Abstract
The emergence of multidrug-resistant parasites is a major concern for malaria control, and development of novel drugs is a high priority. Curcumin, a natural polyphenolic compound, possesses diverse pharmacological properties. Among its antiprotozoan activities, curcumin was potent against both chloroquine-sensitive and -resistant Plasmodium falciparum strains. Consistent with findings in mammalian cell lines, curcumin's prooxidant activity promoted the production in P. falciparum of reactive oxygen species (ROS), whose cytotoxic effect could be antagonized by coincubation with antioxidants and ROS scavengers. Curcumin treatment also resulted in damage of both mitochondrial and nuclear DNA, probably due to the elevation of intracellular ROS. Furthermore, we have demonstrated that curcumin inhibited the histone acetyltransferase (HAT) activity of the recombinant P. falciparum general control nonderepressed 5 (PfGCN5) in vitro and reduced nuclear HAT activity of the parasite in culture. Curcumin-induced hypoacetylation of histone H3 at K9 and K14, but not H4 at K5, K8, K12, and K16, suggested that curcumin caused specific inhibition of the PfGCN5 HAT. Taken together, these results indicated that at least the generation of ROS and down-regulation of PfGCN5 HAT activity accounted for curcumin's cytotoxicity for malaria parasites.
The increasing burden of malaria partially due to parasite drug resistance demands continued and sustained improvement in antimalarial medicines through focused research and development. With the increasing awareness of the significance of natural medicinal plants, considerable efforts have been undertaken towards discovery of natural compounds for malaria chemotherapy (42). Amazingly, some natural compounds such as quinine, artemisinin, and their derivatives still remain the most effective antimalarial drugs. It is anticipated that modern approaches will facilitate the discovery and development process of natural antimalarial medicines (39).
Curcumin (diferuloylmethane) is a natural polyphenolic compound abundant in the rhizome of the perennial herb turmeric, Curcuma longa Linnaeus. It is widely used as a dietary spice and coloring in cooking and as a herb in traditional Indian medicine (41). Curcumin exhibits a wide range of pharmacological activities, including anti-inflammatory, anticarcinogenic, and anti-infectious activities. The anti-inflammatory and anticarcinogenic effects of curcumin largely depend on its antioxidant activity. In addition, curcumin possesses activities against bacteria, fungi, and protozoa. Cytotoxic and parasiticidal effects of curcumin on protozoan parasites have been demonstrated in cultures against Leishmania, Trypanosoma, Giardia, and Plasmodium falciparum (23, 33, 34, 37, 40). In vivo, curcumin also displayed potent activity against Plasmodium berghei, and it was synergistic with artemisinin (32, 38). Although curcumin's pharmacological activities may be partially attributed to its inhibition of several cell signaling pathways and cellular enzymes in various biological systems (19), the molecular mechanism of the parasiticidal activity remains to be explored.
In eukaryotes, the packaging of genomic DNA into chromatin has functional significance for many cellular processes, including transcription, DNA replication, and repair (13). Nucleosome, the building block of chromatin, contains two copies of histones H2A, H2B, H3, and H4. Among the various posttranslational modifications of histone tails that confer epigenetic regulation of gene expression, histone lysine acetylation is the most extensively studied. Histone acetylation is catalyzed by histone acetyltransferases (HATs), and its removal by histone deacetylases (HDACs). Research in this field has established the fundamental importance of histone acetylation in development and revealed its great potential as a novel therapeutic target. To date, a number of HDAC inhibitors have already been in clinical trials for anticancer therapy (29). Like other eukaryotes, malaria parasites have a typical chromatin structure and encode multiple HATs and HDACs (12, 31). The P. falciparum general control nonderepressed 5 (PfGCN5) is a HAT that preferentially acetylates K9 and K14 of histone H3 (12). The importance of epigenetics in malaria parasite development suggests that pathways involved in parasite chromatin modifications may be practical drug targets (36). Indeed, drugs that act on HDACs and interfere with histone acetylation in the parasites have strong antiparasitic effects (2, 10, 28). In search of HAT inhibitors, curcumin has been recognized as an inhibitor of the HAT p300/CREB-binding protein (CBP) (4), suggesting that its effect on HATs may partially account for its biological activities in different systems. Interestingly, the inhibitor activity of curcumin on HAT is selective, since it does not inhibit the p300/CBP-associated factor of the GNAT (Gcn5-related acetyltranferase) superfamily of HATs (4).
To address the anti-Plasmodium effect of curcumin, we have evaluated the dose-dependent activity of curcumin on P. falciparum in in vitro culture. In this paper, we report that curcumin is potent against both chloroquine (CQ)-susceptible (CQS) and -resistant (CQR) P. falciparum strains, and the parasiticidal effect is at least partially due to the generation of reactive oxygen species (ROS), and down-regulation of the PfGCN5 HAT activity.
MATERIALS AND METHODS
Parasites and culture.
Two CQS (3D7 and D10) and two CQR strains (Dd2 and 7G8) of P. falciparum were obtained from the malaria reagent depository MR4 (ATCC, Manassas, VA). Parasites were grown in human O+ erythrocytes at 5% hematocrit in a complete medium (RPMI 1640 medium supplemented with 25 mM HEPES, pH 7.5, 25 mM sodium bicarbonate, 50 mg/liter hypoxanthine, 0.5% Albumax II, and 40 μg/ml gentamicin sulfate) (44). Cultures were maintained at 37°C in a gas mixture of 5% CO2 and 3% O2. Synchronization was done twice at the ring stage by 5% sorbitol treatment (24).
Susceptibility of P. falciparum to curcumin in vitro.
A stock solution of curcumin (Sigma, St. Louis, MO) was made in dimethyl sulfoxide (DMSO) at 100 mM. The effect of curcumin on parasite in vitro growth was tested using a standard [3H]hypoxanthine labeling method (11, 30) with serial dilutions of the drug to final concentrations of 5 to 60 μM. Briefly, ring-stage parasite culture was diluted to 1% parasitemia and 5% hematocrit in a complete low-hypoxanthine medium (5 mg/liter hypoxanthine) and seeded in triplicate in 96-well flat-bottom plates. Curcumin or DMSO was added to 200 μl of culture and incubated at 37°C for 24 h. Subsequently, 100 μl supernatant was replaced with an equal volume of complete low-hypoxanthine medium containing 1 μCi of [3H]hypoxanthine (Amersham, Piscataway, NJ). The culture was incubated for another 20 h and harvested onto glass-fiber filters using a cell harvester. The filters were counted using a TriLux microbeta counter (Perkin-Elmer, Wellesley, MA), and the value from uninfected red blood cell control was subtracted from the experimental values. Inhibitory concentration (IC) values were calculated by a linear regression method using KaleidaGraph version 3.5 (Synergy Software, Reading, PA). The 95% confidence intervals of the IC values were calculated using the inverse prediction from the linear equation. To determine the long-term effects of concentrations of curcumin below the 50% inhibitory concentration (IC50) on parasite growth, equal numbers of parasites were seeded in 24-well plates at 0.5% parasitemia and 5% hematocrit. Two parasite strains (3D7 and 7G8) were treated with or without 5 or 20 μM curcumin. Parasitemia was determined daily for 4 days by counting Giemsa-stained slides.
Measurement of intracellular ROS.
The effect of curcumin on the level of intracellular ROS was measured by the alteration of fluorescence resulting from oxidation of 29,79-dichlorofluorescein diacetate (DCFH-DA) (Molecular Probes, Eugene, OR) (25). Although DCFH-DA is generally thought to react with various types of ROS and used to study oxidative stress in cells, an in vitro study showed that H2O2 is the major ROS that oxidizes DCFH-DA (25). Therefore, DCFH-DA may detect only part of the ROS induced by curcumin. A DCFH-DA stock of 100 mM was made in DMSO. Cells were incubated with 50 μM DCFH-DA at 37°C for 30 min, washed with RPMI 1640 medium, and treated with different concentrations of curcumin for 4 h in the absence or presence of antioxidants (ascorbic acid and reduced glutathione) or an ROS scavenger, mannitol. The intensity of fluorescence was determined by flow cytometry with an excitation filter at 485 nm and an emission filter at 535 nm. The ROS level was calculated as the mean fluorescence intensity of exposed parasites.
The antagonistic effect of mannitol against curcumin in parasite culture was estimated using a parasite maturation assay (43). Synchronized ring-stage parasites were incubated with 50 μM curcumin in 24-well plates with increasing concentrations of mannitol. At the moment when schizonts were mature in the control, the numbers of schizonts in 5,000 red blood cells in each of the control and treated wells were counted.
Effect of curcumin on HAT activity.
The effect of curcumin on PfGCN5 HAT activity was tested using an in vitro HAT assay (4, 12). Briefly, 1 μg of bovine core histones and 30 ng of purified recombinant PfGCN5 HAT domain (recombinant GCN5 [rGCN5]) were incubated in HAT buffer in the absence or presence of curcumin at 30°C for 10 min (12). The reaction mixtures were further incubated with 0.1 μCi of [3H]acetyl coenzyme A (Amersham) for 10 min. Half of the reaction mixture was blotted onto P-81 filters (Whatman, Florham Park, NJ), washed with 50 mM NaHCO3-Na2CO3 buffer (pH 9.2), dried, and the radioactive counts were determined with a Beckman Coulter scintillation counter. The other half was separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and processed for fluorography (12).
To study the inhibition of PfGCN5 by curcumin in cultured parasites, P. falciparum 3D7 trophozoites were treated with 25, 50, or 100 μM curcumin for 8 h. To measure the effects of ROS scavengers on parasite HAT activity, trophozoites were treated with 50 μM curcumin in the presence or absence of 300 μM mannitol, 5,000 units/ml catalase (CAT), or 400 units/ml superoxide dismutase (SOD) (Sigma) for 8 h. Nuclear extracts were prepared from 3 × 108 parasites for each treatment. Parasite pellet released from saponin treatment was resuspended in 100 μl of hypotonic buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.5 mM EDTA, and 1% protease inhibitor cocktail) for 10 min on ice. Nuclei were harvested by centrifugation at 10,000 × g for 2 min. The nuclear pellet was resuspended in 50 μl of buffer B (20 mM HEPES, pH 7.9, 10% glycerol, 200 mM KCl, 0.5 mM dithiothreitol, 0.5 mM EDTA, 1% protease inhibitor cocktail, and 0.5% NP-40) and ground with a Dounce homogenizer. Nuclear extracts were collected after centrifugation at 10,000 × g for 10 min at 4°C. HAT activity of nuclear extracts was determined essentially as described above using 5 μl of nuclear extract and 1 μg of purified recombinant P. falciparum H3 (PfH3) as the substrate (31).
Effect of curcumin on histone acetylation.
To determine whether inhibition of PfGCN5 by curcumin in cultured parasites leads to the change of histone acetylation, unsynchronized 3D7 parasites were treated with 20 μM curcumin for 4 or 12 h. Aliquots of the same culture were treated with or without 20 μM curcumin beginning at 0 and 8 h, respectively, and parasites were harvested at 12 h. Histones were extracted from parasites using an acid extraction method (31). Equal amounts of histones were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with a panel of antibodies specific for histone acetylation on different lysine residues (Upstate Biotechnology, Charlottesville, VA).
Measurement of curcumin-induced DNA damage.
To determine whether curcumin-induced ROS causes mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) damage, a real-time PCR was performed by the method of Yakes and van Houten (45). This technique is based on the principle that lesions in the target DNA block the progression of DNA polymerase and thus are inversely proportional to the amplification efficiency. For this experiment, early trophozoites in 2 ml of complete medium were seeded in 24-well plates at 5% parasitemia and 5% hematocrit and treated with curcumin or DMSO control for 6 h at 37°C. Genomic DNA was isolated from parasite pellets using a proteinase K digestion and phenol-chloroform extraction method (8). DNA concentrations were accurately determined by dot blot analysis. Blotting of serially diluted DNA onto a nylon membrane, labeling of 3D7 genomic DNA with [32P]dATP by a random priming protocol, and molecular hybridization were performed as previously described (8). The hybridization intensity of each dot was measured using a phosphorimager (Molecular Dynamics, Piscataway, NJ). All DNA samples were subsequently adjusted to a final concentration of 50 ng/μl. For mtDNA damage, primers (5′-GGTCATTGATCATTACAT-3′ and 5′-TACATGACTAATTACTCC-3′) were designed to amplify a 214-bp fragment corresponding to 2615 to 2829 of the mtDNA sequence. For nDNA damage, primers (5′-AAAGGAGGGAATCCTGAC-3′ and 5′-CAACATCAGCATTCTTGTC-3′) were used to amplify a 196-bp fragment of the seryl-tRNA synthetase gene (PF07_0073). Real-time PCR was performed on Opticon DNA Engine II (Bio-Rad, Hercules, CA) using a SYBR green PCR kit (Roche Applied Science, Indianapolis, IN) with 1 ng of DNA from each sample (31). The cycle threshold (CT) was defined as the cycle number at which the change of fluorescence in the reaction exceeds 10 standard deviations above the mean fluorescence between cycles 3 and 7. Since we wanted to determine the relative amount of amplifiable targets in the samples, a relative quantification method was used and increase in DNA lesions was expressed as 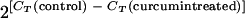 , where CT(control) is the CT of the control and CT(curcumin treated) is the CT of the curcumin-treated sample. The amplification efficiency from the control was arbitrarily set at 1.
, where CT(control) is the CT of the control and CT(curcumin treated) is the CT of the curcumin-treated sample. The amplification efficiency from the control was arbitrarily set at 1.
Detection of curcumin-induced DNA double-strand breaks.
Ring-stage parasites were treated with 20 or 50 μM curcumin with or without 400 μM mannitol for 12 h in a 24-well plate. Parasites were purified with Percoll and adhered to poly-l-lysine-treated slides. Parasites containing apoptotic nuclei were labeled by in situ terminal deoxynucleotidyltransferase-mediated dUTP-nick end labeling (TUNEL) using the ApoAlert DNA fragmentation assay kit (Clontech, Mountain View, CA) according to the manufacturer's instructions. Fragmented DNA was labeled with fluorescein-dUTP, and parasites were counterstained with propidium iodide. Slides were viewed with a Nikon fluorescence microscope, and 300 cells were counted for each treatment to calculate the percentage of fluorescein-labeled cells. Each treatment was performed in three replicate samples.
Statistical analysis.
All experiments were performed in triplicate. The effects among treatments in each experiment were compared using Tukey's pairwise t test.
RESULTS
Parasiticidal effect of curcumin on P. falciparum strains.
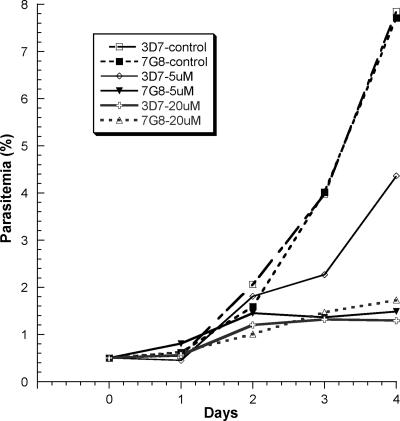
Curcumin was a potent inhibitor for both CQS and CQR parasite strains with IC50s in the 20 to 30 μM range (Table 1). The difference in IC values between parasite strains was probably due to their divergent genetic backgrounds. The long-term growth inhibition effect of curcumin was further tested on two parasite strains at 5 and 20 μM (Fig. 1). These concentrations of curcumin greatly inhibited the growth of parasites of both strains 3D7 and 7G8. In the presence of 20 μM curcumin, there was only a threefold increase in parasitemia by day 4, whereas there was a >15-fold increase in the controls. When synchronized late ring-stage parasites were treated with 20 μM curcumin, gross morphological changes of the parasites were not observed at 12 h. At later times, the development of the parasites was retarded and became less synchronous. At 50 μM, curcumin had more profound effects on parasite morphology even at 12 h (data not shown).
TABLE 1.
Sensitivities of four P. falciparum strains to curcumin in vitro
| Parasite strain | IC50 (μM)a | IC90 (μM)a |
|---|---|---|
| 3D7 | 24.69 ± 0.47 A | 44.45 ± 0.52 A |
| D10 | 22.93 ± 0.40 B | 41.27 ± 0.43 B |
| 7G8 | 29.61 ± 0.66 C | 53.29 ± 0.76 C |
| Dd2 | 27.45 ± 0.65 D | 49.42 ± 0.74 D |
IC50 and IC90 values are shown as means ± 95% confidence intervals. In the same column, values labeled with different letters are significantly different at P = 0.05 (Tukey's t test).
FIG. 1.
Effects of curcumin on in vitro growth of P. falciparum strains 3D7 and 7G8. Synchronized parasites at the ring stage were treated with 5 or 20 μM curcumin. Parasitemia was determined daily. Each point represents the mean of three replicate samples.
Curcumin affects intracellular ROS levels.
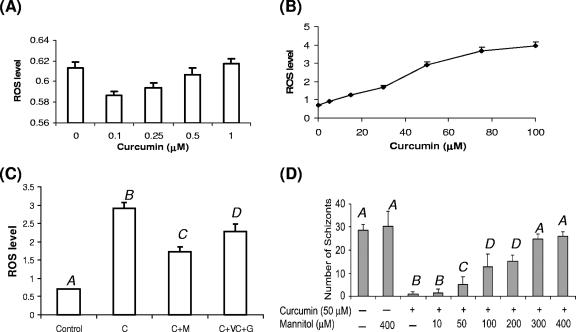
Curcumin has both antioxidant and prooxidant properties depending on its concentration. Its cytotoxic activity on many cancer cells is attributed to its prooxidant activity through the generation of ROS (5). Since the ROS types detected by DCFH-DA in cells are not completely clear, this study determined the DCFH-DA-measurable ROS induced by curcumin in the parasite. At lower concentrations (<1 μM), curcumin slightly reduced intracellular ROS levels (Fig. 2A), whereas at higher concentrations, it promoted intracellular ROS levels in a concentration-dependent manner (Fig. 2B). Apparently, P. falciparum was more sensitive than some mammalian cells, since a curcumin concentration as low as 1 μM had already resulted in the elevation of intracellular ROS. Furthermore, curcumin-induced increase of intracellular ROS could be attenuated by both antioxidants and ROS scavengers. Coincubation with 50 μM mannitol diminished the curcumin-induced ROS level (Fig. 2C). Similarly, 1 mM (each) of ascorbic acid and reduced glutathione also reduced the ROS level to that of controls (Fig. 2C). To determine whether this attenuation of curcumin-induced ROS could lead to improved parasite growth, we compared parasite development after treatment with 50 μM curcumin and different concentrations of mannitol using a parasite maturation assay. The results showed that mannitol alone did not significantly improve the development of untreated parasites, but it improved the growth of curcumin-treated parasites in a concentration-dependent way (Fig. 2D). Whereas treatment with 50 μM curcumin nearly completely blocked parasite maturation, coincubation with 300 or 400 μM mannitol restored the parasite growth almost to the levels of the controls. This suggests that high concentrations of mannitol protected the parasites from all DCFH-DA-measurable ROS.
FIG. 2.
(A) Antioxidant activity of curcumin at concentrations of <1 μM. The y axis indicates the fluorescence intensity of the parasites after treatment with different concentrations of curcumin. (B) Concentration-dependent prooxidant activity of curcumin and elevation of intracellular ROS levels in P. falciparum. (C) Effects of antioxidants and mannitol on the attenuation of curcumin-induced intracellular ROS. C, 50 μM curcumin; C+M, 50 μM curcumin plus 50 μM mannitol; C+VC+G, 50 μM curcumin plus 1 mM vitamin C plus 1 mM reduced glutathione. Bars labeled with different letters indicate significant differences at P = 0.001 (Tukey's HSD test). (D) Effect of mannitol on curcumin-induced parasite growth arrest. The y axis indicates the mean number of schizonts from three replicate samples in 5,000 red cells, while the x axis indicates different treatments (+, present; −, absent). Bars labeled with different letters are significantly different (Tukey's pairwise t test, P = 0.05). Standard deviations were calculated from three experiments.
Curcumin inhibits GCN5 HAT activity.
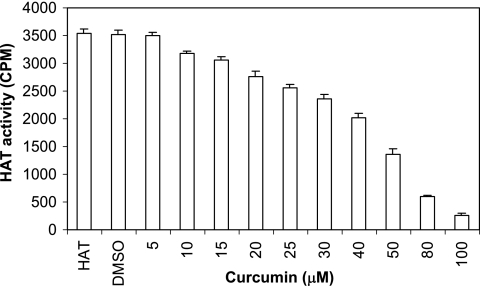
Curcumin was shown to inhibit the p300/CBP HAT activity in vitro and in vivo (4, 22). To determine whether curcumin could inhibit the GNAT superfamily member HAT PfGCN5, we performed an in vitro HAT assay using rGCN5 (12) in the presence of different concentrations of curcumin. As shown in Fig. 3, curcumin strongly inhibited the activity of rGCN5 with an IC50 of ∼48 μM. The presence of 100 μM curcumin abolished 95% of the HAT activity. When the HAT assays were performed with 50 μM curcumin alone or 50 μM curcumin plus 300 μM mannitol, 5,000 units/ml CAT, or 400 units/ml SOD, no significant effect was observed on rGCN5 activity (data not shown), suggesting that curcumin inhibits rGCN5 directly or that the activities of these anti-ROS agents on curcumin require intracellular environments.
FIG. 3.
Inhibition of recombinant PfGCN5 activity by curcumin. HAT assay was performed with rGCN5 in the presence or absence of curcumin using bovine core histones as substrates. DMSO was included as the solvent control. The IC50 calculated from this experiment was ∼48 μM. The means plus standard deviations (error bars) from three replicate samples are shown.
Curcumin affects histone acetylation in cultured parasites.
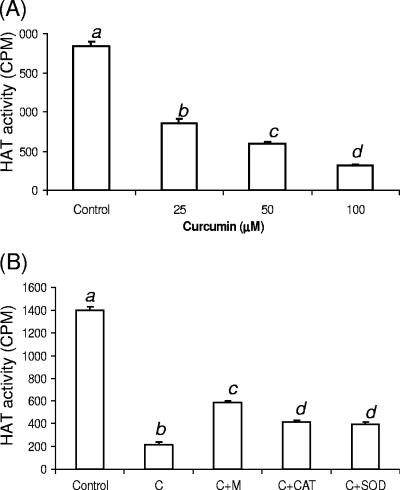
We determined the effect of curcumin on PfGCN5 in the parasite using a liquid HAT assay with parasite nuclear extract and recombinant PfH3. The results showed that brief treatment of the parasite culture for 8 h with 25 to 100 μM curcumin significantly reduced the HAT activity in the parasite nuclear extracts (Fig. 4A). This effect of curcumin on nuclear HAT activity could also be antagonized by treatment with mannitol, CAT, and SOD (Fig. 4B). Inclusion of each of these compounds in the culture medium with 50 μM curcumin restored nuclear HAT activity to different levels. Mannitol had the highest activity, owing probably to its ability to enter the cells. Since PfH3 is the preferred substrate of PfGCN5, the HAT inhibitor activity of curcumin probably acts on the PfGCN5 in vivo.
FIG. 4.
Effects of curcumin on in vivo HAT activity in P. falciparum. (A) Dosage-dependent inhibition of HAT activity in vivo. HAT activity in the same amount of nuclear extracts from control and curcumin-treated parasites for 8 h was measured using PfH3. (B) ROS scavenger and antioxidant enzymes antagonized curcumin-induced inhibition of HAT activity. HAT activity was measured with PfH3 and equal amounts of nuclear extracts from untreated (control) parasites and parasites treated with 50 μM curcumin alone (C) or coincubated with 300 μM mannitol (M), 5,000 units/ml catalase (CAT), or 400 units/ml superoxide dismutase (SOD). Values in each graph labeled with different letters are significantly different (Tukey's HSD test, P < 0.001). The means plus standard deviations (error bars) from three replicate samples are shown.
To more conclusively show selective inhibition of PfGCN5 by curcumin, we evaluated the levels of histone acetylation in P. falciparum using acetylation-specific antibodies. The results showed that treatment with 20 μM curcumin did not cause noticeable changes in the acetylation of H4 in vivo (Fig. 5). Similar levels of acetylation of H3-K9 and H3-K14 were also observed between control and treated parasites at 4 h, but hypoacetylation at these sites became apparent in curcumin-treated parasites at 12 h. Coincubation with mannitol increased acetylation at these two lysines, although the acetylation levels were not completely restored. This suggests that curcumin-induced H3 hypoacetylation only partially resulted from elevated intracellular ROS levels.
FIG. 5.
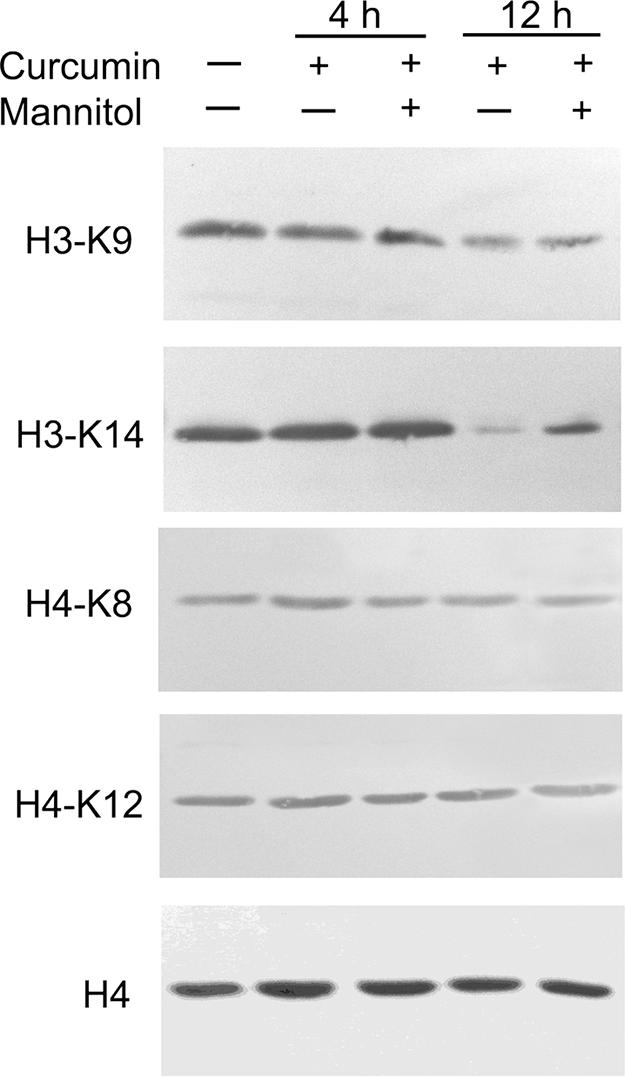
Effects of curcumin (20 μM) and mannitol (300 μM) treatments on histone acetylation in cultured parasites. Western blots were done with approximately equal amounts of histones extracted from control and curcumin-treated parasites at 4 and 12 h using a panel of acetylation-specific antibodies. The presence (+) or absence (−) of curcumin and mannitol is indicated above the gels. Antibodies against H4 were used to indicate approximately equal loads in the lanes.
Curcumin results in parasite DNA damage.
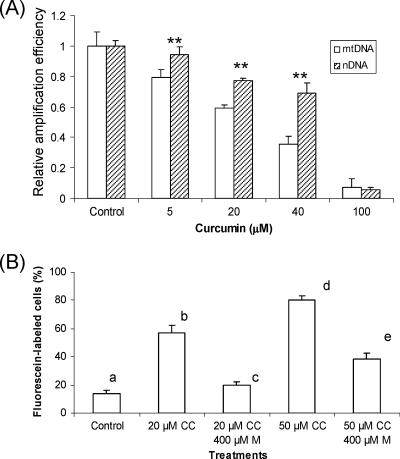
To investigate whether treatment with curcumin causes nDNA and mtDNA damage, we quantified lesions in parasite DNA by real-time PCR. The DNA concentration in each sample was accurately determined by dot blot analysis, and the same amount was used for real-time PCR analysis. The results showed that treatment with increasing concentrations of curcumin for 6 h caused a gradual increase of the CT value of the real-time PCR, indicating increased DNA lesions in the target sequences of both mtDNA and nDNA (Fig. 6). We noted that curcumin at a concentration of <40 μM caused more severe damage to mtDNA than to nDNA. To further demonstrate that curcumin causes DNA double-strand breaks in parasites, we performed in situ TUNEL analysis of parasites. Although we did not observe any apparent morphological changes in parasites after treatment with 20 μM curcumin for 12 h, significantly more parasites were labeled in the treated parasites than in the control parasites (P < 0.001, Tukey's honestly significant difference [HSD] test), indicative of an increase in drug-induced DNA breaks. However, nDNA laddering in treated parasites was not observed (data not shown).
FIG. 6.
Effect of ROS on DNA damage in P. falciparum measured by real-time PCR and TUNEL analysis. (A) Real-time PCR analysis. The relative amplification efficiency is inversely proportional to the frequency of DNA damage. The means plus standard deviations (error bars) from three experiments are shown. Asterisks indicate significant differences between mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) in the same treatment at a P value of 0.001 (Tukey's HSD test). (B) TUNEL analysis. Bars labeled with different letters indicate significant differences at a P value of 0.001 (Tukey's HSD test).
DISCUSSION
Curcumin has been the subject of intensive research due to its diverse pharmacological activities and proven biosafety (41). As a natural antioxidant, curcumin scavenges ROS and has anticarcinogenic activity (19). However, at higher concentrations or under certain conditions (e.g., in the presence of transition metal ions), curcumin also exhibits prooxidant properties and promotes ROS generation mainly in the form of hydroxyl radicals (1, 17). This cytotoxic property of curcumin and its ability to induce apoptosis of tumor cells are explored extensively in anticancer therapy (41). Here we demonstrated parasiticidal activity of curcumin against both CQS and CQR P. falciparum. This inhibitory effect is dosage dependent and evidenced by growth retardation, lack of maturation of parasites, and a decline of parasitemia. The difference in IC50 between our results and the results of others could be due to variations in culture conditions, parasite genetic background, developmental stages, drug purity, solvents for dissolving the drug, etc. Further, we have shown that constant exposure of the parasites to sub-IC50 doses of curcumin in vitro strongly inhibited parasite growth, leading to a sustained reduction in parasitemia. This is particularly relevant, since curcumin normally has low oral bioavailability. In this context, the synergistic effect of orally delivered curcumin with artemisinin in protecting mice against P. berghei is quite promising (32).
Curcumin-induced ROS production is linked directly to cytotoxicity through damage of proteins, DNA, and lipids, often resulting in cell death (5, 15). We have shown that curcumin treatment damaged mtDNA and nDNA, readily detectable by quantitative PCR and TUNEL analysis. However, DNA damage was atypical of apoptosis, without DNA ladder formation. This could be due to the lack in malaria parasites of a histone variant H2AX homologue, which is required for DNA ladder formation in human cells (27). Consistent with findings from human cells following oxidative stress (45), mtDNA damage in P. falciparum was more severe than nDNA damage. Curcumin-induced DNA damage in malaria parasites may be due to ROS production, because curcumin-induced ROS has been shown to cause oxidative DNA damage both in vitro and in vivo (1, 6). It is noteworthy that a curcumin concentration as low as 1 μM could elevate the intracellular ROS level in P. falciparum, whereas 50 μM curcumin caused an increase of >fourfold. This prooxidant activity of curcumin on malaria parasites could be attenuated by antioxidants and ROS scavengers. Culturing of curcumin-treated parasites in the presence of mannitol not only reduced the intracellular ROS levels but also restored parasite growth to certain extents. These results show ROS as a major effector of curcumin in killing malaria parasites.
The diverse pharmacological activities of curcumin have been attributed to its actions on multiple cellular targets. In addition to cyclooxygenase, cytochrome P450, glutathione S-transferase, protein kinase C, and telomerase (9, 19, 41), curcumin can also inhibit HATs and HDACs (4, 26), thereby affecting the status of histone acetylation (16, 18, 20-22, 35). In these cases, histone hypoacetylation occurs on both H3 and H4. Interestingly, we found that curcumin induced significant hypoacetylation of H3-K9 and H3-K14 in P. falciparum, whereas it did not perturb acetylation of the four lysines on H4, the presumable targets of MYST (MOZ, Ybf2/SAS3, SAS2, Tip60) family HATs. In vitro, curcumin directly inhibited the rGCN5 activity with an IC50 (∼48 μM) in a range similar to that for p300/CBP (∼25 μM). Moreover, nuclear extracts from curcumin-treated parasites also displayed reduced HAT activity towards PfH3, the preferred target of PfGCN5 HAT (12). Our results indicate that curcumin specifically inhibits PfGCN5 HAT activity in P. falciparum. Since inhibition of HATs and histone hypoacetylation could be partially reversed by ROS scavengers, it is plausible that ROS inflicts HAT enzymatic activity through oxidation of its essential residues. Collectively, curcumin-induced generation of ROS may lead to histone hypoacetylation and DNA damage that account for the parasiticidal effect of curcumin.
This work and earlier studies on HDAC inhibitors have demonstrated that histone acetylation is a viable target of antimalarial drug development (2, 10, 28). Yet, despite its in vitro parasiticidal activity, there was concern that curcumin's antioxidant and ROS-scavenging ability may overcome the in vivo inhibitory effects of macrophage-produced nitric oxide on protozoan parasites (7). Since nitric oxide has a crucial role in eliminating infections such as malaria parasites (3, 14), the ROS-scavenging activity of curcumin may exacerbate parasitic infections. Nevertheless, the biological effect of curcumin in the mouse malaria model is encouraging (32, 38). Because curcumin's antioxidant and prooxidant properties are dependent on the environment, its in vivo effects on parasitic diseases require further investigations.
Acknowledgments
We thank Guofa Zhou and Xinyi Li for helping with statistical analysis. We also thank Kelli Hoover and Gary Felton for advice on the antioxidant studies.
This work was supported by NIAID, National Institutes of Health.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Ahsan, H., N. Parveen, N. U. Khan, and S. M. Hadi. 1999. Pro-oxidant, antioxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem. Biol. Interact. 121:161-175. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, K. T., A. Walduck, M. J. Kelso, D. P. Fairlie, A. Saul, and P. G. Parsons. 2000. Anti-malarial effect of histone deacetylation inhibitors and mammalian tumour cytodifferentiating agents. Int. J. Parasitol. 30:761-768. [DOI] [PubMed] [Google Scholar]
- 3.Awasthi, A., A. Kumar, S. N. Upadhyay, T. Yamada, and Y. Matsunaga. 2003. Nitric oxide protects against chloroquine resistant Plasmodium yoelii nigriensis parasites in vitro. Exp. Parasitol. 105:184-191. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanyam, K., R. A. Varier, M. Altaf, V. Swaminathan, N. B. Siddappa, U. Ranga, and T. K. Kundu. 2004. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 279:51163-51171. [DOI] [PubMed] [Google Scholar]
- 5.Bhaumik, S., R. Anjum, N. Rangaraj, B. V. V. Pardhasaradhi, and A. Khar. 1999. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 456:311-314. [DOI] [PubMed] [Google Scholar]
- 6.Cao, J., L. Jia, H. M. Zhou, Y. Liu, and L. F. Zhong. 2006. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. 91:476-483. [DOI] [PubMed] [Google Scholar]
- 7.Chan, M. M. Y., N. S. Adapala, and D. Fong. 2005. Curcumin overcomes the inhibitory effect of nitric oxide on Leishmania. Parasitol. Res. 96:49-56. [DOI] [PubMed] [Google Scholar]
- 8.Cui, L., K. A. Rzomp, Q. Fan, S. K. Martin, and J. Williams. 2001. Plasmodium falciparum: differential display analysis of gene expression during gametocytogenesis. Exp. Parasitol. 99:244-254. [DOI] [PubMed] [Google Scholar]
- 9.Cui, S. X., X. J. Qu, Y. Y. Xie, L. Zhou, M. Nakata, M. Makuuchi, and W. Tang. 2006. Curcumin inhibits telomerase activity in human cancer cell lines. Int. J. Mol. Med. 18:227-231. [PubMed] [Google Scholar]
- 10.Darkin-Rattray, S. J., A. M. Gurnett, R. W. Myers, P. M. Dulski, T. M. Crumley, J. J. Allocco, C. Cannova, P. T. Meinke, S. L. Colletti, M. A. Bednarek, S. B. Singh, M. A. Goetz, A. W. Dombrowski, J. D. Polishook, and D. M. Schmatz. 1996. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 93:13143-13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan, Q., L. An, and L. Cui. 2004. Plasmodium falciparum histone acetyltransferase, a yeast GCN5 homologue involved in chromatin remodeling. Eukaryot. Cell 3:264-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsenfeld, G., and M. Groudine. 2003. Controlling the double helix. Nature 421:448-453. [DOI] [PubMed] [Google Scholar]
- 14.Fritsche, G., C. Larcher, H. Schennach, and G. Weiss. 2001. Regulatory interactions between iron and nitric oxide metabolism for immune defense against Plasmodium falciparum infection. J. Infect. Dis. 183:1388-1394. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa, S., T. Atsumi, M. Ishikara, and Y. Kadoma. 2004. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 24:563-569. [PubMed] [Google Scholar]
- 16.Gilmour, P. S., I. Rahman, K. Donaldson, and W. MacNee. 2003. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environment. Am. J. Physiol. Lung Cell Mol. Physiol. 284:L533-L540. [DOI] [PubMed] [Google Scholar]
- 17.Hadi, S. M., S. F. Asad, S. Singh, and A. Ahmad. 2000. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 50:167-171. [DOI] [PubMed] [Google Scholar]
- 18.Ito, K., T. Hanazawa, K. Tomita, P. J. Barnes, and I. M. Adcock. 2004. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem. Biophys. Res. Commun. 315:240-245. [DOI] [PubMed] [Google Scholar]
- 19.Joe, B., M. Vijaykumar, and B. R. Lokesh. 2004. Biological properties of curcumin—cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 44:97-111. [DOI] [PubMed] [Google Scholar]
- 20.Kang, J., J. Chen, Y. Shi, J. Jia, and Y. Zhang. 2005. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem. Pharmacol. 69:1205-1213. [DOI] [PubMed] [Google Scholar]
- 21.Kang, J., Y. Zhang, J. Chen, H. Chen, C. Lin, Q. Wang, and Y. Ou. 2003. Nickel-induced histone hypoacetylation: the role of reactive oxygen species. Toxicol. Sci. 74:279-286. [DOI] [PubMed] [Google Scholar]
- 22.Kang, S. K., S. H. Cha, and H. G. Jeon. 2006. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 15:165-174. [DOI] [PubMed] [Google Scholar]
- 23.Koide, T., M. Nose, Y. Ogihara, Y. Yabu, and N. Ohta. 2002. Leishmanicidal effect of curcumin in vitro. Biol. Pharm. Bull. 25:131-133. [DOI] [PubMed] [Google Scholar]
- 24.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 25.LeBel, C. P., H. Ischiropoulos, and S. C. Bondy. 1992. Evaluation of the probe 29,79-dichlorofluorescein as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 5:227-231. [DOI] [PubMed] [Google Scholar]
- 26.Liu, H. L., Y. Chen, G. H. Cui, and J. F. Zhou. 2005. Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharm. Sin. 26:603-609. [DOI] [PubMed] [Google Scholar]
- 27.Lu, C., F. Zhu, Y.-Y. Cho, F. Tang, T. Zykova, W.-Y. Ma, A. M. Bode, and Z. Dong. 2006. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation caspase-3. Mol. Cell 23:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mai, A., I. Cerbara, S. Valente, S. Massa, L. A. Walker, and B. L. Tekwani. 2004. Antimalarial and antileishmanial activities of aroyl-pyrrolyl-hydroxyamides, a new class of histone deacetylase inhibitors. Antimicrob. Agents Chemother. 48:1435-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mai, A., S. Massa, D. Rotili, I. Cerbara, S. Valente, R. Pezzi, S. Simeoni, and R. Ragno. 2005. Histone deacetylation in epigenetics: an attractive target for anticancer therapy. Med. Res. Rev. 25:261-309. [DOI] [PubMed] [Google Scholar]
- 30.McConkey, G. A., I. Ittarat, S. R. Meshnick, and T. F. McCutchan. 1994. Auxotrophs of Plasmodium falciparum dependent on p-aminobenzoic acid for growth. Proc. Natl. Acad. Sci. USA 91:4244-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao, J., Q. Fan, L. Cui, J. Li, J. Li, and L. Cui. 2006. The malaria parasite Plasmodium falciparum histones: organization, expression, and acetylation. Gene 369:53-65. [DOI] [PubMed] [Google Scholar]
- 32.Nandakumar, D. N., V. A. Nagaraj, P. G. Vathsala, P. Rangarajan, and G. Padmanaban. 2006. Curcumin-artemisinin combination therapy for malaria. Antimicrob. Agents Chemother. 50:1859-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nose, M., T. Koide, Y. Ogihara, Y. Yabu, and N. Ohta. 1998. Trypanocidal effects of curcumin in vitro. Biol. Pharm. Bull. 21:643-645. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Arriaga, L., M. L. Mendoza-Magana, R. Cortes-Zarate, A. Corona-Rivera, L. Bobadilla-Morales, R. Troyo-Sanroman, and M. A. Ramirez-Herrera. 2006. Cytotoxic effect of curcumin on Giardia lamblia trophozoites. Acta Trop. 98:152-161. [DOI] [PubMed] [Google Scholar]
- 35.Rahman, I., J. Marwick, and P. Kirkman. 2004. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-κB and proinflammatory gene expression. Biochem. Pharmacol. 68:1255-1267. [DOI] [PubMed] [Google Scholar]
- 36.Ralph, S. A., and A. Scherf. 2005. The epigenetic control of antigenic variation in Plasmodium falciparum. Curr. Opin. Microbiol. 8:434-440. [DOI] [PubMed] [Google Scholar]
- 37.Rasmusen, H. B., S. B. Christensen, L. P. Kuist, and A. Karazami. 2000. A simple and efficient separation of the curcumins. The antiprotozoal constituents of Curcuma longa. Planta Med. 66:396-398. [DOI] [PubMed] [Google Scholar]
- 38.Reddy, R. C., P. G. Vatsala, V. G. Keshamouni, G. Padmanaban, and P. N. Rangarajan. 2005. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 326:472-474. [DOI] [PubMed] [Google Scholar]
- 39.Rosenthal, P. J. 2003. Antimalarial drug discovery: old and new approaches. J. Exp. Biol. 206:3735-3744. [DOI] [PubMed] [Google Scholar]
- 40.Saleheen, D., A. A. Syed, A. Khalid, A. S. Anwar, A. Ajmal, and M. Y. Muhammad. 2002. Latent activity of curcumin against leishmaniasis in vitro. Biol. Pharm. Bull. 25:386-389. [DOI] [PubMed] [Google Scholar]
- 41.Sharma, R. A., A. J. Gescher, and W. P. Steward. 2005. Curcumin: the story so far. Eur. J. Cancer 41:1955-1968. [DOI] [PubMed] [Google Scholar]
- 42.Tagboto, S., and S. Townson. 2001. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv. Parasitol. 50:199-295. [DOI] [PubMed] [Google Scholar]
- 43.Taguchi, N., T. Hatabu, H. Yamaguchi, M. Suzuki, K. Sato, and S. Kano. 2004. Plasmodium falciparum: selenium-induced cytotoxicity to P. falciparum. Exp. Parasitol. 106:50-55. [DOI] [PubMed] [Google Scholar]
- 44.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 45.Yakes, F. M., and B. van Houten. 1997. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 94:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]