most pathogenic CoNS (9). Indeed, S. lugdunensis infections resemble S. aureus infections (9) in terms of virulence, tissue destruction, and clinical course, particularly for endocarditis (22). Current S. lugdunensis isolates usually remain susceptible to methicillin and other antistaphylococcal antibiotics (9). Thus, the use of glycopeptides for S. lugdunensis infections is usually limited to the initial days of empirical treatment when a possibly methicillin-resistant Staphylococcus infection has to be considered and to patients with a beta-lactam allergy. The fact that S. lugdunensis appears to be less affected by the bactericidal activity of glycopeptides reinforces the need to identify CoNS to the species level for serious infections as well as to consider tests for the detection of tolerance when glycopeptides have to be used for an S. lugdunensis infection. This study also confirms that time-kill curves have the crucial advantage of providing dynamic data (14) and are the most reliable approach to detect tolerance (1, 14), especially by bacterial count reduction after 24 h (14). An expanded use of time-kill curves should lead to an increased appreciation of the magnitude of the glycopeptide tolerance phenomenon in CoNS and thus permit relevant comparisons between studies.
Tolerance mechanisms remain elusive to this day, even if recent works on Streptococcus pneumoniae and S. aureus have suggested the involvement of impaired autolysin regulation systems (3, 18) or modifications in the cell wall composition (7, 17). Studies should be undertaken to explore the mechanism of the phenomenon of S. lugdunensis tolerance to glycopeptides observed in the present work and to evaluate its clinical implications.
Acknowledgments
We thank F. Doucet-Populaire (General Hospital, Versailles, France) for kindly providing three S. lugdunensis isolates. We also express our gratitude to M. F. Hellot and M. Etienne for their assistance with statistical analysis.
Footnotes
Published ahead of print on 20 November 2006.
REFERENCES
- 1.Amsterdam, D. 2005. Susceptibility testing of antimicrobials in liquid media, p. 61-144. In V. Lorian (ed.), Antibiotics in laboratory medicine, 5th ed. Williams & Wilkins, Baltimore, MD.
- 2.Carbon, C. 2000. MRSA and MRSE: is there an answer? Clin. Microbiol. Infect. 6:(Suppl. 2)17-22. [DOI] [PubMed] [Google Scholar]
- 3.Charpentier, E., and E. Tuomanen. 2000. Mechanisms of antibiotic resistance and tolerance in Streptococcus pneumoniae. Microbes Infect. 2:1855-1864. [DOI] [PubMed] [Google Scholar]
- 4.Chu, V. H., C. H. Cabell, E. Abrutyn, G. R. Corey, B. Hoen, J. M. Miro, L. Olaison, M. E. Stryjewski, P. Pappas, K. J. Anstrom, S. Eykyn, G. Habib, N. Benito, and V. G. Fowler, Jr. 2004. Native valve endocarditis due to coagulase-negative staphylococci: report of 99 episodes from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. 39:1527-1530. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing—15th informational supplement. Approved standard, CLSI document M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Comité de l'Antibiogramme de la Société Française de Microbiologie. 2006. Communiqué 2006. Société Française de Microbiologie, Paris, France. http://www.sfm.asso.fr/.
- 7.Filipe, S. R., E. Severina, and A. Tomasz. 2002. The murMN operon: a functional link between antibiotic resistance and antibiotic tolerance in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 99:1550-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freney, J., Y. Brun, M. Bes, F. Meugnier, F. Grimont, P. A. D. Grimont, C. Nervi, and J. Fleurette. 1988. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int. J. Syst. Bacteriol. 38:168-172. [Google Scholar]
- 9.Hellbacher, C., E. Tornqvist, and B. Soderquist. 2006. Staphylococcus lugdunensis: clinical spectrum, antibiotic susceptibility, and phenotypic and genotypic patterns of 39 isolates. Clin. Microbiol. Infect. 12:43-49. [DOI] [PubMed] [Google Scholar]
- 10.Huang, C. R., C. H. Lu, J. J. Wu, H. W. Chang, C. C. Chien, C. B. Lei, and W. N. Chang. 2005. Coagulase-negative staphylococcal meningitis in adults: clinical characteristics and therapeutic outcomes. Infection 33:56-60. [DOI] [PubMed] [Google Scholar]
- 11.Huebner, J., and D. A. Goldmann. 1999. Coagulase-negative staphylococci: role as pathogens. Annu. Rev. Med. 50:223-236. [DOI] [PubMed] [Google Scholar]
- 12.Levine, D. P. 2006. Vancomycin: a history. Clin. Infect. Dis. 42:(Suppl. 1)S5-S12. [DOI] [PubMed] [Google Scholar]
- 13.May, J., K. Shannon, A. King, and G. French. 1998. Glycopeptide tolerance in Staphylococcus aureus. J. Antimicrob. Chemother. 42:189-197. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity antimicrobial agents. Approved guidelines. Document M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard. Document M7, vol. M7-A5. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 16.Perry, J. D., A. L. Jones, and F. K. Gould. 1999. Glycopeptide tolerance in bacteria causing endocarditis. J. Antimicrob. Chemother. 44:121-124. [DOI] [PubMed] [Google Scholar]
- 17.Peschel, A., C. Vuong, M. Otto, and F. Götz. 2000. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob. Agents Chemother. 44:2845-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice, K. C., B. A. Firek, J. B. Nelson, S. J. Yang, T. G. Patton, and K. W. Bayles. 2003. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J. Bacteriol. 185:2635-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherris, J. C. 1986. Problems in in vitro determination of antibiotic tolerance in clinical isolates. Antimicrob. Agents Chemother. 30:633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomasz, A., A. Albino, and E. Zanati. 1970. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227:138-140. [DOI] [PubMed] [Google Scholar]
- 21.Tuomanen, E., D. T. Durack, and A. Tomasz. 1986. Antibiotic tolerance among clinical isolates of bacteria. Antimicrob. Agents Chemother. 30:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandenesch, F., J. Etienne, M. E. Reverdy, and S. J. Eykyn. 1993. Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin. Infect. Dis. 17:871-876. [DOI] [PubMed] [Google Scholar]
- 23.Watanakunakorn, C. 1985. Antibiotic tolerance of Staphylococcus epidermidis. Scand. J. Infect. Dis. 17:59-61. [DOI] [PubMed] [Google Scholar]
- 24.Yugueros, J., A. Temprano, M. Sanchez, J. M. Luengo, and G. Naharro. 2001. Identification of Staphylococcus spp. by PCR-restriction fragment length polymorphism of gap gene. J. Clin. Microbiol. 39:3693-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]