Abstract
RBX11160 (OZ277) is a fully synthetic peroxidic antimalarial in clinical development. To study the possible mechanisms of action of RBX11160, we have examined its ability to inhibit PfATP6, a sarcoplasmic reticulum calcium ATPase and proposed target for semisynthetic peroxidic artemisinin derivatives. RBX11160 inhibits PfATP6 (apparent half-maximal inhibitory constant = 7,700 nM) less potently than artemisinin (79 nM). Inhibition of PfATP6 is abrogated by desferrioxamine, an iron-chelating agent. Consistent with this finding, the killing of Plasmodium falciparum organisms by RBX11160 in vitro is antagonized by desferrioxamine. Artesunate and RBX11160 also act antagonistically against P. falciparum in vitro. A fluorescent derivative of RBX11160 localizes to the parasite cytosol in some parasites and to the food vacuole in other parasites. These data demonstrate that there are both similarities and differences between the antimalarial properties of RBX11160 and those of semisynthetic antimalarials such as artesunate and artemisinin.
Artemisinins are our most important class of antimalarials because they are effective against multidrug-resistant parasites and they can be used to treat severe malaria (13). They kill parasites in asexual stages of infection and prevent the early development of gametocytes, but they also suffer from several limitations. Most artemisinins in current use are semisynthetic derivatives of artemisinin that must be extracted from sweet wormwood (Artemisia annua) (4). As the demand for artemisinins has increased due to widespread use of artemisinin-containing combination therapies to treat malaria, the supply of raw materials has become limiting and has influenced both price and availability. Large-scale production of artemisinins by conventional chemical techniques is currently precluded because it involves many (>10) inefficient steps (3). Biological solutions for engineering the production of artemisinins in microorganisms are being pursued but are not yet at the stage of being commercially viable (10). A fully synthetic antimalarial whose activity matches that of artemisinins would have several potential advantages, including a secure supply and cost.
RBX11160 is one such fully synthetic peroxidic antimalarial under clinical development as an alternative to artemisinins (12). In RBX11160, the critical peroxidic pharmacophore of the artemisinins is present within a 1,2,4-trioxolane rather than a 1,2,4-trioxane heterocycle. As artemisinins are proposed to interact with a Plasmodium falciparum-encoded calcium-transporting ATPase (PfATP6) to kill parasites (1, 8), we tested the idea that PfATP6 may also be inhibited specifically by RBX11160. In addition, other aspects of the behavior of RBX11160, such as subcellular distribution, iron dependency, and interaction with artemisinins, have also been examined to increase the understanding of the mechanism of action of this drug, which is currently in an advanced stage of clinical development.
MATERIALS AND METHODS
ATPase assays.
Xenopus laevis oocytes were microinjected with cRNA (∼25 ng/egg) encoding PfATP6 or control material (RNase-free water) or cRNA encoding PfHT, the P. falciparum hexose transporter (15), and incubated for 4 to 7 days before the membranes were harvested for the assay. Oocyte membranes were prepared by a two-step centrifugation process, and ATPase activity was measured (pCa 5.5, 10 μg protein/assay, 25°C, at 340 nm) using a coupled enzyme assay as described previously (7).
For inhibitor studies, comparisons were made between ATPase activities in preparations without inhibitors (but with solvent) added and those in the same batch of oocytes tested with an inhibitor. Control oocyte membrane preparations were assayed to confirm that PfATP6 was expressed. To estimate apparent Ki (half-maximal inhibition) values for Ca2+-dependent ATPase activity, single-site competition models were fitted to the data (GraphPad Prism, version 3). Control experiments also included a preparation of mammalian sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) (1). RBX11160 tosylate was kindly provided by J. L. Vennerstrom (University of Nebraska) and artesunate by Dafra Pharma, and desferrioxamine (DFO) was from Sigma.
PfHT glucose uptake experiments.
Competitive uptake on glucose was carried out as described previously (15) and assayed between 36 and 48 h after microinjection, at room temperature for 20 to 30 min, on groups of eight oocytes in Barth's medium containing d-glucose (d-[U-14C]glucose; specific activity, 10.4 GBq/mmol; Amersham Biosciences) and unlabeled glucose (35 μM) with 50 μM RBX11160. At least three independent experiments were carried out.
Culture of parasites and isobolograms.
Parasites (clone 3D7) were cultured using standard techniques (11). After determination of 50% inhibitory concentration (IC50) values (within 48 h of addition of antimalarials/inhibitors), fixed ratios of concentrations of inhibitor A to those of inhibitor B were used (A/B ratios of 10:0, 9:1, 7:3, 5:5, 3:7, 1:9, and 0:10). The middle concentration of the inhibitors (5:5) was aimed to be the IC50 of each drug. Subsequently, the molar ratios of each drug were calculated, and the IC50s of the mixtures are presented in relation to the IC50s of the individual drugs.
Dye loading of P. falciparum.
P. falciparum clone 3D7 was washed twice in Ringer's solution (122.5 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 0.8 mM MgCl2, 11 mM d-glucose, 10 mM HEPES, 1 mM NaHPO4, pH 7.4) and incubated in 500 nM endoplasmic reticulum (ER)-Tracker Blue-White Dapoxyl (DPX) in Ringer's solution in the presence of Pluronic F-127 (0.025%, 45 min, 37°C) and in 500 nM TAMRA (6-carboxytetramethylrhodamine)-RBX11160 (20 min, 37°C).
Immunofluorescence microscopy.
Confocal laser scanning fluorescence microscopy was performed using a Zeiss LSM510 META (Carl Zeiss, Jena, Germany). Images were obtained with a 63× lens and an image resolution of 512 by 512 pixels. The laser lines and filter settings used in Multi-Track mode were as follows: ER-Tracker Blue-White DPX was excited at 364 nm, with emission detected using a band-pass 505- to 530-nm filter (blue channel), and TAMRA-RBX11160 was excited at 543 nm, with emission detected using a band-pass 560- to 615-nm filter (green channel). Throughout, a pinhole of 1.5 μm was chosen. Conventional immunofluorescence microscopy was performed after NF54 cells were incubated in 600 nM TAMRA-RBX11160 (40 min, 37°C, slow shaking) and then washed three times with culture medium, diluted 1:6 in a 50% glycerine-50% phosphate-buffered saline solution.
Statistical analyses.
Parametric comparisons were made with Student's unpaired t test, and nonparametric comparisons were made with the Mann-Whitney U statistic.
RESULTS
Inhibition of PfATP6 activity by RBX11160.
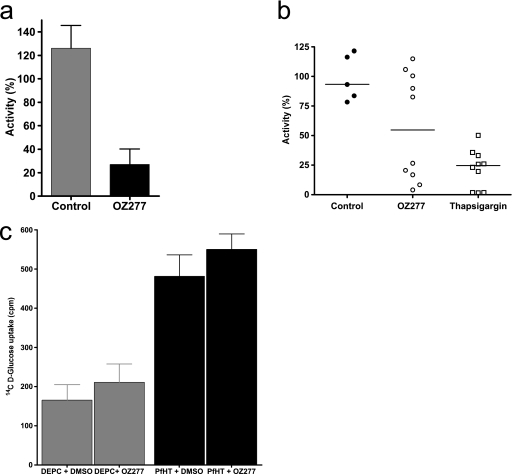
In the initial screening, the inhibition of PfATP6 activity by RBX11160 at relatively high concentrations (50 μM) was assayed after expression in Xenopus oocytes. Figure 1a shows significant inhibition of PfATP6 by RBX11160 (P = 0.0018). To examine the specificity of this inhibition, RBX11160 was also applied to rabbit muscle SERCA, and the inhibition of calcium-dependent ATPase activity was compared with that caused by thapsigargin, a highly specific and potent inhibitor of this class of transporter. Figure 1b shows that thapsigargin significantly reduces mammalian ATPase activity (by a median of 75%; P was 0.0007 for comparison with control preparations). In contrast, RBX11160 inhibits mammalian SERCA less potently (50%; P = 0.13), although there is a dichotomous pattern to the degrees of inhibition and these can be >80% in some experiments.
FIG. 1.
RBX11160 inhibition of transporter activities. (a) Inhibition of PfATP6 activity by RBX11160 (50 μM) compared with results for control preparations (n = 6 for both groups; P = 0.0018). (b) Inhibition of mammalian SERCA by RBX11160 (100 μM; P = 0.13) and thapsigargin (10 μM; P = 0.0007) compared with results for control experiments. (c) Uptake of d-glucose in water-injected (diethyl pyrocarbonate [DEPC]) and PfHT-expressing oocytes is shown. There is no inhibition of PfHT activity by RBX11160 (50 μM, n ≥ 9 per column; P > 0.5). DMSO, dimethyl sulfoxide.
To confirm that RBX11160 inhibits PfATP6 and not other P. falciparum-encoded transport proteins, the inhibition of the falciparum hexose transporter was also studied after heterologous expression in oocytes. Figure 1c shows that PfHT significantly increases uptake of d-[14C]glucose into oocytes (approximately threefold; P was <0.001 for comparison with water-injected controls, with or without exposure to dimethyl sulfoxide used as a solvent for RBX11160). There is no inhibition of PfHT-mediated glucose uptake (P > 0.5) by relatively high concentrations (50 μM) of RBX11160.
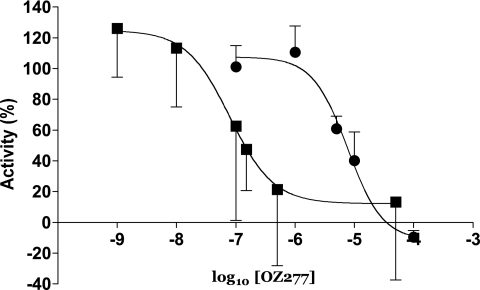
An assay of the apparent inhibitory constants (Ki) of RBX11160 and artemisinin for PfATP6 (Fig. 2) was carried out as previously described (7). The Ki for RBX11160 is 7,700 nM, compared with a value of 79 nM for artemisinin, showing that PfATP6 is less susceptible to inhibition by RBX11160 than by artemisinin in these assays. The differences in Ki for RBX11160 and artemisinin are in contrast to the relative potencies of these drugs in killing parasites. For example, the IC50 values for parasite strain NF54 (means ± standard errors of the means) are 1.6 ± 0.21 nM for RBX11160, compared to 4.2 ± 0.26 nM for artesunate (12).
FIG. 2.
Apparent inhibitory constants for PfATP6 of RBX11160 and artemisinin. The apparent Ki values are 7,700 nM for RBX11160 (filled circles) (n = 3 for each value) and 79 nM for artemisinin (filled squares) (n = 5 for each value).
Iron dependency of RBX11160 activity.
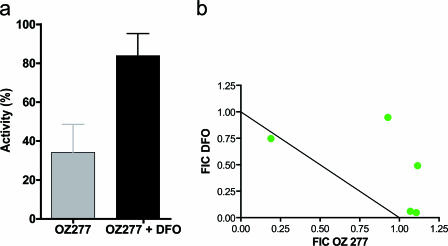
To examine the iron dependency of RBX11160, a tight-binding Fe3+-chelating agent (DFO) was used in assays of inhibition of PfATP6. There was abrogation of inhibition of PfATP6 by RBX11160 after the addition of DFO (100 μM; P was 0.037 for differences between groups) (Fig. 3a).
FIG. 3.
Effects of desferrioxamine on RBX11160. (a) Inhibition of PfATP6 activity by RBX11160 (gray column) (10 μM, n = 5) is abrogated by desferrioxamine (black column) (100 μM, P = 0.037, n = 9). (b) Isobologram of desferrioxamine and RBX11160. The summed fractional inhibitory concentration index (FIC) for this assay [geometric mean (range)] is 1.36 (0.97 to 2).
To see whether DFO effects extend from assays with oocytes to parasites in culture, isobolograms were constructed with RBX11160 and DFO. Figure 3b shows results from one of three independent experiments. These results are consistent with an antagonistic effect between DFO and RBX11160, as most points on the isobologram lie in the region indicating antagonism (above the line that indicates additive effects).
Localization of RBX11160 in parasites.
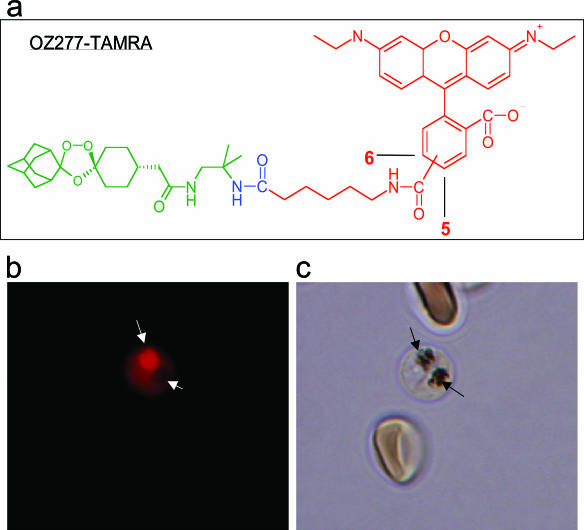
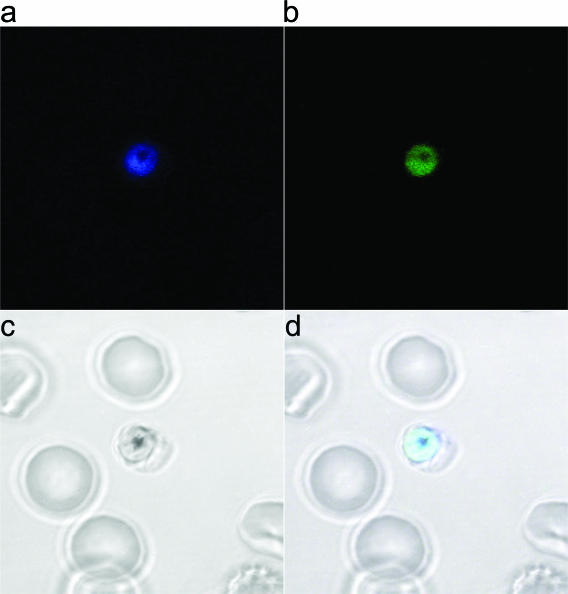
P. falciparum (NF54) was exposed to TAMRA-labeled RBX11160 (whose structure is shown in Fig. 4a) to determine the subcellular distribution of the fluorescent derivative. The IC50 value for TAMRA-RBX11160 (85 ± 3 nM [mean ± standard error of the mean]) is about 50-fold higher than that for unlabeled RBX11160 (see above). Parasites display two different patterns of distribution, observed in at least three independent experiments. Some parasites show the label in the cytosol only, with no staining of the food vacuole. Others show more-intense staining of the food vacuole, with relatively poorly stained cytosol. This differential pattern of staining does not appear to be a result of differences in parasite growth or metabolism, as illustrated by the staining of two parasites that are at similar stages of development in a single erythrocyte (Fig. 4b and c), where one shows diffuse cytosolic staining and the other predominant staining of the food vacuole.
FIG. 4.
Immunofluorescence labeling of trophozoites. (a) Chemical structure of RBX11160-TAMRA. (b) Immunofluorescent staining of two parasites in a single erythrocyte by labeled RBX11160 (a). One trophozoite shows intense staining of the food vacuole (indicated by a long white arrow), whereas the other shows negative staining of the pigment-containing food vacuole (short white arrow) with diffuse cytosolic staining. (c) Bright-field view of panel b. Black arrows indicate parasite pigments in the food vacuole.
Confocal images with ER-Tracker Blue (Fig. 5a) and RBX11160-TAMRA (Fig. 5b) show that in parasites with cytosolic labeling with RBX11160, this fluorescence signal coincides with that from ER-Tracker Blue (Fig. 5d) and suggests that RBX11160 is associated with the parasite endoplasmic reticulum when in the cytosol.
FIG. 5.
Confocal imaging of trophozoite stage. (a) Trophozoite labeled with ER-Tracker Blue showing cytosolic staining with negative staining of the food vacuole. (b) Corresponding bright-field image of panel a. (c) Parasite from panel a showing labeling with RBX11160-TAMRA. (d) Merged images (panels a to c).
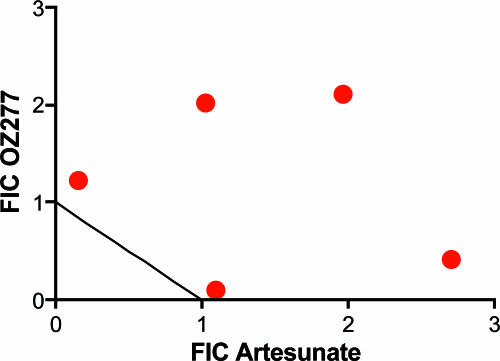
Isobologram of artesunate and RBX11160.
Previous studies with Gabonese isolates of P. falciparum showed a significant correlation between IC50 values for artesunate and RBX11160 (5). We extended these studies to include isobologram analysis for artesunate and RBX11160 with clone 3D7. The results suggest that artesunate and RBX11160 antagonize each others' activities (Fig. 6) .
FIG. 6.
Isobologram of RBX11160 and artesunate. Points lying above the line of additivity indicate antagonistic effects. The summed fractional inhibitory concentration index (FIC) for this assay [geometric mean (range)] is 2.4 (1.85 to 4.2).
DISCUSSION
New drugs are urgently needed to treat multidrug-resistant P. falciparum malaria (6, 14). Peroxidic antimalarials are still effective against parasites that have evolved resistance to aminoquinolines, folate antagonists, and the arylamino alcohols. The most important endoperoxides in use are semisynthetic artemisinin derivatives (13). Their mechanisms of action are being intensively investigated, and accumulating evidence suggests that they act by inhibition of PfATP6, a sarcoplasmic endoplasmic reticulum calcium ATPase encoded by P. falciparum (8). RBX11160, a fully synthetic trioxolane, is in advanced stages of clinical development, but its mechanism of action has not been fully characterized (12). As with trioxane artemisinins, an endoperoxide bond is critical for the antimalarial activity of RBX11160. The remainder of the molecule shares little by way of structural features with the sesquiterpene lactone that is found in artemisinin derivatives. It was therefore of interest to determine those aspects in which the mechanism of action of RBX11160 is distinguishable from that of the artemisinins and those where there may be overlap.
RBX11160 inhibits PfATP6 with relatively low potency (apparent Ki = 7,700 nM) compared with artemisinins. This activity is similar to that observed for artemisinin inhibition of Plasmodium berghei SERCA (Ki = 6,000 nM) and is approximately 2 log orders less potent than the activity against PfATP6 (Ki ∼ 100 nM). The difference between the concentrations of drug needed to kill parasites (as shown in measurements of IC50 values that are approximately 1.5 nM for RBX11160) and the potency of inhibition of PfATP6 suggests that there may be different mechanisms of action for RBX11160 and artemisinins. However, there are also many aspects that overlap in their mechanisms of action. The antimalarial activities of artemisinin and RBX11160 are both abrogated by DFO, which also inhibits the activity of RBX11160 against PfATP6 (Fig. 3). RBX11160 inhibits mammalian SERCA variably (by a median of ∼50%) at relatively high concentrations (100 μM), with dichotomous results in experiments carried out on the same SERCA preparations (some data points showing little inhibition and others showing obvious inhibition). However, RBX11160 does not inhibit the malarial hexose transporter PfHT at all, confirming that these inhibitory properties are not seen with all integral membrane transport proteins.
Both types of antimalarial (artemisinins and RBX11160) are concentrated in intraerythrocytic parasites 200- to 300-fold compared with extracellular concentrations (2, 9). The subcellular distribution of RBX11160 is similar to that of artemisinin in some parasite preparations where it is found in the parasite cytosol. Under identical circumstances, however, some parasites show accumulation of RBX11160 in the parasite's food vacuole whereas this is not a feature of the subcellular distribution of artemisinins (1). Also consistent with these findings is the observation that artesunate antagonizes the antimalarial activity of RBX11160 when this is examined in isobologram analysis of cultured parasites.
Other studies show that there is a positive correlation between the IC50 values for artesunate and RBX11160 determined for field isolates in Gabon (r2 = 0.5, P = 0.002, n = 38) and not, for example, between those for chloroquine or mefloquine and RBX11160 (5). Also, the stage specificity of antimalarial action of RBX11160 is very similar to that assessed for artemisinins (such as artemisinin itself and artemether) (9, 11).
Knowledge of the mechanism of action of antimalarials is critical to many aspects of their development and use. Drug prototypes may be improved by increasing their efficacy, minimizing their toxicity, and improving their pharmacokinetic behavior. If resistance to a drug is mediated by alterations in a target, this can be monitored once a target is identified (14). It may also be possible to bypass this type of resistance mechanism by appropriate alterations in drug structure.
These preliminary studies on the mechanism of antimalarial activity of RBX11160 illustrate many aspects that overlap with the mechanisms of action for artemisinin derivatives. However, there are also interesting differences in the behavior of the two types of antimalarial, particularly in the potencies of their inhibition of PfATP6 and the variability in the subcellular localization pattern of RBX11160. It will be of interest to test their relative potencies against stable artemisinin resistance parasites, a resource that is becoming available in animal models as well as more recently in in vitro studies of parasites obtained from French Guiana (8). In this way, the understanding of both mechanisms of action and resistance may be improved.
Acknowledgments
We thank Malcolm East (University of Southampton) for mammalian SERCA preparations.
This work was supported by Medicines for Malaria Venture.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Eckstein-Ludwig, U., R. J. Webb, I. D. van Goethem, J. M. East, A. G. Lee, M. Kimura, P. M. O'Neill, P. G. Bray, S. A. Ward, and S. Krishna. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957-961. [DOI] [PubMed] [Google Scholar]
- 2.Gu, H. M., D. C. Warhurst, and W. Peters. 1984. Uptake of [3H] dihydroartemisinine by erythrocytes infected with Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 78:265-270. [DOI] [PubMed] [Google Scholar]
- 3.Haynes, R. K. 2006. From artemisinin to new artemisinin antimalarials: biosynthesis, extraction, old and new derivatives, stereochemistry and medicinal chemistry requirements. Curr. Top. Med. Chem. 6:509-537. [DOI] [PubMed] [Google Scholar]
- 4.Haynes, R. K., and S. Krishna. 2004. Artemisinins: activities and actions. Microbes Infect. 6:1339-1346. [DOI] [PubMed] [Google Scholar]
- 5.Kreidenweiss, A., B. Mordmuller, S. Krishna, and P. G. Kremsner. 2006. Antimalarial activity of a synthetic endoperoxide (RBx-11160/OZ277) against Plasmodium falciparum isolates from Gabon. Antimicrob. Agents Chemother. 50:1535-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremsner, P. G., and S. Krishna. 2004. Antimalarial combinations. Lancet 364:285-294. [DOI] [PubMed] [Google Scholar]
- 7.Krishna, S., C. Woodrow, R. Webb, J. Penny, K. Takeyasu, M. Kimura, and J. M. East. 2001. Expression and functional characterization of a Plasmodium falciparum Ca2+-ATPase (PfATP4) belonging to a subclass unique to apicomplexan organisms. J. Biol. Chem. 276:10782-10787. [DOI] [PubMed] [Google Scholar]
- 8.Krishna, S., C. J. Woodrow, H. M. Staines, R. K. Haynes, and O. Mercereau-Puijalon. 2006. Re-evaluation of how artemisinins work in light of emerging evidence of in vitro resistance. Trends Mol. Med. 12:200-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maerki, S., R. Brun, S. A. Charman, A. Dorn, H. Matile, and S. Wittlin. 2006. In vitro assessment of the pharmacodynamic properties and the partitioning of OZ277/RBx-11160 in cultures of Plasmodium falciparum. J. Antimicrob. Chemother. 58:52-58. [DOI] [PubMed] [Google Scholar]
- 10.Ro, D. K., E. M. Paradise, M. Ouellet, K. J. Fisher, K. L. Newman, J. M. Ndungu, K. A. Ho, R. A. Eachus, T. S. Ham, J. Kirby, M. C. Chang, S. T. Withers, Y. Shiba, R. Sarpong, and J. D. Keasling. 2006. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940-943. [DOI] [PubMed] [Google Scholar]
- 11.ter Kuile, F., N. J. White, P. H. Holloway, G. Pasvol, and S. Krishna. 1993. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 76:85-95. [DOI] [PubMed] [Google Scholar]
- 12.Vennerstrom, J. L., S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. Chiu, J. Chollet, Y. Dong, A. Dorn, D. Hunziker, H. Matile, K. McIntosh, M. Padmanilayam, J. Santo Tomas, C. Scheurer, B. Scorneaux, Y. Tang, H. Urwyler, S. Wittlin, and W. N. Charman. 2004. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 430:900-904. [DOI] [PubMed] [Google Scholar]
- 13.Woodrow, C. J., R. K. Haynes, and S. Krishna. 2005. Artemisinins. Postgrad. Med. J. 81:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodrow, C. J., and S. Krishna. 2006. Antimalarial drugs: recent advances in molecular determinants of resistance and their clinical significance. Cell. Mol. Life Sci. 63:1586-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodrow, C. J., J. I. Penny, and S. Krishna. 1999. Intraerythrocytic Plasmodium falciparum expresses a high-affinity facilitative hexose transporter. J. Biol. Chem. 274:7272-7277. [DOI] [PubMed] [Google Scholar]